Abstract
Coordination of swallowing and breathing is an important airway-protecting mechanism. Although it is regulated by the interaction between central pattern generators of swallowing and breathing within the brainstem, various factors modulate the coordination of swallowing and breathing. Swallowing normally occurs during expiration, and respiration after swallowing resumes with expiration. However, swallowing can occur immediately following inspiration (I-SW pattern), and respiration can resume with inspiration (SW-I pattern). Such atypical breathing–swallowing coordination occurs when the timing of swallows is inappropriate and tends to increase due to diseases and aging. In patients with chronic obstructive pulmonary disease (COPD), swallowing physiology is altered, and breathing–swallowing coordination is impaired. COPD patients with a higher frequency of I-SW and/or SW-I patterns have a higher frequency of exacerbations. Thus, we suggest that the impairment of breathing–swallowing coordination can cause exacerbations and may influence the prognosis of diseases. Two modalities, swallowing rehabilitation and low-level continuous positive airway pressure (CPAP), may ameliorate breathing–swallowing coordination. More studies are needed to elucidate whether such interventions improve outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Breathing–swallowing coordination
- Chronic obstructive pulmonary disease
- Continuous positive airway pressure
- Exacerbation
- Aspiration
1 Introduction
Pneumonia is a major cause of death in the elderly worldwide. In a case-control study, the incidence of aspiration pneumonia was 18% in nursing home patients and 15% in community-acquired pneumonia patients [1]; however, the incidence of aspiration pneumonia is thought to be much greater in the elderly population. Aspiration pneumonia occurs recurrently because it is due to the functional impairment of swallowing. Chronic obstructive pulmonary disease (COPD) is another major cause of death worldwide, and exacerbation seriously affects its morbidity and mortality [2]. A number of studies suggest that the functional impairment of swallowing is associated with exacerbations of COPD [3,4,5]. Therefore, understanding the physiology and pathophysiology underlying swallowing malfunction is essential for clinicians who treat these patients. A variety of pathological and physiological factors cause swallowing disorder, but the two major causes of swallowing disorder in the elderly are muscle weakness and the inappropriate timing of swallowing relative to the respiratory cycle. Thus, in this chapter, we will focus on how the timing of swallowing and the coordination between swallowing and breathing are regulated and discuss the clinical consequences of the alteration of coordination due to aging, diseases, and interventions.
2 Swallowing Physiology
2.1 Swallowing as an Airway Protecting Reflex
The pharynx is the common pathway of breath and swallowed food. During swallowing, the airway is securely protected against aspiration by the epiglottis, vocal cords, and vestibular folds that cover the laryngeal orifice. In addition, the airway is protected physiologically by “deglutition apnea,” the phenomenon in which the respiration is stopped during the event of swallowing. Deglutition apnea is regulated by the interaction between central pattern generators (CPGs) for swallowing and respiration in the brainstem [6].
During swallowing, the hyoid bone first slowly moves in the rostral and posterior direction and then rapidly moves toward the anterior direction. By this movement, the larynx elevates, the epiglottis falls to cover the larynx, and the upper esophageal sphincter opens to let pass a food bolus. After the completion of swallowing, the hyoid bone descends to its original position. The elevation of the larynx often starts before the provisional arrest of respiratory flow. This phenomenon is one of the remarkable characteristics of patients with stroke [7] and can be interpreted as a behavior to compensate for the delayed triggering of the swallowing reflex. The restoration of respiration usually begins with expiration (SW-E pattern), and expiration begins earlier than the completion of the laryngeal descent to its original position [8]. This can also be interpreted as an airway-protecting behavior to avoid aspirating a food residue within the pharynx.
2.2 Importance of Subglottic Pressure in Swallowing Efficiency
Gross et al. [9, 10] postulated that subglottic pressure plays an important role in swallowing efficiency. The reduction of subglottic pressure prolongs the pharyngeal contraction in healthy subjects [9]. In patients with tracheostomy, the reduction of subglottic pressure lengthens the pharyngeal transit time and increases the chance of pharyngeal residue and aspiration. Closing the tracheostomy tube during swallowing improves swallowing dynamics and reduces aspiration. The mechanisms by which subglottic pressure affects swallowing efficiency remain to be elucidated.
