Opinion statement
Patients with severe ischemic and hemorrhagic stroke may require tracheostomy in the course of their disease. This may apply to stroke unit patients whose deficits include a severe dysphagia posing such risk of aspiration as it cannot be sufficiently counteracted by tube feeding and swallowing therapy alone. More often, however, tracheostomy is performed in stroke patients so severely afflicted that they require intensive care unit treatment and mechanical ventilation. In these, long-term ventilation and prolonged insufficient airway protection are the main indications for tracheostomy. Accepted advantages are less pharyngeal and laryngeal lesions than with prolonged orotracheal intubation, better oral hygiene and nursing care, and higher patient comfort. Optimal timing of tracheostomy is unclear, in general, as in stroke intensive care unit patients. Potential benefits of early tracheostomy concerning ventilation duration and length of stay, respirator weaning, airway safety, rate of pneumonia, and other complications, outcome and mortality have been suggested in studies on non-neurologic subgroups of critical care patients. Stroke patients have hardly been investigated with regard to these aspects, and mainly retrospectively. A single randomized pilot trial on early tracheostomy in 60 ventilated patients with severe hemorrhagic and ischemic stroke demonstrated feasibility, safety, and less need of sedation. Regarding the technique, bedside percutaneous dilational tracheostomy should be preferred over surgical tracheostomy because of several reported advantages. As the procedural risk is low and early tracheostomy does not seem to worsen the clinical course of the ventilated stroke patient, it is reasonable to assess the need of further ventilation at the end of the first week of intensive care and proceed to tracheostomy if extubation is not feasible. Reliable prediction of prolonged ventilation need and outcome benefits of early tracheostomy, however, await further clarification. Decannulation of stroke patients after discontinued ventilation has to follow reliable confirmation of swallowing ability, as by endoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke candidates for tracheostomy
The need to tracheostomize a patient with ischemic or hemorrhagic stroke results from the patient´s prolonged inability to breath and/or protect his airway sufficiently. This can be caused by various types of stroke, such as severe acute ischemic stroke (AIS, eg, large hemispheric stroke, space-occupying cerebellar stroke, basilar thrombosis or embolism with brainstem infarction), large or brainstem intracerebral hemorrhage (ICH), intraventricular hemorrhage, severe cerebral sinus venous thrombosis, and subarachnoid hemorrhage (SAH). It appears less important what the particular type of cerebrovascular pathology is but rather, how extensive the brain damage and its sequelae (brain edema, secondary ischemia) are and what parts of the brain are affected. The latter include those regulating the level of consciousness (reticular formation in the brain stem, thalami, limbic system), breathing (respiratory centers in the cortex, pons, and medulla), and swallowing (medulla and brain stem connections). Principally, there are two main scenarios in which a tracheostomy (TT) is usually considered. The first is in a patient with a moderate stroke just resulting in stroke unit care but affecting swallowing centers of the brain (such as infarcts of the brain stem or the medulla oblongata) thus producing dysphagia. If the dysphagia is then found to be severe by specific testing and the patient is at risk of aspiration, TT might be indicated to bridge swallowing therapy during rehabilitative care. The second scenario is in a patient that suffered from a stroke so severe to make admission to the intensive care unit (ICU) and mechanical ventilation necessary. Here, TT will be chosen if extubation failed or is deemed not feasible (ie, as part of weaning from the ventilator). This review will mainly focus on the intensive care unit tracheostomy.
Tracheostomy in the stroke unit patient
Dysphagia after stroke is very common, reported incidences for the acute phase range from 30 % to 80 % [1–3]. Numerous predictors have been investigated but their validity remains limited as a recent systematic review showed [4]. Posterior circulation stroke involving the brainstem is placing the patient at particular risk to develop dysphagia [5], and within that subgroup, stroke affecting the pons, the medial medulla, or the lateral medulla (highest risk) seems to be most relevant, as a recent MRI-based meta-analysis demonstrated [6]. Some stroke patients might otherwise have a quite unremarkable course, ie, not necessarily have very disabling deficits, going through regular stroke unit care, not requiring ventilatory support. However, a severe dysphagia might lead to recurrent aspiration pneumonia, thus impeding rehabilitative progress and increasing morbidity and mortality [5, 7–11]. For the detection of dysphagia in stroke, numerous clinical and apparative tests have been described, such as different versions of bedside screening tests, videofluoroscopic, or fiberoptic endoscopic tests [12–17]. A systematic review comparing three of these methods favored a simple water swallowing test combined with pulse oxymetry [18], but the endoscopic tests might be of great value in less cooperative patients. Screening for dysphagia and subsequent management thereof was shown to prevent pneumonia [19]. Management comprises special oral hygiene, nasogastric. or percutaneous tube feeding, very diverse forms of nutritional support, and swallowing therapies that are beyond the scope of this review and are still subject to considerable controversy [20]. One randomized control trial showed “high-intensity” behavioral intervention to lead to better outcomes in dysphagic stroke patients [21].
