Abstract
The purpose of this study was to compare the swallowing function and kinematics in stroke patients with and without tracheostomies. In this retrospective matched case–control study, we compared stroke patients with (TRACH group, n = 24) and without (NO-TRACH group, n = 24) tracheostomies. Patients were matched for age, sex, and stroke-type. Swallowing function was evaluated using the videofluoroscopic dysphagia scale (VDS) and functional oral intake scale (FOIS) obtained from videofluoroscopic swallow study (VFSS) images. Swallowing kinematics were evaluated using a two-dimensional kinematic analysis of the VFSS images. Mean duration of tracheostomy was 132.38 ± 150.46 days in the TRACH group. There was no significant difference in the total VDS score between the TRACH (35.17 ± 15.30) and NO-TRACH groups (29.25 ± 16.66, p = 0.247). FOIS was significantly lower in the TRACH group (2.33 ± 1.40) than in the NO-TRACH group (4.33 ± 1.79, p = 0.001). The TRACH group had a significantly lower maximum vertical displacement (15.23 ± 7.39 mm, p = 0.011) and velocity (54.99 ± 29.59 mm/s, p = 0.011), and two-dimensional velocity (61.07 ± 24.89 mm/s, p = 0.013) of the larynx than the NO-TRACH group (20.18 ± 5.70 mm, 82.23 ± 37.30 mm/s, and 84.40 ± 36.05 mm/s, respectively). Maximum horizontal velocity of the hyoid bone in the TRACH group (36.77 ± 16.97 mm/s) was also significantly lower than that in the NO-TRACH group (47.49 ± 15.73 mm/s, p = 0.032). This study demonstrated that stroke patients with tracheostomies had inferior swallowing function and kinematics than those without tracheostomies. A prospective longitudinal study is needed to elucidate the effect of a tracheostomy on swallowing recovery in stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rate of tracheostomy placement after a stroke has been reported as 1.3–2.8% [1, 2]. The typical indication for a tracheostomy is maintenance of upper airway patency, specifically for positive-pressure ventilation when extubation has failed or is deemed unfeasible in the intensive care unit [3, 4]. Although the risk of aspiration is also considered an indication for a tracheostomy in stroke patients [4], the tracheostomy itself can predispose patients to dysphagia and aspiration [5–9]. Previous studies suggest that the presence of a tracheostomy has structural or functional effects on swallowing function. A tracheostomy can have an anchoring effect on laryngeal elevation and interrupt pulmonary positive airway pressure during the swallowing reflex [10–13].
In recent years, several studies have contradicted the traditional notion that tracheostomies hamper swallowing and increase the risk of aspiration. Studies using fiberoptic endoscopy have shown that a tracheostomy was not associated with aspiration [14, 15]. Kinematic studies evaluating the swallowing function in the presence of a tracheostomy tube have shown no relationship between a tracheostomy and dysphagia [16, 17]. These studies investigated the immediate or short-term effects of a tracheostomy tube on swallowing function and kinematics in patients with various disease entities. On the other hand, recent studies continue to report that the components of a tracheostomy tube, such as the speaking valve or cuff, affect the physiology of swallowing [18, 19].
The incidence of dysphagia after a stroke have been reported as up to 80% using instrumental testing [20], but most patients recover their swallowing function within 6 months [21, 22]. Although the presence of a medical tube, including a tracheostomy, was reported to be associated with poor functional outcomes after a stroke [1], the relationship between the presence of a tracheostomy and swallowing function has not been investigated in stroke patients. To elucidate this relationship, we compared the swallowing function of dysphagic stroke patients with and without tracheostomies. This study utilized swallowing kinematics and functional outcomes to reveal the functional difference in swallowing mechanism between two groups.
