Abstract
Superior and anterior hyoid movements are important events in pharyngeal deglutition. This cross-sectional study uses a cadaver model to document the structural properties of the muscles underlying these movements in an effort to understand how their morphology influences function. Measurements to determine physiological cross-sectional areas (PCSAs) of swallowing muscles were taken from hemisected head and neck formalin-fixed cadaver specimens (n = 13). Coordinates of muscle attachment sites and PCSAs were used to calculate \( \hat{i} \) and \( \hat{j} \) unit force vectors, where \( \hat{i} \) and \( \hat{j} \) represent anterior-posterior and superior-inferior directions, respectively. The suprahyoid muscle subsamples were grouped for analysis as follows: digastric (DG), geniohyoid (GH), mylohyoid (MH), and stylohyoid (SH). The ANOVA with Tukey HSD post hoc analysis of unit force vectors showed the following results: GH (−0.44 ± 0.15 cm2) >MH (−0.02 ± 0.21 cm2), DG (−0.05 ± 0.11 cm2), SH (0.14 ± 0.04 cm2), with negative values representing the anterior direction (p < 0.01); and MH (0.91 ± 0.28 cm2) >DG (0.29 ± 0.14 cm2), SH (0.22 ± 0.08 cm2), GH (12 ± 0.08 cm2), with positive values representing the superior direction (p < 0.01). The morphology of the suprahyoid muscles suggests that based on structural properties, the geniohyoid has the most potential to displace the hyoid in the anterior direction and the mylohyoid has the most potential to displace the hyoid in the superior direction. These data in complement with physiological findings may provide greater insight into these movements for those developing novel treatments for dysphagia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
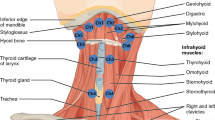
Prior to fluoroscopy and electromyography, the function of the anatomy underlying hyoid movement was poorly understood. The 1918 edition of Gray’s anatomy describes the upward and forward motion of the hyoid in deglutition, but then erroneously states “the hyoid bone is carried upward and backward by the posterior bellies of the Digastrici and the Stylohyoidei, which assist in preventing the return of the food into the mouth” [1]. Videofluoroscopy has since verified and quantified the superior and anterior movement of the hyoid [2–5] (Fig. 1). The suprahyoid muscles are the presumed muscles affecting this movement, as shown in animal studies correlating electromyography and fluoroscopy [6]. However, the structural properties of muscles that determine their potential contribution to the superior and anterior movement of the hyoid are not documented. Understanding the structural contributions of individual muscles in the context of functional findings may have important clinical implications for surgeons concerned with retaining function, clinicians seeking to improve swallowing function with exercise regimens, and investigators hoping to augment function technologically.
Current swallowing theory suggests that the suprahyoid muscles, along with the thyrohyoid muscle, are primarily responsible for the opening of the upper esophageal sphincter [7, 8]. Perlman et al. [9] used electromyography to show that activation of the submental muscles, including the geniohyoid, mylohyoid, and anterior digastric muscle, correlates with the inhibition of the cricopharyngeus and the opening of the sphincter in humans. Kurt et al. [10] demonstrated that the stylohyoid and posterior digastric muscles as a complex are activated in human deglutition. The purpose of the present investigation is to document the structural properties of the suprahyoid muscles using human cadavers to better understand their potential relative contribution to the movement of the hyoid.
The suprahyoid muscles include the geniohyoid, stylohyoid, mylohyoid, anterior digastric, and posterior digastric (Fig. 2). The geniohyoid and stylohyoid originate from the mandible and cranial base, respectively. The mylohyoid, consisting of an anterior and posterior portion, arises from the mandible and inserts on a midline raphé and the body of the hyoid. The anterior and posterior digastrics arise from the mandible and cranial base, respectively, and share a common tendon that translates force to the hyoid through a fibrous band of connective tissue.
It should be underscored that findings based on structure alone will not explain or predict what actually happens in nature. The composition of fiber types, passive forces of a muscle, and motor units recruited for a specific task such as swallowing are essential to understanding function, and conclusions cannot be drawn from mere structural data. However, muscle function is limited by its structural properties. What we can say is that based on structural data, muscles have the potential for certain actions and that these data may then be helpful in understanding the foundation of swallowing movements and treating swallowing dysfunction.