However, at times it might not be enough to divert nutrition by means of gastric tubes and to initiate swallowing therapy, as some of these patients might persistently fail to handle their saliva, thus demanding ongoing suctioning. In these patients at risk of aspiration TT is indicated. Unfortunately, there exist hardly any systematic studies on TT in the non-ICU stroke patient with dysphagia. It is unclear when to tracheostomize, which technique to choose, and when and how to decannulate. Pragmatically, it seems reasonable to do a clinical bedside swallowing test in any stroke patient even slightly suggestive of dysphagia [12, 22, 23], proceed to endoscopic swallowing tests if screening was pathological [24, 25], keep the patient nil by mouth, place a nasogastric tube if endoscopic tests are pathological as well [26, 27] and apply a penetration/aspiration score [13, 28]. If aspiration is confirmed persistently despite supportive measures and initiation of swallowing therapy, the patient should be tracheostomized. If long-term dysphagia is estimated, surgical TT may be preferable as it makes changing cannulas during rehabilitation easier and safer. As decannulation has hardly been assessed in non-ICU dysphagic stroke patients, indirect evidence on how best to confirm the absence of dysphagia may cautiously be transferred from patients previously ventilated (see below).
Tracheostomy in the intensive care unit patient
Rates and rationale
The prognosis of stroke patients requiring ICU management and mechanical ventilation was suggested to be generally poor by retrospective studies in the past with reported mortality rates ranging between 40 % and 80 % [29–32]. These studies, however, were conducted before new insights on options to reduce these stroke patients´ morbidity and mortality (as by decompressive surgery etc.) were gained. Given that the most frequent extracerebral complications of neurologic ICU patients are respiratory [33, 34] measures to improve airway and ventilation management might theoretically transduce in better outcome. A retrospective analysis revealed that good outcome is possible in severely afflicted cerebrovascular patients after TT, even after long-term ventilation [35].
It can only be estimated how many ICU-dependent stroke patients receive a tracheostomy in the course of their ICU stay. While TT in the general ICU is performed in about 10 %–15 % of patients, the rate in ICU stroke patients seems to range between 15 %–35 % [34, 36]. In general critical care, it is customary to do a TT if ventilated patients cannot be weaned from the respirator and extubated quickly but require long-term ventilation and airway support. A common practice is to estimate chances of extubation at the end of the first week of ventilation and proceed to TT if this is not deemed feasible for the following week. Practices, however, vary greatly between centers and how to decide on the need of TT is not even standardized in general ICU patients [37], not to speak of stroke ICU patients. Accepted advantages of a short tracheal cannula compared with a long orotracheal tube are improved oral hygiene and nursing measures, avoidance of lesions to pharynx and larynx, and a higher patient comfort [38–42], the latter of which alone might justify the procedure. More meaningful reputed benefits of tracheostomy such as reduction of ventilatory dead space and thus of work of breathing, improved patient safety, faster weaning, and shorter ventilation duration, reduced ICU-length of stay, lower risk of ventilator-associated pneumonia, or even lower mortality and improved outcome have been suggested by studies of varying sizes and numbers but have largely remained controversial until today [37]. These aspects have hardly been investigated in ICU stroke patients (see below).
Decision
Accepted indications for TT in general critical care are long-term ventilation due to prolonged respiratory failure; demand to protect the airway in increased risk of aspiration or functional/mechanical obstruction, prolonged demand of suctioning of tracheal secretions, and the presence of dysphagia. In ICU-dependent, ventilated stroke patients any single or the combination of these can be caused either directly by the brain lesion (eg, in brainstem ICH) or indirectly by complications thereof (eg, by prolonged coma or intracranial pressures (ICP) crises in SAH). Studies on predictors of TT need and prolonged mechanical ventilation in non-neurologic ICU patients have yielded parameters of compromised circulation, and oxygenation, organ failure, and infection status [43, 44]. These may not be too helpful in stroke patients, many of which do not present with these systemic problems, at least not in the early phase of their ICU stay. Two retrospective studies have investigated predictors for the need of TT in ICU stroke patients, but only for patients with supratentorial ICH. Independent predictors of TT were low Glasgow Coma Scale (GCS), presence of Chronic Obstructive Pulmonary Disease (COPD), volume and thalamic location of the ICH, midline shift, presence of intraventricular blood, and hydrocephalus [45, 46]. Some of these parameters have been combined to form the TRACH score with a reported positive predictive value of 95 % and a negative one of 83 % [46]. That score awaits, however, prospective validation. Qureshi and colleagues have retrospectively assessed predictors of TT in patients with infratentorial lesions (mainly vascular) and found brainstem dysfunction, a low GCS and additional supratentorial lesions to either predict TT or alternatively death [47]. At the authors´ own institution, an in-house score (the “SET score”) based on some of the retrospectively found TT predictors named above, is used to identify possible candidates for TT among ICU stroke patients. The score has not been formally validated yet, but has proved helpful in 50 prospectively studied ventilated stroke patients (unpublished data) and been used as a screening tool in a randomized pilot trial [48]. Our decision to tracheostomize is based on the score combined with the judgment of two experienced intensivists (Table 1). Studies on clinical judgment with regard to the need of prolonged ventilation (ie, at least 14 days) have yielded conflicting results in prospective TT trials in non-neurologic patients. In one trial, this resulted in only 10 % of patients deemed long-term actually not needing a TT, while in another, the large TracMan trial, the rate was 50 % [63•].