Materials and Methods
Subjects
In this retrospective study, we reviewed records from March 2005 to January 2012 in our hospital’s videofluoroscopic swallowing study (VFSS) database. Patients included in the study group were 24 stroke patients, who were diagnosed by a neurologist as having an acute stroke that had been confirmed by computed tomography (CT) or magnetic resonance imaging (MRI), with tracheostomies (TRACH group). All the patients underwent tracheostomy for weaning from ventilatory support. Cuff pressure was monitored routinely using a cuff manometer (Posey, Arcadia, CA, USA) and maintained in the recommended range (22–32 cm H2O). Exclusion criteria were patients with (1) a history of a recurrent stroke; (2) other diseases that could affect swallowing function, such as Parkinson’s disease, bulbar palsy, a brain tumor, or a head and neck cancer; and (3) poor cooperation during VFSS. The control group was composed of 24 sex, age, and stroke-type matched stroke patients without tracheostomies (NO-TRACH group), who were selected from the database by a blind researcher. The database was divided into subgroups according to sex and stroke-type, and then sorted by age. The matched stroke patients were randomly selected in the same age group divided by decade. Each patient’s demographic data, including age, sex, type of stroke, interval between stroke onset and VFSS, mean duration of tracheostomy, type of cannula, underlying cardiovascular disease, and Modified Barthel Index (MBI) at the time of VFSS, were obtained by reviewing the patient’s medical records. We certified that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. This study was approved by the Institutional Review Board of Seoul National University Hospital [1312-061-541].
VFSS
All subjects were tested with foods in various forms, including 2, 5, and 10 ml of thin liquids, pudding, honey, and nectar-thick liquids, a semi-blended food, and boiled rice. Thin liquids were diluted barium solution (35% w/v), and all other foods were mixed with undiluted barium sulfate (Solotop suspension®, Taejoon Pharm Co., Ltd., Seoul, Republic of Korea) to allow for fluoroscopic visualization. Each form of food was given twice using a spoon. The subjects swallowed the fluid or food naturally without any additional cues. Two coins with a diameter of 24 mm were attached under the chin and below the mastoid process to serve as a ruler for radiographic magnification. Under the lateral projection of videofluoroscopy, the modified barium swallow results were saved as digital image files (.avi) using a digital computer frame grabber board (Pinnacle Studio MovieBox DV, Pinnacle System, Inc., Mountain View, CA; Pegasus HD/SD Board, Grass Valley Inc., Honorine, France) and image processing software (Pinnacle Studio 9.0, Pinnacle System, Inc.; EDIUS 4.5, Grass Valley Inc.).
The interpretation of the results of VFSS required the consensus of two physiatrists who had at least 2 years of experience in dysphagia management. The videofluoroscopic dysphagia scale (VDS) [23] and functional oral intake scale (FOIS) [24] were rated for functional evaluation of swallowing based on the VFSS findings. The VDS includes 14 items that represent oral and pharyngeal swallowing functions in the VFSS. Each item was evaluated in consideration of all tested food consistencies. The VDS is a negative rating system and provides a maximum possible score of 100. A score of zero reflects a completely normal finding. The FOIS is a 7-point ordinal scale developed to document the functional level of oral intake of solid foods and liquids in stroke patients.
Two-Dimensional Kinematic Analysis
The VFSS video clips were analyzed by two-dimensional motion analysis. VFSS images were recorded at 30 frames per second. The lateral projection of the VFSS taken during the 2-ml thin-liquid swallowing was analyzed as previously described [25, 26]. All kinematic analyses were performed by a blinded researcher. The initial frame for the analysis was the 15th frame (0.5 s) before the frame in which the head of the bolus passed the angle of the mandibular ramus at the base of the tongue. The last frame was the frame in which the tail of the bolus passed the upper esophageal sphincter.
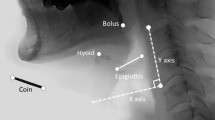
Motion analysis was performed using a motion analysis software system (Ariel Performance Analysis System; Ariel Dynamics, Inc., Trabuco Canyon, CA, USA) using the landmarks shown in Fig. 1. The anterior–superior margin of the hyoid bone, the larynx represented by the anterior–superior margin of the subglottic airway column, and base-to-tip margin of the epiglottis were digitally coordinated at each frame. We defined the zero point as the anterior–inferior margin of the fourth cervical vertebral body. The y-axis was defined as the straight line connecting the zero point and the anterior–inferior margin of the second cervical vertebral body and the x-axis as the line perpendicular to the y-axis, passing the zero point.
