Abstract
Clinical tongue-strengthening therapy programs are generally based on the principles of exercise and motor learning, including the specificity paradigm. The aim of this study was to investigate the specific effect of anterior and posterior tongue-strengthening exercises (TSE) on tongue strength (TS) in healthy older adults and to measure possible detraining effects. Sixteen healthy elderly completed 8 weeks of TSE by means of the Iowa Oral Performance Instrument (IOPI). They were distributed in two different treatment arms and performed either exclusively anterior or posterior TSE (ATSE, n = 9 or PTSE, n = 7) depending on the treatment arm. Anterior and posterior maximal isometric pressures (MIPA, MIPP) were measured at baseline, halfway, and after completion of the training sessions. Detraining was measured by repeating MIPA and MIPP measures 4 weeks after the last session of TSE. MIPA and MIPP increased significantly in both treatment arms. MIPA was significantly higher in the ATSE group compared to the PTSE group across all measures in time. No significant differences were observed in MIPP between the ATSE and PTSE groups. Regardless of treatment arm, there was no significant detraining effect measured 4 weeks after the last TSE session. This study suggests that TSE show partial specificity concerning bulb position. We conclude that especially anterior training results in higher anterior TS in comparison with posterior exercises. Furthermore, we found no detraining effects, independent of bulb location.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tongue strength (TS) contributes to an efficient and safe swallowing function, since it is the main driving force for food propulsion from the oral cavity to pharynx [1]. Previous research shows that insufficient TS is associated with aspiration and endangers adequate oral nutrition [2, 3].
It is generally accepted that tongue-strengthening exercises (TSE) should be based on the principles of motor learning and international guidelines concerning muscular training to be effective. These principles are rooted in the physical rehabilitation literature, based on studies in major skeletal muscles [4]. The human tongue, however, is a muscular hydrostat, an organ without skeletal support, with muscle fiber populations aligned both perpendicular and parallel to the axis of the tongue. In contrast to skeletal muscles, a hydrostat keeps the volume constant during muscle contractions [5,6,7]. The muscular tissue is organized into both intrinsic and extrinsic muscle fibers, which function synergistically. In general, the contractions produced by the intrinsic elements are augmented and modified by extrinsic muscular elements. These combined contractions allow changes in both tongue position and shape, thus maintaining the hydrostatic condition [6, 8]. Therefore, it can be hypothesized that certain principles of exercise may not be applicable to the tongue musculature.
One of the principles of motor learning concerns the specificity paradigm. Specificity refers to how closely the exercise task corresponds with the targeted outcome and thus implies that training effects are most explicit when the task resembles the end goal as much as possible [4, 9, 10]. Limited and preliminary evidence is available that training specificity may be observed in the lingual musculature. Research showed an indication of task specificity in training the tongue musculature in rats [11]. Human data show task specificity in TSE [12, 13], but without targeting the specific location of the tongue. However, research has shown that different regions in the tongue act diversely through different stages during swallowing, although it cannot be determined with certainty how many functional segments might exist [14,15,16,17,18,19]. Most remarkable differences in movement characteristics are found between the anterior and the posterior tongue [15]. These areas play an important role in the front-to-back bolus propulsion, which leads the bolus to the pharynx [1, 16].
Research has clearly demonstrated the association between TSE and increased isometric tongue-to-palate pressure (maximal isometric pressure, MIP) [2, 20,21,22,23,24]. Consequently, there is an increased interest in developing efficient and scientifically based TSE as shown in Table 1. Listed training schemes result in positive effects on TS, with detraining effects measurable from 4 weeks after finishing TSE [25], but the composition of the different protocols is quite variable. One of the variables is location of the MIP, anterior or posterior (resp. MIPA or MIPP). Most training schemes combine both locations based on the presumption that different locations activate different muscle groups. Up to now, there is limited attention to the relationship between the specific location and the development of TS.
Concerning the described specificity paradigm, we could expect different results on anterior and posterior maximal isometric tongue pressures (MIPA and MIPP) when training exclusively the anterior or posterior part of the tongue.
Therefore, the purpose of this study was to investigate the training and detraining effects of anterior and posterior tongue strength exercises (TSE) on anterior and posterior maximum tongue pressures (MIPA and MIPP).