In cerebrovascular ICU patients, a frequent reason for physicians to delay extubation or decide for TT is a reduced level of consciousness. In fact, a GCS >8 is part of the classical ICU extubation criteria. However, there are data suggesting that brain-lesioned patients with a reduced level of consciousness but otherwise stable (with regard to circulation, respiration, and protective reflexes) can often be successfully extubated [49, 50]. Thus, coma on its own should not necessarily be regarded as an indication for TT. However, if there are several arguments to doubt extubation success (no cough effectiveness, high quantity of secretions, high viscosity of secretions, etc.) TT might be the better option, as both extubation delay and extubation failure with re-intubation worsen the prognosis of the ICU patient [51, 52].
A common and justified reason to tracheostomize ICU stroke patients is to facilitate the process of respirator weaning. Although discontinuous methods of weaning, ie, those involving extended spontaneous breathing trials and as such wake-up trials can be successful [53], these can be problematic in cerebrovascular ICU patients. Brain-damaged ICU patients may respond to wake-up trials with stress reactions and increases in ICP [54–56]. It may be more adequate to apply a continuous way [57] of >weaning (such as gradually reduced pressure support ventilation) to a tracheostomized patient at higher comfort but still less sedation, and thus able to better participate in ventilation. In fact, data suggest faster weaning after TT in non-neurologic ICU patients [58, 59], but this has not been sufficiently clarified for ICU stroke patients yet. At least, in a retrospective subgroup analysis of 129 patients of a mixed ICU, the 31 neurologic/neurosurgical patients were the fastest to be weaned from the ventilator after TT compared with other subgroups [60].
Timing
The optimal timing might be the aspect most actively studied in ICU tracheostomy. Studies in very different populations of non-neurologic critical care patients have addressed this question, among these countless retrospective observational studies and a few randomized controlled trials (RCTs). In 2005, a systematic review of the latter culminated in significantly reduced duration of mechanical ventilation (weighted mean difference −8.5 days, 95 % COI −15.3 to −1.7) and ICU length of stay (LOS) (− 15.3 days, -24.6 to −6.1) [61]. Since then, a few larger RCTs have been conducted in broader mixed ICU populations with largely disappointing results, only showing increased patient comfort [42] and failing to demonstrate reduced pneumonia rate [62]. The largest RCT so far, the UK TracMan trial on TT at day 4 vs day 10 (or more) in 909 mixed ICU patients, demonstrated no other relevant benefit of early tracheostomy than less sedation in the early TT group [63•]. Mortality and most other relevant secondary outcomes were identical between the groups. Two results of the TracMan trial are of particular interest: First, TT (about 90 % percutaneous and the vast majority in single-tapered dilator technique at the bedside) was safe with very few minor TT-related complications and no TT-related deaths. Secondly, clinicians were not very good at estimating the need of 14 day ventilation, as 50 % of patients deemed in need thereof could eventually be extubated. The to-date largest retrospective analysis in more than 10,000 mixed ICU patients showed only a marginal survival benefit through early TT (37 % vs 35 % at 30 days, P = 0.032/46.5 % vs 49.8 % at 1 year, P = 0.001/63.9 % vs 67.2 % study mortality, P < 0.001) in addition to slightly reduced times of ventilation and ICU–LOS [64]. This might indicate that recent RCTs were underpowered to show benefits in mixed populations and it could be more relevant (and rewarding) to investigate the matter in special ICU subgroups. The optimal time point for a TT in general ICU patients remaining thus unclear, it is wide-spread critical care practice to perform the procedure after 2 to 3 weeks from intubation, often after failed weaning or extubation attempts. A common recommendation is to estimate the need of prolonged (>14 days) ventilation (how to do this remains elusive) after 7 days of ventilation and proceed to TT if that is deemed likely [37].