A digital correlation of the hyoid bone (black arrow), larynx (black arrowhead), epiglottis (black dotted line with white arrow), with the control frame for the x- and y-axes. The zero point was defined as the anterior–inferior margin of the fourth cervical vertebral body, the y-axis as the straight line connecting the zero point with the anterior–inferior margin of the second cervical vertebral body, and the x-axis as the line perpendicular to the y-axis. Coins were used as the scale for length
Data were transformed into actual distances and angles using the known diameter of the coin attached to the subject’s chin and mastoid process. Spatial and temporal measurements were performed automatically using our in-house software built-in MATLAB R2009a (The MathWorks Inc., Natick, MA, USA). The maximum distances (mm) of the vertical and horizontal displacements of the hyoid bone and larynx during swallowing were calculated and defined as the differences between the highest and lowest values. The two-dimensional distances from the initial points to the maximum excursion points of the hyoid bone and the larynx during swallowing were also calculated as the shortest distance between the two points. Maximum velocities of the hyoid and laryngeal displacements during swallowing were calculated in the vertical, horizontal, and two-dimensional directions. Instantaneous velocity was calculated using the distance between two adjacent time points throughout the swallowing cycle, and then the maximal value was obtained. The maximum angle (°) of the epiglottic tilt was measured from the initial position.
Intra- and inter-rater reliabilities of the kinematic analysis showed good-to-excellent agreement, with ICC values ranging from 0.752 to 0.996, in our previous study [25]. The accuracy of our method in measuring distances, linear velocity, and angular velocity has also been described previously [27].
Statistical Analysis
Since this was a matched case–control study, continuous variables in the patients’ characteristics and the kinematic measurements of swallowing were analyzed using a paired t test [28, 29]. McNemar’s and Wilcoxon signed ranks tests were used to compare the dichotomous variables and ordinal scales between the TRACH and NO-TRACH groups, respectively. As a subgroup analysis, the kinematic difference between the cuffed and uncuffed cannula in the TRACH group was analyzed using an independent t test. A p value less than 0.05 was considered significant.
Results
The patient demographics are shown in Table 1. There was no difference in age, sex, and type of the stroke between the two groups. The mean interval between stroke onset and VFSS was 152.42 ± 153.60 days in the TRACH group and 195.54 ± 356.08 in the NO-TRACH group (p = 0.612). The number of patients with underlying cardiopulmonary disease was also comparable between the two groups. MBI at the time of VFSS was 21.76 ± 25.23 in the TRACH group and 47.75 ± 27.95 in the NO-TRACH group (p = 0.019). Mean duration of presence of the tracheostomy was 132.38 ± 150.46 days in the TRACH group. Mean inner diameter of the cannulas was 6.90 ± 0.66 (ranging from 5.0 to 7.5) mm. In the TRACH group, 11 subjects had cuffed cannulas and 3 subjects had fenestrated cannulas. Tracheostomy tubes were removed within 2 weeks after VFSS in 11 patients. The other patients remained tracheostomy-dependent due to aspiration in 7, secretion control in 4, and other airway problems in 2 patients.
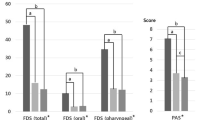
Table 2 presents the differences of swallowing function, including each component of the VDS, VDS total score, and FOIS between the TRACH and NO-TRACH groups. Among the components of the VDS, bolus formation and mastication were significantly more deteriorated in the TRACH group than in the NO-TRACH group. There was no significant difference in the total VDS score between the TRACH (35.17 ± 15.30) and NO-TRACH groups (29.25 ± 16.66, p = 0.247). FOIS was significantly lower in the TRACH group (2.33 ± 1.40) than that in the NO-TRACH group (4.33 ± 1.79, p = 0.001).
Table 3 shows the differences of swallowing kinematics between stroke patients with and without tracheostomies. The TRACH group showed significantly lower maximum vertical displacement (15.23 ± 7.39 mm, p = 0.011) and velocity (54.99 ± 29.59 mm/s, p = 0.011), and two-dimensional velocity (61.07 ± 24.89 mm/s, p = 0.013) of the larynx than the NO-TRACH group (20.18 ± 5.70 mm, 82.23 ± 37.30 mm/s, and 84.40 ± 36.05 mm/s, respectively). Maximum horizontal velocity (36.77 mm/s ± 16.97) of the hyoid bone was also significantly lower than NO-TRACH group (47.49 ± 15.73 mm/s, p = 0.032). However, there was no significant difference in the maximum angle of epiglottic tilt between two groups.