Our research hypotheses are as follows:
-
(1)
Anterior TSE will preferentially increase MIPA.
-
(2)
Posterior TSE will preferentially increase MIPP.
-
(3)
Detraining effects will be measurable 4 weeks after finishing tongue strength training.
Methods
Participants
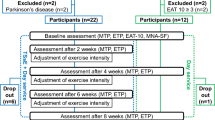
Initially 18 patients were included, 9 per treatment arm. Two persons in treatment arm 1 (ATSE: anterior tongue-strengthening exercises) ended training sessions at their own initiative before 4 weeks of exercising. Data of these subjects were excluded from further statistical analysis. Consequently, 16 subjects (8 males and 8 females) with a mean age of 84 years (range 70–95 years) were included for final analysis. Seven subjects performed ATSE and 9 subjects PTSE. Subjects were healthy elderly with a minimum age of 70 years who were recruited in nursing homes. All participants met the following inclusion criteria: no cognitive deficit, as measured by the Mini Mental State Examination [35]; Belgian origin, with Dutch as native language allowing comparison with the Belgian normative data [36]; MIPA and MIPP are within the 95% prediction range of the patients’ age and gender [36]; and able to complete the Yale Swallowing Protocol [37], thereby ruling out silent aspiration. Subjects having a history of neurogenic disorders [38,39,40], head and neck cancer [41], and other diseases with a possible influence on tongue strength were excluded from this study.
Instrumentation
The Iowa Oral Performance Instrument version 2.3 (IOPI) was used for both anterior and posterior MIP measurements and for monitoring tongue–palate pressure values during training sessions. The location of the bulb is based on the findings of Steele et al. [15] and similar to the procedure previously described by Van Nuffelen et al. [10]. MIPA was measured at the level of the tongue blade, by placing the most distal part of the bulb in contact with the frontal incisors and thereby positioning the bulb against the hard palate, just posterior to the alveolar ridge. MIPP was measured at the level of the tongue dorsum, by placing the top of the bulb against the transition of the hard to soft palate. A permanent mark on the connecting tube just anterior to the incisors assures accurate placement for each MIPP repetition and measurement [10]. The same bulb, with permanent mark, is used during training sessions to maintain the accurate posterior location. The LCD screen displays the exerted pressure in kPa and the LED lights display the exercise target of 80% MIP as visual feedback during training.
Study Design and Interventions
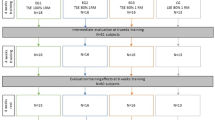
Participants were assigned to two different treatment arms by means of convenience sampling. The ATSE group performed solely anterior TSE, and the PTSE group performed solely posterior TSE. Both treatment groups completed 3 sessions per week on nonconsecutive days for 8 weeks [42, 43]. Training sessions were conducted at the patients’ nursing home and supervised by the researcher without additional homework. Each training session involved 120 tongue-pressure tasks divided in 24 sets of 5 repetitions with 30-s rest following each repetition, with the target level set at 80% of the 1RM [26, 27, 31, 32, 34]. Patients were instructed to hold this effort for 3 s based on the LEDs. Exercise levels were recalculated every 2 weeks according to the principle of progressive overload. In order to maximize gains over time, the absolute value of load placed on the muscle must exceed the demand typically encountered and progressively adjusted over the course of the exercise program, to maintain the relative physiologic load as a proportion of the maximal force-generating capacity [4]. This described study design is represented schematically in Fig. 1.
Outcome Measures
Regardless of the treatment arm, anterior as well as posterior MIP values were measured. The highest MIP value obtained over the first three repetitions was recorded. A 3-second time frame per attempt was used to reach the maximum pressure. MIPA as well as MIPP was performed at baseline, after 4 and 8 weeks of training and 4 weeks after the last training session (outcome 1 (O1), outcome 2 (O2), outcome 3 (O3) resp.) to document possible detraining effects similar to the study by Clark [25]. A margin of 48 h was tolerated for O1, O2, and O3 (Fig. 1).
Data Analysis
Statistical analysis was performed by means of SPSS v21. Descriptive statistics and tests for normality (Kolmogorov–Smirnov), Mixed Models Effects, and post hoc analysis with Holm–Bonferroni correction were performed for 16 participants (n = 7 for ATSE and n = 9 for PTSE).