Early TT in stroke ICU patients has hardly been investigated prospectively. Although burdened with high mortality rates, ventilated survivors of ischemic or hemorrhagic stroke who had undergone long-term ventilation leading to TT had a favorable outcome in about 25 % of the cases in a retrospective study of 97 patients [35]. The same study suggested earlier tracheostomy to be associated with shorter ICU-LOS, as was likewise demonstrated in another retrospective study on 69 ventilated stroke patients with infratentorial lesions [47]. A third retrospective analysis in 28 ICU-patients with nontraumatic brain injuries suggested lower mortality through early TT [65]. That study, however, included not only “cerebrovascular accidents”, but brain pathologies as diverse as meningitis, epilepsy, or hypoxic encephalopathy that were not very evenly distributed between the study groups, and thus, these results have to be interpreted with great caution. In the only RCT on the question of early TT in stroke ICU patients to date, in the monocentric pilot study SETPOINT, we randomized 60 ventilated patients with severe intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), or acute ischemic stroke (AIS) to either early TT (within 3 days from intubation) or prolonged intubation (weaning and extubation or, if not successful, TT between day 7 and 14). Early TT was feasible, safe, and resulted in less sedation and a more patient-dominated ventilation. The primary endpoint ICU-LOS, however, was identical between the groups, as were many other secondary endpoints. ICU-mortality was significantly lower in the early TT group, but this has to be regarded with caution as the small trial was not designed to robustly investigate this and as this difference could not be consistently explained by findings in the other endpoints [66••]. The results of SETPOINT need to be confirmed and extended in a larger multi-center trial, before early TT can be sufficiently judged in the ICU stroke patient. Relevant studies on TT in ICU stroke patients are listed in Table 2.
The way to tracheostomize
The procedure of tracheostomy dates back to Egyptian times, but the first record of a medically sound and persistently successful TT in a human is from 16th century Italy. Since then, the surgical version of the procedure has been continuously improved and made a standard procedure among surgeons and ear-nose-throat specialists. The principle of surgical TT is to open the anterior trachea under sight and suture the tracheal wall to the external skin, thus creating a “permanent” tracheostoma [67, 68]. In 1985, Ciaglia revolutionized TT by introducing the (Seldinger-derived) guide-wire technique [69]. The principle of this percutaneous dilational tracheostomy (PDT) is to puncture the trachea, insert a guide-wire and use this to dilate the tracheal opening and to introduce the tracheal cannula. PDT, as its surgical counterpart, has been considerably developed and found increasing popularity in ICUs worldwide. Today, 6 methods of PDT are available, the classical Ciaglia method using several dilators of increasing sizes, the Griggs method employing a cutting forceps [70], the Fantoni method of retrograde inside-outside cannula placement [71], the Ciagla Blue Rhino single dilator [72] method, the PercuTwist method employing a screw-like device [73], and finally the Blue Dolphin method where dilation is accomplished by a high-pressure balloon [74]. The most popular and widespread version of PDT worldwide appears to be the single-tapered dilator method under bronchoscopic control. A recent meta-analysis on 13 comparative PDT studies found some advantages regarding the latter approach, but overall the PDT techniques appeared comparable [75]. Details of surgical and percutaneous TT and details of peri-procedural measures are nicely summarized and illustrated in a review by Durbin [76]. The intervention of PDT in ICU stroke patients is further presented below.
Treatment
Percutaneous dilational tracheostomy
Treatment aims
-
The supreme goal of PDT is to establish a safe airway in stroke patients that either cannot protect their airway and/or are in need of prolonged mechanical ventilation.
-
Direct aims of the exchange of a long orotracheal tube against a short tracheal cannula below the larynx are better oral hygiene, avoidance of pharyngeal/laryngeal lesions, more efficient nursing care and higher patient comfort.
-
Indirect aims particularly of early tracheostomy include a reduced sedation demand, easier and faster weaning from the ventilator, a reduced rate of pneumonia, a shorter duration of ventilation, reduced ICU-length of stay and benefits in mortality and outcome. These, however, await prospective confirmation or demonstration in ICU stroke patients.