The actions of the muscles examined in the present study are described using force vectors, which describe the magnitude and direction of muscle pull (Fig. 2). To isolate the potential structural contributions of muscles in the anterior and superior direction, unit force vectors are calculated for each muscle. This approach provides a quantitative way to evaluate the potential effect of individual muscles on a movable structure such as the hyoid [11]. By calculating the unit force vectors for each of the suprahyoid muscles, we tested the hypotheses that, structurally, (1) the geniohyoid has the most potential to affect the anterior displacement of the hyoid, and (2) the mylohyoid has the most potential to affect the superior displacement of the hyoid.
Methods
Subjects
The Boston University School of Medicine Anatomical Gifts program granted permission to conduct this research, which was in full compliance with the intent and permissions of their program. The Boston University Medical Campus Institutional Review Board (BUMC IRB) holds that cadaveric research on bodies donated to Boston University School of Medicine Anatomical Gifts program is ethical and exempt from the IRB process. Thirteen hemisections of head and neck formalin-fixed cadaver specimens were selected for this study. Structural properties of the suprahyoid muscles, including the geniohyoid, stylohyoid, anterior digastric, posterior digastric, and the anterior and posterior portions of the mylohyoid, were recorded.
Physiological Cross-sectional Area (PCSA)
Maximum potential magnitude of muscle pull is calculated using physiological cross-sectional area (PCSA), which is a proxy for potential maximum tetanic tension [12]. PCSA is calculated from muscle mass (g), pennation angle (Θ), fiber length (FL) (cm), and density (ρ) as follows (see Table 1):
Mass and Density
Muscles were harvested and soaked in 0.2 M phosphate buffered saline for 48 h according to a protocol that calibrates tissue density to 1.112 g/cm3 [13]. Muscle mass was measured with a Mettler-Toledo PB602-S digital balance (Mettler-Toledo, Greifensee, Switzerland).
Pennation Angles
Surface pennation angles of muscles were measured by photomacrographic digital image analysis. The digital photomacrograph workstation includes a photomacrography table (Polaroid, Cambridge, MA) with a mounted 10-megapixel Canon EOS Rebel XTi digital camera with a 35-mm lens (Canon, Tokyo, Japan). Images were analyzed using Image J software (http://rsbweb.nih.gov/ij) and calibrated to a standard recorded on the image.
Fiber Length (FL)
PSCA calculations measure fiber length functionally as bundles of fibers or muscle fascicles. Fascicle bundles were separated with an Olympus OME surgical dissecting microscope at 2.5 × magnification (Olympus, Tokyo, Japan) and measured using photomacrographic digital image analysis with Image J software (http://rsbweb.nih.gov/ij). Since muscles fix at either concentric or eccentric contraction, measured FLs were adjusted to optimal sarcomere length (SL) using the following equation to reflect maximum tension [14]:
Sarcomere Length (SL)
Muscle tissue samples from distal, proximal, and midpoints of muscle fascicles were wet mounted with glycerol to avoid tissue-altering processing [15]. Photomicrographs were captured under a 60 × objective. Sarcomere lengths were measured by photomicrographic digital image analysis with an average length of ten sarcomeres in series reported. The digital photomicrograph workstation includes a Nikon Eclipse 80i microscope (Nikon, Lake Placid, NY) equipped with an Optronics MicroFire digital camera (Optronics, Goleta, CA) that is connected to a Dell Optiplex GX270 computer (Dell Computer Co., Round Rock, TX) equipped with Stereo Investigator 7 software (MicroBrightField Inc., Williston, VT). Images were analyzed using Image J software (http://rsbweb.nih.gov/ij) and calibrated to a calibration scale recorded on the image.
Direction of Force
Lines of action were described by connecting three dimensional coordinates of muscle attachment sites. Muscle attachments were marked with pins and photographed. Photomacrographic images of the medial and lateral surfaces of the bisected skull and attached swallowing apparatus generated x and y coordinates of muscle attachments by digital image analysis. Coordinates in two dimension of muscle attachments of the sample are shown in Fig. 3. The z coordinates were measured with a Mitutoyo digital caliper model No. CD-600 CSX (Mitutoyo Corp., Kanogawa, Japan).