2.3 Swallowing Frequency
Spontaneous swallow frequency rates (swallows per minute: SPM) in healthy subjects are 1.01 in young subjects and 0.58 in elderly subjects [11]. SPM is diminished in patients with acute stroke, and the reduction is correlated with the severity of dysphagia [12]. Healthy subjects swallow 5–6 times per hour during sleep [13]. Swallows occur when the subject arises, which accompanies body movement and changes in breathing pattern, or in an arousal state, as identified by an electroencephalogram, that does not accompany body movement or changes in breathing pattern [14]. The frequency of swallows is highest at Stage I sleep and decreases as the depth of sleep progresses [14]. Therefore, the clearance of the upper airway dramatically deteriorates during sleep, particularly in elderly subjects. Saliva secretion also decreases, which may result in bacterial growth and accumulation of gastric juice and refluxed gastric contents.
3 Neural Substrates of Swallowing Reflex
3.1 Swallowing CPG and Its Interaction with Respiratory CPG
The swallowing CPG consists of the dorsal swallowing group (DSG) located within the nucleus of the solitary tract (NTS) and the ventral swallowing group (VSG) located in the ventral reticular formation adjacent to the nucleus ambiguus (AMB) [15]. These swallowing neuron groups anatomically overlap with the dorsal and ventral respiratory groups, and CPGs for swallowing and respiration functionally share a common neuronal network [16, 17].
Figure 3.1 illustrates the interaction between CPGs of swallowing and breathing in a simplified model. Here, we consider a respiratory CPG model that consists of preinspiratory/inspiratory (pre-I/I) neurons in the pre-Bötzinger complex (preBötC); expiratory-decrementing (E-DEC), expiratory-augmenting (E-AUG), and inspiratory-decrementing (I-DEC) neurons in the Bötzinger complex (BötC); and inspiratory augmenting (I-AUG) neurons in the rostral ventral respiratory group (rVRG). The preBötC is the kernel of the inspiratory rhythm generation, whereas E-DEC, E-AUG, and I-DEC neurons in the BötC shape the respiratory pattern by constituting an “inhibitory ring.” I-AUG neurons are inspiratory premotor neurons. For simplicity, the swallowing CPG is composed of two interneuron groups (SW-1 and SW-2) and one premotor neuron group (SW-3).
Interaction between respiratory and swallowing CPGs. Excitatory neuron pools and projections are colored in red (Glu; glutamatergic neurons), whereas inhibitory neuron pools and projections are colored either in blue (Gly: glycinergic neurons) or in purple (GABA/Gly: GABA/glycine-coreleasing neurons). See text for detailed explanation
When a liquid or a food bolus reaches the pharynx (the valleculae or the pyriform recess), the signal is transmitted to the primary afferent relay neurons in the NTS (SW-1) via the superior laryngeal nerve (SLN) and the glossopharyngeal nerve, then activates swallowing interneurons (SW-2) in the DSG that gate the afferent information and trigger the swallowing reflex by a burst of firings. The swallowing reflex is a programmed sequence of muscle activities, where premotor neurons (SW-3) in the VSG generate a stereotypic motor pattern almost irrespective of the strength of the afferent stimulus. When a swallow is triggered, E-DEC neurons are activated to suppress respiratory activity (deglutition apnea). During a volitional swallow, swallowing-related cortical areas (SW cortex) activate (presumably) E-DEC neurons to suppress respiration (both inspiration and expiration). Thus, in the case of a volitional swallow, respiration stops before the swallowing reflex occurs. Simultaneously, the SW cortex lowers the threshold of SW-2 neurons to facilitate the swallowing reflex.