From Table 4, the patients with cuffed cannulas had a lower horizontal velocity of the larynx (13.59 ± 6.71 mm/s) than those with uncuffed cannulas (23.66 ± 10.91 mm/s, p = 0.014). There was no difference in hyoid bone movement and the angle of the epiglottis between the two subgroups.
Discussion
Our study demonstrated that compared to the NO-TRACH group, the TRACH group had poorer functional outcome of swallowing and impaired hyolaryngeal movements during swallowing. In particular, distance of laryngeal elevation and the velocity of hyoid horizontal excursion and laryngeal elevation during swallowing were shorter and slower, respectively, in the TRACH group. Patients with cuffed cannulas had an even lower velocity of horizontal laryngeal movement during swallowing. Because laryngeal elevation is an important mechanism for airway protection, reduced laryngeal elevation may contribute to a higher risk of aspiration/penetration in the TRACH group.
Results from the present study are consistent with the results of prior studies that also show that the swallowing mechanism is significantly disturbed by a tracheostomy [10–13, 30, 31]. It has been reported that a tracheostomy structurally restricts laryngeal elevation [11] and could change vocal cord kinetics [31]. Goldsmith summarizes that tracheostomy tubes have a negative physiological and mechanical impact on swallowing and airway protection due to an impaired glottis closure reflex, diversion of pulmonary airflow, reduced cough at the glottis, reduced subglottic pressure, reduced laryngeal elevation, disuse muscle atrophy, and desensitization of larynx and hypopharynx [30]. Recently, Prigent et al. [18] demonstrated that expiratory flow towards the upper airway after swallowing was negligible in patients with an open tracheostomy tube and was restored by adding a speaking valve. Therefore, an open tracheostomy may abolish several benefits of expiration during swallowing for laryngeal protection. In addition, Ding et al. [32] proposed that the cuffed cannula lowered the laryngeal elevation and increased the aspiration risk. All these structural and functional changes by a tracheostomy might have a detrimental effect on swallowing in stroke patients.
However, differing opinions on whether a tracheostomy directly impairs swallowing still exists. Donzelli et al. [14] reported that the incidence of aspiration and/or penetration was not changed after tracheostomy tube removal. Leder et al. [15] also demonstrated that aspiration status did not change in most patients between pre- and post-tracheostomy. They evaluated the immediate and short-term (mean of 9.0 days) effect of tracheostomy on aspiration in tracheostomized patients due to various etiologies. Terk et al. [16] reported that the presence of a tracheostomy tube did not significantly alter hyoid bone movement and laryngeal excursion during swallowing immediately following placement; however, this study only had 7 patients. Kang et al. [17] reported no significant changes in swallowing kinematics, including time variables and the extent of laryngeal elevation, before and after tracheostomy removal (mean interval of 6.6 days). These results suggest that there is no causal relationship between tracheostomies, aspiration events, and swallowing kinematics, at least during a short period.
On the other hand, in patients with post-stroke dysphagia, most problems resolved over a 6-month period [21], and about 90% of stroke patients returned to their pre-stroke diet [22]. Both compensatory and rehabilitative approaches are necessary for swallowing recovery in stroke patients. Tracheostomy tubes disturb compensatory maneuvers such as chin tuck and supraglottic swallow. A tracheostomy causes several physiological changes, such as reduced laryngeal elevation, disuse muscle atrophy, and desensitization of larynx and hypopharynx [30]. These changes may also impair swallowing recovery after stroke when a tracheostomy is needed for a longer period of time. Results from the present study suggest that a tracheostomy could be an inhibiting factor for recovery of the swallowing function in patients with post-stroke dysphagia.