Unit Force Vectors
Unit force vectors were calculated to isolate the potential contribution of each muscle to the relevant anatomical movements. The anterosuperior border of the hyoid was set as the origin. The \( \hat{i} \) unit vectors were calculated from the x coordinates to represent the anterior-posterior portion of a vector, with anterior indicated in the negative direction (−x). The \( \hat{i} \) unit vectors were calculated from the y coordinates to represent the superior-inferior portion of a vector, with superior indicated in the positive direction (+y):
where \( d = \sqrt {\left( {x_{2} - x_{1} } \right)^{2} + \left( {y_{2} - y_{1} } \right)^{2} + \left( {z_{2} - z_{1} } \right)^{2} } . \) The \( \hat{i} \) and \( \hat{j} \) unit vectors multiplied by the PCSA as a proxy for force provides the potential maximum tension of a given muscle in the anterior-posterior and the superior-inferior directions:
is the PCSA force unit vector for the anterior-posterior direction and
is the PCSA force unit vector for the superior-inferior direction.
Statistics
Statistical tests used for these studies include a one-way analysis of variance with Tukey HSD post hoc analysis performed using web-based computer software: [©R. Lowry (1998-2010), VassarStats: Website for Statistical Computation. Available at http://faculty.vassar.edu/lowry/VassarStats.html].
Results
The mean and standard deviation of the PCSA values (in cm2) are as follows: posterior portion of mylohyoid (0.43 ± 0.12), anterior portion of mylohyoid (0.82 ± 0.18), posterior digastric (0.64 ± 0.16), anterior digastric (0.55 ± 0.12), stylohyoid (0.27 ± 0.09), and geniohyoid (0.46 ± 0.16) (Table 1).
From the three-dimensional coordinates collected, \( \hat{i} \) unit vectors representing the anterior-posterior direction, with −\( \hat{i} \) representing the anterior direction, were calculated. The mean and standard deviation (all in cm2) are as follows: posterior portion of mylohyoid (0.09 ± 0.13), anterior portion of mylohyoid (−0.09 ± 0.21), posterior digastric (0.75 ± 0.06), anterior digastric (−0.95 ± 0.03), stylohyoid (0.53 ± 0.10), and geniohyoid (−0.96 ± 0.03). The \( \hat{j} \) unit vectors representing the superior-inferior direction, with +\( \hat{j} \) representing the superior direction, were calculated. The mean and standard deviation are as follows: posterior portion of mylohyoid (0.85 ± 0.05), anterior portion of mylohyoid (0.66 ± 0.18), posterior digastric (0.58 ± 0.06), anterior digastric (−0.15 ± 0.13), stylohyoid (0.80 ± 0.05), and geniohyoid (0.24 ± 0.13).
The \( \hat{i} \) and \( \hat{j} \) unit vectors were multiplied by the PCSA values to calculate a PCSA force unit vector in the anterior-posterior and the superior-inferior direction, respectively. The mean and standard deviation (in cm2) of the anterior-posterior PCSA force unit vectors, with −\( \hat{i} \) representing the anterior direction, were calculated as follows: posterior portion of mylohyoid (0.04 ± 0.06), anterior portion of mylohyoid (−0.07 ± 0.18), posterior digastric (0.48 ± 0.12), anterior digastric (−0.53 ± 0.12), stylohyoid (0.14 ± 0.04), and geniohyoid (−0.44 ± 0.15). The mean and standard deviation (in cm2) of the superior-inferior PCSA force unit vectors, with +\( \hat{j} \) representing superior direction, were calculated as follows: posterior portion of mylohyoid (0.37 ± 0.10), anterior portion of mylohyoid (0.55 ± 0.22), posterior digastric (0.38 ± 0.12), anterior digastric (−0.09 ± 0.08), stylohyoid (0.22 ± 0.08), and geniohyoid (0.11 ± 0.07).