Since the coupling of swallowing and respiratory rhythms occurs in decerebrate animals, the coordination of swallowing and breathing is primarily regulated by the interaction between these CPGs of swallowing and respiration. When the synaptic transmission of the Kölliker–Fuse nucleus in the pons is disrupted, the airway-protecting laryngeal adduction during swallowing and the coordination between swallowing and breathing is impaired [18], suggesting that the Kölliker–Fuse nucleus is required for these functions.
Electrical stimulation of the SLN elicits swallows in cats and rats [16, 19, 20]. During continuous electrical SLN stimulation, swallows preferentially occur at respiratory phase transitions (the phase transition between stage II expiration and inspiration, the transition between inspiration and stage I expiration, and the transition between stage I and II of expiration), where E-DEC neurons are disinhibited. This is physiologically reasonable because E-DEC neurons are inhibitory neurons that have broad projections to other respiratory neurons and thus can contribute to deglutition apnea when disinhibited.
3.2 Swallowing-Related Cortical and Subcortical Areas
Functional brain mapping has revealed that swallowing activates cortical areas (sensorimotor cortex, premotor cortex, cingulate cortex, and insulate cortex) and subcortical areas (internal capsule, hypothalamus, amygdala, substantia nigra, and midbrain reticular formation) [15, 21]. These cortical and subcortical areas not only facilitate the swallowing reflex but also modulate swallow-related muscles by receiving sensory feedback [22].
The incidence of dysphagia after stroke varies between 41% and 78% [23,24,25]. Therefore, patients with stroke have a high incidence of dysphagia and a risk of aspiration pneumonia. Swallowing-related cortical areas display interhemispheric asymmetry, and right hemisphere strokes tend to produce pharyngeal dysphagia, whereas left hemisphere strokes tend to produce oral dysphagia [26]. Recovery of swallowing function after stroke is associated with an increase in the activation of swallow-related cortical areas in the unaffected hemisphere [27], suggesting that functional recovery depends on the reorganization of neuronal circuits, i.e., projections from the unaffected hemisphere to the swallowing CPG. A short-term pharyngeal sensory stimulation causes a persistent augmentation of swallowing motor representation in the cortex [27], which might be applicable to the neurorehabilitation of dysphagia after stroke.
4 Physiological Basis of Coordination Between Breathing and Swallowing
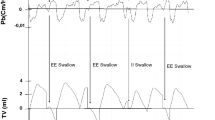
In conscious humans, swallows preferentially occur during the early-middle phase of expiration and respiration after the swallow is resumed with expiration (E-SW-E pattern) [28, 29]. In relation to the lung volume, swallows preferentially occur at a specific range of lung volume (200–370 ml above the functional residual capacity) that is optimal for fundamental swallowing functions—laryngeal elevation, upper airway closure, the opening of the upper and lower esophageal sphincters [30]. However, other types of coordination are observed, i.e., swallowing occurring immediately following inspiration (I-SW pattern) and respiration resuming with inspiration (SW-I pattern), resulting in additional three (I-SW-E, E-SW-I, I-SW-I) patterns [31]. We recruited 269 healthy elderly subjects with a 10-item eating assessment tool (EAT-10) score less than 3, as well as 30 dysphagic patients, to evaluate breathing–swallowing coordination [32]. Figure 3.2 shows the relative frequency of I-SW/SW-I patterns in healthy elderly subjects and dysphagic patients. In general, healthy subjects had a lower occurrence rate of I-SW/SW-I patterns; however, even in healthy subjects, 20 (7.5%) subjects had a high (>40%) I-SW rate, and 25 (9.4%) subjects had a high SW-I rate in water swallows.
Swallowing is a physiological perturbation of the respiratory rhythm [33]. Swallowing causes phase resetting of the respiratory cycle and changes the interval to the next breath depending on the timing in the respiratory cycle when the swallow is initiated. Swallows initiated at late times in the respiratory cycle have the shortest interval to the next breath and tend to happen with the SW-I pattern, whereas swallows initiated at early times naturally tend to happen in the I-SW pattern [32] (Fig. 3.3).