During swallowing, the closure of laryngeal vestibule occurs by contact of the epiglottic petiole with the arytenoids, which makes laryngeal elevation an important mechanism for airway protection [33]. Although there was no significant difference in the maximum angle of epiglottic tilt, which is also related to the risk of aspiration in stroke patients [34], it is most likely the decreased laryngeal elevation in the TRACH group that is contributing to worse swallowing function. Investigators have recently started using kinematic analysis to evaluate the velocity of hyolaryngeal movements during swallowing [34, 35]. Seo et al. [34] reported that sluggish hyolaryngeal movements during swallowing are a remarkable feature of post-stroke dysphagia. Nagi et al. [35] suggested that increased hyoid velocity with thickened liquids may be a mechanism contributing to improved airway protection by facilitating more timely laryngeal vestibule closure. Therefore, decreased velocity of laryngeal elevation and hyoid horizontal excursion in the TRACH group and decreased velocity of horizontal laryngeal movement in patients with cuffed cannulas may reflect the negative impact a tracheostomy has on the swallowing function of dysphagic stroke patients.
Significant difference in MBI between the two groups may represent more severe medical or neurological conditions in the TRACH group than in the NO-TRACH group, although tracheostomy itself can limit functional activities. Inferior swallowing function and kinematics in the TRACH group could in part result from the patient’s premorbid or post-stroke disease condition, independent of the effect of the tracheostomy. Our results also show that the TRACH group had some decreased oral motor functions, such as bolus formation and inadequate mastication, although the tracheostomy does not appear to have a direct effect on oral motor function. This could be attributed to the TRACH group’s limited oral intake, as indicated by the lower FOIS. However, a decline in general functional or cognitive status may also contribute to poor oral motor function in TRACH group. Therefore, the results of the present study should be interpreted cautiously by considering the potential limitations of a retrospective study.
Our study has certain limitations. First, our main finding was a more deteriorated swallowing function and kinematics in dysphagic stroke patients with tracheostomies compared to those without. These differences found between the two groups may have been a manifestation of their disease rather than an effect from the tracheostomy since the central control of swallowing and respiration are closely interrelated [36]. As mentioned above, the probable differences in medical and neurological status between the two groups may also contribute to the results. Second, this was a retrospective case–control study. Although age, sex, and stroke-type were matched and there was no significant difference in the duration of disease, there could have been differences in their clinical status, such as cognitive state and respiratory function, that contributed to the differences seen in their swallowing function. Adequate clinical information could have allowed for better statistical matching, such as propensity score matching, which would have produced a more reliable result.
Conclusions
This study demonstrated that stroke patients with tracheostomies had inferior swallowing function and kinematics compared to those without tracheostomies. Tracheostomy weaning and cuff deflation may help them overcome their swallowing difficulties. Based on our findings, a prospective longitudinal study is warranted in order to elucidate the effects of a tracheostomy on swallowing recovery in stroke patients.
References
Roth EJ, Lovell L, Harvey RL, Bode RK, Heinemann AW. Stroke rehabilitation: indwelling urinary catheters, enteral feeding tubes, and tracheostomies are associated with resource use and functional outcomes. Stroke. 2002;33:1845–50.
Walcott BP, Kamel H, Castro B, Kimberly WT, Sheth KN. Tracheostomy after severe ischemic stroke: a population-based study. J Stroke Cerebrovasc Dis. 2014;23:1024–9.
Hess DR, Altobelli NP. Tracheostomy tubes. Respir Care. 2014;59:956–71 discussion 971-953.
Bosel J. Tracheostomy in stroke patients. Curr Treat Options Neurol. 2014;16:274.
DeVita MA, Spierer-Rundback L. Swallowing disorders in patients with prolonged orotracheal intubation or tracheostomy tubes. Crit Care Med. 1990;18:1328–30.
Nash M. Swallowing problems in the tracheotomized patient. Otolaryngol Clin N Am. 1988;21:701–9.
Cameron JL, Reynolds J, Zuidema GD. Aspiration in patients with tracheostomies. Surg Gynecol Obstet. 1973;136:68–70.
Muz J, Mathog RH, Nelson R, Jones LA Jr. Aspiration in patients with head and neck cancer and tracheostomy. Am J Otolaryngol. 1989;10:282–6.
Elpern EH, Scott MG, Petro L, Ries MH. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest. 1994;105:563–6.
Feldman SA, Deal CW, Urquhart W. Disturbance of swallowing after tracheostomy. Lancet. 1966;1:954–5.