For the statistical analysis of PCSA force unit vectors, the mylohyoid was considered to perform as a singular muscle. The analysis of variance of the PCSA force unit vectors in the anterior-posterior direction for the mylohyoid (MH), posterior digastric (PDG), anterior digastric (ADG), stylohyoid (SH), and geniohyoid (GH) showed a statistically significant difference between all groups (p < 0.0001). A Tukey HSD post hoc analysis showed that there were significant differences in the anterior-posterior PCSA force unit vector between each muscle sample, except for the ADG and GH, which are indicated to possess the greatest potential tension in the anterior direction (MH vs. PDG, p = 0.01; MH vs. ADG, p = 0.01; MH vs. SH, p = 0.05; MH vs. GH, p = 0.01; PDG vs. ADG, p = 0.01; PDG vs. SH, p = 0.01; PDG vs. GH, p = 0.01; ADG vs. SH, p = 0.01; SH vs. GH p = 0.01).
The literature reports that both the anterior and posterior digastrics are active in deglutition [9, 10]. Electromyography data alone does not specify if muscles are in eccentric or concentric contraction. For the purposes of morphological analysis, if it is assumed that in the overall process of deglutition, the posterior and anterior digastrics concentrically contract in tandem to translate force to the hyoid bone through a fibrous loop to elevate the hyoid, then the digastric muscles counterbalance each other in the anterior-posterior direction. Under these assumptions, the PCSA force unit vector for the geniohyoid is greater than all other muscles (p = 0.01) (Fig. 4).
Means and standard deviations of anterior-posterior PCSA force unit vectors (cm2), with anterior = −, posterior = +, and hyoid position = 0. An analysis of variance with Tukey HSD of the unit force vector in the anterior-posterior direction shows that the geniohyoid has the most potential structurally to effect the anterior displacement of the hyoid (p < 0.01)
The analysis of variance of the PCSA force unit vectors in the superior-inferior direction for the mylohyoid, posterior digastric, anterior digastric, stylohyoid, and geniohyoid showed a statistically significant difference between all groups (p < 0.0001). A Tukey HSD post hoc analysis showed that there were significant differences in the superior-inferior PCSA force unit vector between each muscle sample, except for the PDG and SH and the SH and GH, with the MH greater than all others (MH vs. PDG, p = 0.01; MH vs. ADG, p = 0.01; MH vs. SH, p = 0.01; MH vs. GH, p = 0.01; PDG vs. ADG, p = 0.01; PDG vs. GH, p = 0.01; ADG vs. SH, p = 0.01; ADG vs. GH, p = 0.05). Even when it is assumed that the posterior and anterior digastrics act in tandem to translate force to the hyoid bone, the mylohyoid is structured to translate a greater potential superior tension on the hyoid bone than the digastrics. In either case, the PCSA force unit vector in the superior direction for the mylohyoid is greater than that for all other muscles (p = 0.01) (Fig. 5).
Means and standard deviations of superior-inferior PCSA force unit vectors (cm2), with superior = + and hyoid position = 0. An analysis of variance with Tukey HSD of the unit force vector in the superior-inferior direction shows that the mylohyoid has the most potential structurally to effect the superior displacement of the hyoid (p < 0.01)
Discussion
Understanding the potential relative contribution of each of the suprahyoid muscles to the function of swallowing may advance the treatment of dysphasia in several ways. Since the geniohyoid and mylohyoid have more structural potential for displacing the hyoid anteriorly and superiorly, respectively, these two muscles could be preferentially targeted for neuromuscular stimulation [16]. Studies have also shown that exercise can increase motor unit recruitment of muscles for particular functions [17]. By understanding the potential for hyoid excursion demonstrated by the structures of these muscles, therapists could target specific muscles with exercises designed to promote hyoid excursion. In addition, awareness of the function of these muscles by radiation and surgical oncologists may reduce treatment-induced dysphagia [18, 19]. For example, greater efforts might be made during neck dissection to preserve the nerve to the mylohyoid or the C1 branch coursing as the descendens hypoglossi nerve, which supplies the geniohyoid. Finally, these structural data may be useful to those using computers to model deglutition.