Results
As illustrated in Table 2, the results of this study reveal the increases in MIP during training for the ATSE as well as the PTSE group. Significant effects of treatment arm are represented in Fig. 2. For MIPA, there are significant effects of treatment arm (F (1,14) = 12.863, p = .003), outcome (F (3,42) = 27.279, p = .000), and the interaction of group by time (F (3,42) = 9.105, p = .000). Post hoc tests revealed that only MIPA was significantly higher in the ATSE group in comparison with the PTSE group at all measure moments, except baseline.
Analyzing MIPP as an outcome parameter (Fig. 3) reveals significant effects of group (F (1,14) = 4.889, p = .044) and time (F (3,42) = 37.137, p = .000). Post hoc tests verified significant increases in MIPP for both groups between baseline, O1, and O2. No significant differences were observed in MIPP between the ATSE and PTSE groups at any measure moment.
When post-treatment TS (outcome 2) is expressed as a percent of TS at baseline, the degree of increase of MIPA is 72% (+ 26.00 kPa) in the ATSE group and 15% (+ 5.11 kPa) in the PTSE group. The degree of increase of MIPP is 60% (+ 19.29 kPa) in the ATSE group and 44% (12.67 kPa) in the PTSE group.
Regardless of training condition, there were small, but insignificant changes in MIPA and MIPP 4 weeks after training (outcome 3). When O3 is expressed as a percentage of MIP after TSE, the degree of decrease of MIPA is 6% for the ATSE group. MIPP decreases by 1% in the ATSE group and by 5% in the PTSE group. There is a slight, but limited increase of MIPA in the PTSE group (+ 5 kPa, 12%), even without specific training.
Discussion
Since Steele (2016) showed that the increase of TS in patients ameliorates their swallowing function [33], the development of efficient training schemes based on the insights of motor learning is crucial to their rehabilitation. Clark et al. [13] demonstrated task specificity for TSE in comparison with endurance and power-targeted exercises. The main purpose of our current study was to further explore this principle of training specificity in the lingual musculature.
The findings of this study suggest a specific training effect from TS depending on the localization of the bulb. According to the results in Table 1, anterior as well as posterior TSE result in an increase of TS, but exclusively anterior training results in a much larger gain (+ 72% MIPA) in comparison with only posterior practice (+ 15% MIPA). This may indicate a specificity effect of ATSE on MIPA. Note that the amount of increase in MIPA measured in this study is much higher than the increase observed in other studies exclusively containing ATSE, as discussed in Table 1 [12, 25, 26, 29]. Comparison is difficult due to the different training parameters, but a possible explanation could be the difference in repetitions per session, which is much higher in the present study. This hypothesis should be explored in future research.
A surprising finding in this study is the lack of this specificity effect for PTSE. Training the anterior part of the tongue results in higher posterior TS values (+ 60% MIPP) than posterior training (+ 44% MIPP), although the difference is less explicit than the anterior effect discussed before. Since there is actually no study available concerning exclusively posterior training, comparison of the increment from MIPP with other data is impossible. Perhaps, the anterior bulb position is easier to tolerate by the subjects and thus leads to a better exercise effect. Although previous research in head and neck cancer patients does not show a different feasibility of tongue strength measures between anterior and posterior bulb locations [44], maybe the total length of the full training protocol gives different results. Another possible explanation for the lack of specificity during posterior training is based on the characteristics of the tongue as muscular hydrostat. Training on any part of the tongue causes adaption of other muscle fibers to keep the volume of this organ constant during movement and exercising [6,7,8]. Subsequently, ATSE can lead to posterior compensation and possible training of the muscular tissue, unlike the skeletal muscle groups.
Considering the rather limited research on the long-term effects of swallowing rehabilitation and TSE [45], the second purpose of our study was to investigate a possible detraining effect of TS. Detraining in healthy adult subjects was previously investigated by Clark [25] and Oh [31]. Clark [25] described significant decreases in lingual strength 4 weeks post TSE, with remaining TS not differing from baseline. Oh [31], on the other hand, reported no significant detraining until 8 weeks post treatment. But reported measurements post 8 weeks remained significantly greater than pre-training values. Present data resemble the study of Oh [31], with no significant decreases post 4 weeks.