Standard procedure
The orotracheally intubated and ventilated patient is positioned supine and supported from below for perfect exposure of the trachea. Sufficient analgesia, sedation, and relaxation are administered. Full systemic and ideally cerebral monitoring is installed. An emergency difficult airway trolley has to be at hand. Barrier precautions, sterile skin preparation, and infiltration of the skin with local anesthetic and epinephrine are applied. Preparatory peri-tracheal ultrasound is recommended to detect vessels and thyroid pathology in the intervention zone. The orotracheal tube is retracted to position the cuff just underneath the vocal chords, preferably under bronchoscopic control. The principle of every PDT then is to puncture the trachea between two tracheal rings below the larynx and introduce a guide-wire. Different techniques (most of these variations of the Ciaglia method, see above) can be used to dilate the tracheal puncture, with the single-tapered dilator technique being the most popular. The dilator and eventually the tracheal cannula are passed over the wire, which is finally removed, as well as the orotracheal tube after confirmation of proper intratracheal cannula placement, ideally by bronchoscopy. The cannula-cuff is inflated. The ventilator is connected to the cannula. The cannula is fixed to the neck and the cannula position re-confirmed by chest-x-ray.
Contraindications
The following are contraindications against PDT, some of which have been regarded absolute in the past and are now regarded relative.
Anatomy
-
Gross anatomical distortion of the neck.
-
Previous neck surgery (not by TT), burns, radiotherapy.
-
Instable or rigid cervical spine.
-
Tracheal distortion, stenosis, or malacia.
-
Upper airway tumor or stenosis.
-
Morbid obesity (>35 kg/m2).
-
Large thyroid gland or vessels in the intervention territory.
Physiology
-
Relevant oxygenation compromise (PEEP >12 cmH20, FiO2 >0.6).
-
Hemodynamic instability, high demand of vasopressors.
-
Coagulopathy (aPTT >50 s, INR >1.5, thrombocyte count <50.000/μL)
-
Increased ICP (>20 mm Hg).
Other
-
Emergency situation.
-
Very difficult airway, expected (re-)intubation problems.
-
Need for permanent tracheostoma.
Complications
Overall, PDT is a very safe procedure with a rate of procedure-related complications of 3 %–4 % [77–79]. In a 6-year prospective follow-up study of 572 PDTs, Dempsey and colleagues reported only 3 % early and 0.7 % late complications. However, two patients (0.35 %) died due to bleeding from erosions of the brachiocephalic truncus [79].
In essence, total complication rate is low, more frequent complications are of minor relevance and very rare complications can be severe. Complications can be related to the TT procedure itself, to manipulation and care of the tracheal cannula, and long-term consequences of this artificial airway.
Short-term complications that are frequent but of minor relevance are venous bleeding, transient desaturations, transient hypotension, and skin infection; those rare but of higher or highest relevance include arterial bleeding, false passage, unintentional decannulation, pneumothorax, esophageal damage, and death. Should the cannula be unintentionally removed at the end of the procedure after extubation, it must not be tried to push it back in but instead orotracheal intubation executed immediately. Thereafter, PDT can be repeated in controlled fashion.
Some long-term complications are rare but relevant, such as tracheal stenosis, tracheomalacia, vocal chord injury, and accidental decannulation [37, 80]. The latter can be a particular problem, also when changing the cannula. While early changing is not a problem in the surgically formed tracheostoma, it can be very dangerous soon after PDT, when the tracheostomy canal is not yet granulated. Hence, the cannula should not be changed before 2 weeks after PDT, and if this cannot be avoided (due to leakage, infection, or occlusion) measures like using an exchanger and having emergency orotracheal intubation gear ready are paramount.
Special points
Surgical (“open”) and percutaneous TT have been compared in meta-analyses and the percutaneous technique found advantageous [81, 82], particularly with regard to less overall complications, less wound infections, less unfavorable scarring, and higher cost-effectiveness, as well as a trend toward reduced relevant bleeding.
Studies have demonstrated that PDT on ICU stroke patients can be performed quickly and safely at the bedside by neurointensivists [66••, 83].
In brain-lesioned patients, PDT can at times result in transient increases in ICP during the procedure [84, 85], therefore measures to avoid or manage this situation should be undertaken (slight elevation of head of bed, sufficient sedation and analgesia, avoidance of hypoventilation (and hence, hypercapnia) as can happen bronchoscopic tube occlusion, etc.).
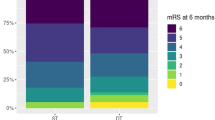
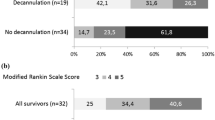
After completed ventilator weaning, a tracheostomized stroke patient can be decannulated as soon as his ability to swallow and handle his saliva is re-established. Dysphagia is very common after stroke, as discussed above, but can also be a result of prolonged orotracheal intubation and ventilation themselves. Clinical swallowing evaluation (CSE) in tracheostomized patients can be unreliable and lead to both inadequately early decannulation in dysphagic patients and (more often) unnecessarily delayed decannulation. Endoscopic tests such as bedside FEES have proven valuable to detect or exclude dysphagia in stroke patients after ventilation and TT, even if the patient is not fully cooperative. In a recent prospective study in 100 such stroke patients, more than 80 % more patients could be successfully decannulated than by relying on CSE alone [86•].