Force vectors determined by PCSA measurements alone cannot predict function since motor unit recruitment of these multifunctional muscles will vary according to the task and stimuli. The pattern of recruitment likely varies when these muscles perform their various roles in deglutition, mastication, phonation, or respiration. While structural properties do constrain the limits of function, they cannot be taken on their own to interpret function. Instead, these data must be complemented by insight from various other studies. For example, fluoroscopy studies have shown that superior movement of the hyoid tends to precede anterior movement [3, 5]. Electromyography data in animal studies have shown that the activation of mylohyoid precedes that of the geniohyoid [6, 20]. These results correlate nicely with the implications of the structural findings of the current investigation.
There are some potential limitations to the methods used for this study. The structural properties of noncontractile tissue such as tendon and fascia were not included in this study. The passive properties of these tissues may influence the position of the hyoid and be an important consideration for future studies. There is also some question about the best way to generate PCSA data [21]. Some have suggested using MRI to assess PCSA in vivo using muscle volumes [22]. However, this method at present cannot account for sarcomere lengths, which puts the reliability of the measure into question as a representation of maximum tetanic tension. Finally, the structural properties reported here were measured from a sample of subjects who were older than 65 years of age. It is estimated that 16–20% of patients over 50 have swallowing difficulties [23]. Therefore, we cannot assume that the individuals in our study enjoyed normal swallowing during life and that the structural properties of these muscles may not be equivalent to those in individuals with normal swallowing in the general population, but may instead represent the structural properties of muscles in an elderly population.
Conclusions
The morphology of the suprahyoid muscles suggests that based on structural properties alone, the geniohyoid has the most potential to displace the hyoid in the anterior direction and the mylohyoid has the most potential to displace the hyoid in the superior direction.
References
Lewis WH (Ed) Gray’s anatomy of the human body, 20th ed. Philadelphia: Lea and Febiger; 1936. p. 393.
Perlman AL, VanDaele DJ, Otterbacher MS. Quantitative assessment of hyoid bone displacement from video images during swallowing. J Speech Hear Res.1995;38:579–85.
Leonard RJ, Kendall KA, McKenzie S, Gonçalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:146–52.
Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23:274–9.
Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154:953–63.
German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102:1017–25.
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707.
Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Brasseur JG, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 1989;257:G748–59.
Perlman AL, Palmer PM, McCulloch TM, Vandaele DJ. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–9.
Kurt T, Gürgör N, Seçil Y, Yıldız N, Ertekin C. Electrophysiologic identification and evaluation of stylohyoid and posterior digastricus muscle complex. J Electromyogr Kinesiol. 2006;16:58–65.
van Eijden T, Korfage J, Brugman P. Architecture of the human jaw-closing and jaw-opening muscles. Anat Rec. 1997;248:464–74.
Lieber R, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66.
Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–20.
Walker SM, Schrodt GR. I segment lengths and thin filament periods in skeletal muscle fibers of the Rhesus monkey and the human. Anat Rec. 1974;178:63–81.
Kamibayashi LK, Richmond FJR. Morphometry of human neck muscles. Spine. 1998;23:1314–23.
Burnett TA, Mann EA, Stoklosa JB, Ludlow CL. Self-triggered functional electrical stimulation during swallowing. J Neurophysiol. 2005;94:4011–8.
Robbins JA, Butler SG, Daniels SK, Gross RD, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek J. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. J Speech Lang Hear Res. 2008;51:S276–97.
Kronenberger MB, Meyers AD. Dysphagia following head and neck cancer surgery. Dysphagia. 1994;9:236–44.
Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, Chepeha DB, Eisbruch A. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–98.
Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol. 2007;102:587–600.
Cutts A, Seedhom BB. Validity of cadaveric data for muscle physiological cross-sectional area ratios: a comparative study of cadaveric and in vivo data in human thigh muscles. Clin Biomech. 1993;8:156–62.
Maganaris CN, Baltzopoulos V, Ball D, Sargeant AJ. In vivo specific tension of human skeletal muscle. J Appl Physiol. 2001;90:865–72.
Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455–78.
Acknowledgments
The authors acknowledge Rob Bouchie and Lee Iacopucci, who administer the Boston University School of Medicine Anatomical Gifts Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pearson, W.G., Langmore, S.E. & Zumwalt, A.C. Evaluating the Structural Properties of Suprahyoid Muscles and their Potential for Moving the Hyoid. Dysphagia 26, 345–351 (2011). https://doi.org/10.1007/s00455-010-9315-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-010-9315-z