A difference however between the present study and Oh [31] is the mean age of participants. Our population is remarkably older than the subjects in the study of Clark (mean age 37.8 years) and the study from Oh (mean age 25.8 years). Even though older people are expected to be more vulnerable to detraining effects [46, 47] than younger subjects, the present study cannot support this statement.
Although we found a clear specificity effect from ATSE and no significant detraining effect at four weeks of detraining for anterior as well as posterior TS, our study has some limitations. First, our population is rather limited with only 7 subjects in the ATSE group. But this amount seems tolerable in comparison with other studies on specificity, where the mean number of participants per treatment arm is situated between 5 and 10 [12, 13, 25]. Second, our specificity effect from bulb localization links up with previous conclusions about the different anatomic regions in the tongue [15], but we cannot assume that these selected training locations necessarily represent distinct, homologous functional units in the tongue. Until now, it is unknown which muscle fibers within the tongue body were most affected by TSE. Therefore, more profound physiologically based research in addition to behavioral outcomes is necessary. Perhaps, other exercises rather than isolated TSE are more appropriate to affect posterior tongue strength. A third limitation that must be pointed out is our choice to exclusively analyze possible training effects of isolated TSM. If the results of our study can be generalized to patients with dysphagia, they suggest to limit TSE to anterior training. But effects on swallowing pressures, coordination of swallowing, and swallowing safety are interesting topics to consider before we can eventually decide to solely train anterior tongue strength. A fourth and last limitation is the limited duration in time considering the detraining period. On the basis of studies on general physical rehabilitation, Burkhead et al. [4] pointed out that older people do show a significantly greater decrease in TS than younger subjects, but the detraining period was longer than ours with 52 weeks instead of 4. Future research on detraining in TSE effects should implement a longer follow-up period.
As speech-language pathologists, we aim to provide the most effectively and efficiently based dysphagia rehabilitation [4, 21]. This study aimed to contribute to the development of scientifically based rehabilitation schemes for TS and consequently dysphagia depending on the specific performing goals set up by the clinician. The provided insights into specificity and detraining in healthy older subjects, which will enhance the knowledge upon which interventions can be built concerning dysphagia in disordered populations.
Conclusion
In summary, the results of the present study show task specificity in TSE depending on the localization of the bulb, with higher increases of anterior TS when training exclusively the anterior tongue. A remarkable finding is the higher increase of posterior TS when training exclusively anteriorly compared to posterior exercises. Our data confirm the possibility of TSE, but reinforce the significance of the difference in training schemes between skeletal muscles and the tongue as a muscular hydrostat.
References
De Bodt M, Guns C, D’Hondt M, Vanderwegen J, Van Nuffelen G. Dysfagie. Handboek voor de klinische praktijk. Antwerp: Garant; 2015.
Butler SG, Stuart A, Leng X, Wilhelm E, Rees C, Williamson J, Kritchevsky SB. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol Ser A. 2011;66:452–8.
Ono T, Kumakura I, Arimoto M, Hori K, Dong J, Iwata H, Nokubi T, Tsuga K, Akagawa Y. Influence of bite force and tongue pressure on oro-pharyngeal residue in the elderly. Gerontology. 2007;24:143–50.
Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia. 2007;22:251–65. doi:10.1007/s00455-006-9074-z.
Sanders I, Mu L. A 3-dimensional atlas of the human tongue muscles. Anat Rec. 2013;296:1102–14. doi:10.1002/ar.22711.
Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol. 2007;210:4069–82.
Kier WM, Smith KK. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool J Linn Soc. 1985;83:307–24.
Felton SM, Gaige TA, Reese TG, Wedeen VJ, Gilbert RJ. Mechanical basis for lingual deformation during the propulsive phase of swallowing as determined by phase contrast magnetic resonance imaging. J Appl Physiol. 2007;103:255–65.
Sale D. Specificity of training of training. Can J Sports Sci. 1992;17:71.
Van Nuffelen G, Van den Steen L, Vanderveken O, Specenier P, Van Laer C, Van Rompaey D, Guns C, Mariën S, Peeters M, Van de Heyning P, Vanderwegen J, De Bodt M. Study protocol for a randomized controlled trial: tongue strengthening exercises in head and neck cancer patients, does exercise load matter? Trials. 2015;16:395. doi:10.1186/s13063-015-0889-5.
Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. J Appl Physiol. 2013;114:472–81. doi:10.1152/japplphysiol.01370.2012.
Lazarus C, Logemann JA, Huang CF, Rademaker W. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatr Logop. 2003;55:199–205.
Clark HM. Specificity of training in the lingual musculature. J Speech Lang Hear Res. 2012;55:657–76. doi:10.1044/1092-4388(2011/11-0045).
Hori K, Taniguchi H, Hayashi H, Magara J, Minagi Y, Li Q, Ono T, Inoue M. Role of tongue pressure production in oropharyngeal swallow biomechanics. Physiol Rep. 2013;. doi:10.1002/phy2.167.
Steele CM, Van Lieshout P. Tongue movements during water swallowing in healthy young and older adults. J Speech Lang Hear Res. 2009;52:1255–67. doi:10.1044/1092-4388(2009/08-0131).
Napadow VJ, Chen Q, Wedeen VJ, Gilbert RJ. Intramural mechanics of the human tongue in association with physiological deformations. J Biomech. 1999;32:1–12.
Chi-Fishman G, Stone M, Mc Call GN. Lingual action in normal sequential swallowing. J Speech Lang Hear Res. 1998;41:771–85. doi:10.1044/jslhr.4104.771.
Tasko SM, Kent RD, Westbury JR. Variability in tongue movement kinematics during normal liquid swallowing. Dysphagia. 2002;17:126–38. doi:10.1007/s00455-001-0112-6.
Wilson EM, Green JR. Coordinative organization of lingual propulsion during the normal adult swallow. Dysphagia. 2006;21:226–36. doi:10.1007/s00455-006-9053-4.
Stierwalt JA, Youmans SR. Tongue measures in individuals with normal and impaired swallowing. Am J Speech Lang Pathol. 2007;16(2):144–56.
Clark HM, Henson PA, Barber WD, Stierwalt JA, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech Lang Pathol. 2003;12:40–50. doi:10.1044/1058-0360(2003/051).
Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50(5):257–62.
Yoshida M, Kikutani T, Tsuga K, Utanohara Y, Hayashi R, Akagawa Y. Decreased tongue pressure reflects symptom of dysphagia. Dysphagia. 2006;21:61–5.
Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51:828–35. doi:10.1044/1092-4388(2008/060).
Clark HM, O’Brien K, Calleja A, Corrie SN. Effects of directional exercise on lingual strength. J Speech Lang Hear Re. 2009;52:1034–47. doi:10.1044/1092-4388(2009/08-0062).
Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53:1483–9. doi:10.1111/j.1532-5415.2005.53467.x.
Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Arch Phys Med Rehabil. 2007;88:150–8. doi:10.1016/j.apmr.2006.11.002.
Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: three case reports. Clin Interv Aging. 2008;3:735–47.
Lazarus CL, Husaini H, Falciglia D, DeLacure M, Branski RC, Kraus D, Lee N, Ho M, Ganz C, Smith B, Sanfilippo N. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg. 2014;43:523–30. doi:10.1016/j.ijom.2013.10.023.
Steele CM, Bailey GL, Polacco RE, Hori SF, Molfenter SM, Oshalla M, Yeates EM. Outcomes of tongue-pressure strength and accuracy training for dysphagia following acquired brain injury. Int J Speech Lang Patholog. 2013;15:492–502. doi:10.3109/17549507.2012.752864.
Oh JC. Effects of tongue strength training and detraining on tongue pressures in healthy adults. Dysphagia. 2015;30:315–20. doi:10.1007/s00455-015-9601-x.
Park JS, Kim HJ, Oh DJ. Effect of tongue strength training using the Iowa Oral Performance Instrument in stroke patients with dysphagia. J Phys Ther Sci. 2015;27:3631–2. doi:10.1589/jpts.27.3631.
Steele CM, Bayley MT, Peladeau-Pigeon M, Nagy A, Namasivayam AM, Stokely SL, Wolkin T. A randomized trial comparing two tongue pressure resistance training protocols for post-stroke dysphagia. Dysphagia. 2016;31:452–61. doi:10.1007/s00455-016-9699-5.