Cost/cost-effectiveness
Cost of bedside PDT is moderate. Absolute amounts are difficult to present as these depend on a lot of center-specific aspects. A comparison of total ICU charges in neurologic ICU patients (mainly cerebrovascular) receiving PDT (n 67) with those receiving a surgical TT (n 68) favored PDT, with mean total savings of 32,900 USD between the cohorts [83]. A meta-analysis on percutaneous vs surgical TT in ICU patients compared four trials and demonstrated significant cost-effectiveness in terms of savings just regarding the procedures itself of 450 USD (and 5 minutes less duration and one individual less in personnel) per case in favor of PDT [81].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •Of importance •• Of major importance
Bath PM, Bath FJ, Smithard DG. Interventions for dysphagia in acute stroke. Cochrane Database Syst Rev. 2000;CD000323.
Martino R, Foley N, Bhogal S, et al. Dysphagia. after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
Falsetti P, Acciai C, Palilla R, et al. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18:329–35.
Daniels SK, Anderson JA, Willson PC. Valid items for screening dysphagia risk in patients with stroke: a systematic review. Stroke. 2012;43:892–7.
Meng NH, Wang TG, Lien IN. Dysphagia. in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil. 2000;79:170–5.
Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R. MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2011;32:1–10.
Doggett DL, Tappe KA, Mitchell MD, et al. Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: an evidence-based comprehensive analysis of the literature. Dysphagia. 2001;16:279–95.
Smithard DG, Smeeton NC, Wolfe CD. Long-term outcome after stroke: does dysphagia matter? Age Ageing. 2007;36:90–4.
McMicken BL, Muzzy CL. Prognostic indicators of functional outcomes in first time documented acute stroke patients following standard dysphagia treatment. Disabil Rehabil. 2009;31:2196–203.
Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke. 2007;38:2284–91.
Ickenstein GW, Stein J, Ambrosi D, et al. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252:1510–6.
Trapl M, Enderle P, Nowotny M, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007;38:2948–52.
Dziewas R, Warnecke T, Olenberg S, et al. Towards a basic endoscopic assessment of swallowing in acute stroke - development and evaluation of a simple dysphagia score. Cerebrovasc Dis. 2008;26:41–7.
Warnecke T, Teismann I, Oelenberg S, et al. Towards a basic endoscopic evaluation of swallowing in acute stroke - identification of salient findings by the inexperienced examiner. BMC Med Educ. 2009;9:13.
Lin YN, Chen SY, Wang TG, et al. Findings of videofluoroscopic swallowing studies are associated with tube feeding dependency at discharge in stroke patients with dysphagia. Dysphagia. 2005;20:23–31.
Daniels SK, Ballo LA, Mahoney MC, Foundas AL. Clinical predictors of dysphagia and aspiration risk: outcome measures in acute stroke patients. Arch Phys Med Rehabil. 2000;81:1030–3.
Daniels SK, Schroeder MF, McClain M, et al. Dysphagia in stroke: Development of a standard method to examine swallowing recovery. J Rehabil Res Dev. 2006;43:347–56.
Bours GJ, Speyer R, Lemmens J, Limburg M, de Wit R, et al. Bedside screening tests vs videofluoroscopy or fibreoptic endoscopic evaluation of swallowing to detect dysphagia in patients with neurological disorders: systematic review. J Adv Nurs. 2009;65:477–93.
Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36:1972–6.
Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10, CD000323.
Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomized controlled trial. Lancet Neurol. 2006;5:31–7.
Perry L. Screening swallowing function of patients with acute stroke. Part one: identification, implementation and initial evaluation of a screening tool for use by nurses. J Clin Nurs. 2001;10:463–73.
Perry L. Screening swallowing function of patients with acute stroke. Part two: detailed evaluation of the tool used by nurses. J Clin Nurs. 2001;10:474–81.
Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17:308–15.
Warnecke T, Teismann I, Oelenberg S, et al. The safety of fiberoptic endoscopic evaluation of swallowing in acute stroke patients. Stroke. 2009;40:482–6.
Dziewas R, Warnecke T, Hamacher C, et al. Do nasogastric tubes worsen dysphagia in patients with acute stroke? BMC Neurol. 2008;8:28.
Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multi-center randomized controlled trial. Lancet. 2005;365:764–72.
Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–8.
Steiner T, Mendoza G, De Georgia M, et al. Prognosis of stroke patients requiring mechanical ventilation in a neurological critical care unit. Stroke. 1997;28:711–5.