Bonanno PC. Swallowing dysfunction after tracheostomy. Ann Surg. 1971;174:29–33.
Eibling DE, Gross RD. Subglottic air pressure: a key component of swallowing efficiency. Ann Otol Rhinol Laryngol. 1996;105:253–8.
Clarett M, Andreu MF, Salvati IG, Donnianni MC, Montes GS, Rodriguez MG. Effect of subglottic air insufflation on subglottic pressure during swallowing. Med Intensiva. 2014;38:133–9.
Donzelli J, Brady S, Wesling M, Theisen M. Effects of the removal of the tracheotomy tube on swallowing during the fiberoptic endoscopic exam of the swallow (FEES). Dysphagia. 2005;20:283–9.
Leder SB, Ross DA. Confirmation of no causal relationship between tracheotomy and aspiration status: a direct replication study. Dysphagia. 2010;25:35–9.
Terk AR, Leder SB, Burrell MI. Hyoid bone and laryngeal movement dependent upon presence of a tracheotomy tube. Dysphagia. 2007;22:89–93.
Kang JY, Choi KH, Yun GJ, Kim MY, Ryu JS. Does removal of tracheostomy affect dysphagia? A kinematic analysis. Dysphagia. 2012;27:498–503.
Prigent H, Lejaille M, Terzi N, Annane D, Figere M, Orlikowski D, Lofaso F. Effect of a tracheostomy speaking valve on breathing-swallowing interaction. Intensive Care Med. 2012;38:85–90.
Amathieu R, Sauvat S, Reynaud P, Slavov V, Luis D, Dinca A, Tual L, Bloc S, Dhonneur G. Influence of the cuff pressure on the swallowing reflex in tracheostomized intensive care unit patients. Br J Anaesth. 2012;109:578–83.
Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
Smithard DG, O’Neill PA, England RE, Park CL, Wyatt R, Martin DF, Morris J. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–93.
Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–8.
Han TR, Paik NJ, Park JW, Kwon BS. The prediction of persistent dysphagia beyond six months after stroke. Dysphagia. 2008;23:59–64.
Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516–20.
Seo HG, Oh BM, Han TR. Longitudinal changes of the swallowing process in subacute stroke patients with aspiration. Dysphagia. 2011;26:41–8.
Paik NJ, Kim SJ, Lee HJ, Jeon JY, Lim JY, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kinesiol. 2008;18:329–35.
Lee SH, Oh BM, Chun SM, Lee JC, Min Y, Bang SH, Kim HC, Han TR. The accuracy of the swallowing kinematic analysis at various movement velocities of the hyoid and epiglottis. Ann Rehabil Med. 2013;37:320–7.
Pearce N. Analysis of matched case–control studies. BMJ. 2016;352:i969.
Conway A, Rolley JX, Fulbrook P, Page K, Thompson DR. Improving statistical analysis of matched case–control studies. Res Nurs Health. 2013;36:320–4.
Goldsmith T. Evaluation and treatment of swallowing disorders following endotracheal intubation and tracheostomy. Int Anesthesiol Clin. 2000;38:219–42.
Shaker R, Milbrath M, Ren J, Campbell B, Toohill R, Hogan W. Deglutitive aspiration in patients with tracheostomy: effect of tracheostomy on the duration of vocal cord closure. Gastroenterology. 1995;108:1357–60.
Ding R, Logemann JA. Swallow physiology in patients with trach cuff inflated or deflated: a retrospective study. Head Neck. 2005;27:809–13.
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19:691–707.
Seo HG, Oh BM, Han TR. Swallowing kinematics and factors associated with laryngeal penetration and aspiration in stroke survivors with dysphagia. Dysphagia. 2016;31:160–8.
Nagy A, Molfenter SM, Peladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus consistency on hyoid velocity in healthy swallowing. Dysphagia. 2015;30:445–51.
Martin-Harris B. Coordination of respiration and swallowing. GI Motility online, 2006. doi: 10.1038/gimo10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Seo, H.G., Kim, JG., Nam, H.S. et al. Swallowing Function and Kinematics in Stroke Patients with Tracheostomies. Dysphagia 32, 393–400 (2017). https://doi.org/10.1007/s00455-016-9767-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-016-9767-x