Rogus-Pulia N, Rusche N, Hind JA, Zielinski J, Gangnon R, Safdar N, Robbins J. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J Am Geriatr Soc. 2016;64:417–24. doi:10.1111/jgs.13933.
Folstein MF, Folstein SE, McHugh PR. “Mini mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Vanderwegen J, Guns C, Van Nuffelen G, Elen R, De Bodt M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy Belgian adults. Dysphagia. 2013;28:159–66. doi:10.1007/s00455-012-9425-x.
Suiter DM, Sloggy J, Leder SB. Validation of the Yale Swallow Protocol: a prospective double-blinded videofluoroscopic study. Dysphagia. 2014;29:199–203. doi:10.1007/s00455-013-9488-3.
Hiraoka A, Yoshikawa M, Nakamori M, Hosomi N, Nagasaki T, Mori T, Oda M, Maruyama H, Yoshida M, Izumi Y, Matsumoto M, Tsuga K. Maximum tongue pressure is associated with swallowing dysfunction in ALS patients. Dysphagia. 2017;. doi:10.1007/s00455-017-9797-z.
O’Day C, Frank E, Montgomery A, Nichols M, McDade H. Repeated tongue and hand strength measurements in normal adults and individuals with Parkinson’s disease. Int J Orofac Myol. 2005;31:15–25.
Murdoch BE, Spencer TJ, Theodoros DG, Thompson EC. Lip and tongue function in multiple sclerosis: a physiological analysis. Mot Control. 1998;2(2):148–60.
Lazarus CL, Husaini H, Hu K, Culliney B, Li Z, Urken M, Jacobson A, Persky M, Tran T, Concert C, Palacios D, Metcalfe-Klax R, Kumar M, Bennett B, Harrison L. Functional outcomes and quality of life after chemoradiotherapy: baseline and 3 and 6 months post-treatment. Dysphagia. 2014;29(3):365–75. doi:10.1007/s00455-014-9519-8.
Schoenfeld BJ, Ratamess NA, Peterson MD, Contreras B, Tiryaki-Sonmez G. Influence of resistance training frequency on muscular adaptations in well-trained men. J Strength Cond Res. 2015;29(7):1821–9. doi:10.1519/JSC.0000000000000970.
Fernández-Lezaun E, Schumann M, Mäkinen T, Kyröläinen H, Walker S. Effects of resistance training frequency on cardiorespiratory fitness in older men and women during intervention and follow-up. Exp Gerontol. 2017;95:44–53. doi:10.1016/j.exger.2017.05.012.
Van den Steen L, Vanderveken O, Vanderwegen J, Van Gestel D, Daisne JF, Allouche J, Delacroix L, Van Rompaey D, Beauvois S, Cvilic S, Mariën S, Desuter G, Vermorken JB, Van den Weyngaert D, Specenier P, Van Laer C, Peeters M, Van de Heyning P, Chantrain G, Lawson G, Lazarus C, De Bodt M, Van Nuffelen G. Feasibility of tongue strength measurements during (chemo)radiotherapy in head and neck cancer patients. Support Care Cancer. 2017;. doi:10.1007/s00520-017-3761-1.
Langmore SE, Pisegna JM. Efficacy of exercises to rehabilitate dysphagia: a critique of the literature. Int J Speech Lang Pathol. 2015;17:222–9. doi:10.3109/17549507.2015.1024171.
Ivey FM, Tracy BL, Lemmer JT, NessAiver M, Metter J, Fozard JL, Hurley BF. Effects of strength training and detraining on muscle quality: age and gender comparison. J Gerontol. 2000;55:152–9.
Toraman NF. Short term and long term detraining: is there any difference between young-old and old people? Br J Sports Med. 2005;39:561–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethical Committee of the Antwerp University Hospital (B300201421549). All subjects agreed voluntarily to participate in this study and signed an informed consent.
Rights and permissions
About this article
Cite this article
Van den Steen, L., Schellen, C., Verstraelen, K. et al. Tongue-Strengthening Exercises in Healthy Older Adults: Specificity of Bulb Position and Detraining Effects. Dysphagia 33, 337–344 (2018). https://doi.org/10.1007/s00455-017-9858-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-017-9858-3