Wijdicks EF, Scott JP. Causes and outcome of mechanical ventilation in patients with hemispheric ischemic stroke. Mayo Clin Proc. 1997;72:210–3.
Berrouschot J, Rossler A, Koster J, Schneider D. Mechanical ventilation in patients with hemispheric ischemic stroke. Crit Care Med. 2000;28:2956–61.
Santoli F, De Jonghe B, Hayon J. Mechanical ventilation in patients with acute ischemic stroke: survival and outcome at one year. Intens Care Med. 2001;27:1141–6.
Holland MC, Mackersie RC, Morabito D, et al. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma. 2003;55:106–11.
Pelosi P, Ferguson ND, Frutos-Vivar F, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–92.
Rabinstein AA, Wijdicks EF. Outcome of survivors of acute stroke who require prolonged ventilatory assistance and tracheostomy. Cerebrovasc Dis. 2004;18:325–31.
Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit vs a general ICU? Neurocrit Care. 2011;15:477–80.
Durbin Jr CG. Tracheostomy: why, when, and how? Respir Care. 2010;55:1056–68.
MacIntyre N. Discontinuing mechanical ventilatory support. Chest. 2007;132:1049–56.
Davis Jr K, Campbell RS, Johannigman JA, Valente JF, Branson RD. Changes in respiratory mechanics after tracheostomy. Arch Surg. 1999;134:59–62.
Shapiro M, Wilson RK, Casar G, Bloom K, Teague RB. Work of breathing through different sized endotracheal tubes. Crit Care Med. 1986;14:1028–31.
Wilson AM, Gray DM, Thomas JG. Increases in endotracheal tube resistance are unpredictable relative to duration of intubation. Chest. 2009;136:1006–13.
Blot F, Similowski T, Trouillet JL, et al. Early tracheotomy vs prolonged endotracheal intubation in unselected severely ill ICU patients. Intens Care Med. 2008;34:1779–87.
Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med. 1999;27:1714–20.
Seneff MG, Zimmerman JE, Knaus WA, Wagner DP, Draper EA. Predicting the duration of mechanical ventilation. The importance of disease and patient characteristics. Chest. 1996;110:469–79.
Huttner HB, Kohrmann M, Berger C, Georgiadis D, Schwab S. Predictive factors for tracheostomy in neurocritical care patients with spontaneous supratentorial hemorrhage. Cerebrovasc Dis. 2006;21:159–65.
Szeder V, Ortega-Gutierrez S, Ziai W, Torbey MT. The TRACH score: clinical and radiological predictors of tracheostomy in supratentorial spontaneous intracerebral hemorrhage. Neurocrit Care. 2010;13:40–6.
Qureshi AI, Suarez JI, Parekh PD, Bhardwaj A. Prediction and timing of tracheostomy in patients with infratentorial lesions requiring mechanical ventilatory support. Crit Care Med. 2000;28:1383–7.
Bösel J, Schiller P, Hacke W, Steiner T. Benefits of early tracheostomy in ventilated stroke patients. Current evidence and study protocol of the randomized pilot trial SETPOINT (Stroke-related Early Tracheostomy vs Prolonged Orotracheal Intubation in Neurocritical care Trial). Int J. Stroke. 2012;7:173–82.
Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161:1530–6.
King CS, Moores LK, Epstein SK. Should patients be able to follow commands prior to extubation? Respir Care. 2010;55:56–65.
Esteban A, Alia I, Gordo F, Fernandez R, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156:459–65.
Epstein SK. Decision to extubate. Intens Care Med. 2002;28:535–46.
Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–50.
Skoglund K, Enblad P, Hillered L, Marklund N. The neurological wake-up test increases stress hormone levels in patients with severe traumatic brain injury. Crit Care Med. 2012;40:216–22.
Skoglund K, Enblad P, Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care. 2009;11:135–42.
Helbok R, Kurtz P, Schmidt MJ, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care. 2012;16:R226.
Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896–903.
Govindan SV, Goldenberg DM, Elsamra SE, et al. Radionuclides linked to a CD74 antibody as therapeutic agents for B-cell lymphoma: comparison of Auger electron emitters with beta-particle emitters. J Nucl Med. 2000;41:2089–97.
Boynton JH, Hawkins K, Eastridge BJ, O'Keefe GE. Tracheostomy timing and the duration of weaning in patients with acute respiratory failure. Crit Care. 2004;8:R261–7.
van der Lely AJ, Veelo DP, Dongelmans DA, et al. Time to wean after tracheotomy differs among subgroups of critically ill patients: retrospective analysis in a mixed medical/surgical intensive care unit. Respir Care. 2006;51:1408–15.
Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243.
Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303:1483–9.
Young D, Harrison DA, Cuthbertson BH, Rowan K. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309:2121–9. Largest (n > 900) randomized controlled trial on early vs late tracheostomy in mixed ICU patients.
Scales DC, Thiruchelvam D, Kiss A, Redelmeier DA. The effect of tracheostomy timing during critical illness on long-term survival. Crit Care Med. 2008;36:2547–57.
Pinheiro Bdo V, Tostes Rde O, Brum CI, et al. Early vs late tracheostomy in patients with acute severe brain injury. J Bras Pneumol. 2010;36:84–91.
Bösel J, Schiller P, Hook Y, et al. Stroke-related Early Tracheostomy vs Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT): a randomized pilot trial. Stroke. 2012;44:21–8. Only prospective randomized trial on early vs late tracheostomy ventilated ICU stroke patients.
Price DG. Techniques of tracheostomy for intensive care unit patients. Anaesthesia. 1983;38:902–4.
McGregor IA, Neill RS. Tracheostomy and the Bjork flap. Lancet. 1983;2:1259.
Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest. 1985;87:715–9.
Griggs WM, Worthley LI, Gilligan JE, Thomas PD, Myburg JA. A simple percutaneous tracheostomy technique. Surg Gynecol Obstet. 1990;170:543–5.
Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: the translaryngeal method. Intens Care Med. 1997;23:386–92.
Byhahn C, Lischke V, Halbig S, Scheifler G. Westphal K [Ciaglia blue rhino: a modified technique for percutaneous dilatation tracheostomy. Technique and early clinical results]. Anaesthesist. 2000;49:202–6.
Frova G, Quintel M. A new simple method for percutaneous tracheostomy: controlled rotating dilation. A preliminary report. Intens Care Med. 2002;28:299–303.
Zgoda MA, Berger R. Balloon-facilitated percutaneous dilational tracheostomy tube placement: preliminary report of a novel technique. Chest. 2005;128:3688–90.
Cabrini L, Monti G, Landoni G, et al. Percutaneous tracheostomy, a systematic review. Acta Anaesthesiol Scand. 2012;56:270–81.
Durbin Jr CG. Techniques for performing tracheostomy. Respir Care. 2005;50:488–96.
Polderman KH, Spijkstra JJ, de Bree R, et al. Percutaneous dilatational tracheostomy in the ICU: optimal organization, low complication rates, and description of a new complication. Chest. 2003;123:1595–602.
Diaz-Reganon G, Minambres E, Ruiz A, et al. Safety and complications of percutaneous tracheostomy in a cohort of 800 mixed ICU patients. Anaesthesia. 2008;63:1198–203.
Dempsey GA, Grant CA, Jones TM. Percutaneous tracheostomy: a 6 yr prospective evaluation of the single tapered dilator technique. Br J Anaesth. 2010;105:782–8.
Durbin Jr CG. Early complications of tracheostomy. Respir Care. 2005;50:511–5.
Higgins KM, Punthakee X. Meta-analysis comparison of open vs percutaneous tracheostomy. Laryngoscope. 2007;117:447–54.
Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412–8.
Seder DB, Lee K, Rahman C, et al. Safety and feasibility of percutaneous tracheostomy performed by neurointensivists. Neurocrit Care. 2009;10:264–8.
Kocaeli H, Korfali E, Taskapilioglu O, Ozcan T. Analysis of intracranial pressure changes during early vs late percutaneous tracheostomy in a neuro-intensive care unit. Acta Neurochir (Wien). 2008;150:1263–7. discussion 1267
Kleffmann J, Pahl R, Deinsberger W, Ferbert A, Roth C. Effect of percutaneous tracheostomy on intracerebral pressure and perfusion pressure in patients with acute cerebral dysfunction (TIP Trial): an observational study. Neurocrit Care. 2012;17:85–9.
Warnecke T, Suntrup S, Teismann IK, et al. Standardized endoscopic swallowing evaluation for tracheostomy decannulation in critically ill neurologic patients. Crit Care Med. 2013;41:1728–32. Very interesting prospective study on endoscopic swallowing assessment prior to decannulation in 68 tracheotomized (post-)ICU stroke patients.
Compliance with Ethics Guidelines
Conflict of Interest
Julian Bösel has received speaker honoraria and had travel/accommodations expenses covered/reimbursed by Covidien, Sedana, and Orion Pharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with animal subjects performed by the author. With regard to the author’s research cited in this paper, all procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Bösel, J. Tracheostomy in Stroke Patients. Curr Treat Options Neurol 16, 274 (2014). https://doi.org/10.1007/s11940-013-0274-1
Published:
DOI: https://doi.org/10.1007/s11940-013-0274-1