Frequency distributions of different breathing–swallowing coordination patterns in early (old phase <0.4), intermediate (old phase between 0.4 and 1.0), and late (old phase >1.0) timings of swallowing. The old phase represents the timing of swallowing in the respiratory cycle, which is expressed as the time from the preceding inspiration, where the mean length of the respiratory cycle is normalized to 1 [33]. ##: P value vs. Intermediate <0.0001 (chi-square test followed by Haberman’s residual test)
Swallows with the I-SW-E pattern accompany the prolonged latency to the onset of swallowing from the onset of deglutition apnea and the prolongation of the duration of deglutition apnea, suggesting that this pattern is an adaptive response to compensate for the delayed onset of the swallowing reflex [32]. In contrast, the E-SW-I pattern tends to occur when the timing of swallows in the respiratory cycle is delayed. Patients with dysphagia often have difficulty initiating swallows, and the timing of swallows is delayed, which may result in E-SW-I-pattern swallows.
5 Impaired Swallowing in COPD: Its Pathophysiology and Consequences
5.1 Swallowing Function in COPD
COPD is a lung disease characterized by chronic obstruction of lung airflow that interferes with normal breathing and is not fully reversible. Since breathing and swallowing cannot be done simultaneously, a prolonged time to empty the inspired air due to an expiratory flow limitation may interfere with the act of swallowing. Therefore, it is reasonable to hypothesize that swallowing is impaired in patients with COPD, though such a phenomenon is poorly recognized by clinicians. Coelho [34] was the first to report that patients with COPD have impaired swallowing function: “The overall picture was one of reduced strength in all aspects of the swallow, coupled with a reduced ability to use pulmonary air to clear the larynx and ensure airway protection.” Subsequent studies revealed a higher (17–20%) prevalence of dysphagia in patients with COPD [35] compared with control subjects.
The impairment of swallowing function can be evaluated by the latency from water injection into the pharynx to the onset of the swallowing reflex (e.g., the simple two-step swallowing-provocation test; STS-SPT) [36] or the repetitive saliva swallowing test (RSST) [37]. In moderate and severe stages of COPD, a higher incidence of abnormal swallowing function is observed by both tests; however, even in the mild stages of COPD, the incidence of abnormal RSST count (<3 voluntary swallows within 30 s) is higher than in controls [38].
5.2 Altered Swallowing Physiology in COPD
Swallowing physiology in patients with COPD is different from that of healthy subjects. The maximal laryngeal elevation during swallowing is reduced in patients with COPD [35]. Some patients use voluntary airway-protecting maneuvers during swallowing, such as prolonged airway closure and earlier laryngeal closure relative to the cricopharyngeal opening [35]. Patients with COPD are more likely to have either penetration of pharyngeal contents into the larynx or actual aspiration when swallowing large fluid volumes and favor an I-SW-E pattern with larger boluses [39]. Further, patients with COPD have a longer pharyngeal swallowing phase than normal subjects, which is associated with a decrease in the difference between the duration of maximal laryngeal elevation and the duration of pharyngeal transit [40]. As described in Sect. 4, the latency to the onset of swallowing from the onset of deglutition apnea and the duration of deglutition apnea are lengthened in swallows with the I-SW-E pattern compared to the E-SW-E pattern [32]. Therefore, the I-SW-E pattern observed in patients with COPD while swallowing large boluses is interpreted as an airway-protecting behavior to compensate for the delayed triggering of the swallowing reflex and a longer pharyngeal transit time.
5.3 Influences of Smoking on Swallowing Function
Smoking, the leading cause of COPD, also causes swallowing abnormalities. Smoking increases both the threshold volume for triggering the pharyngo-upper esophageal sphincter contractile response and the threshold volume for reflexive pharyngeal swallowing [41]. In addition, smoking lowers the upper and lower esophageal sphincter pressures [42]. Therefore, smoking triggers or aggravates gastroesophageal reflux and adversely affects the clearance of refluxed gastric contents. This may be related to a decreased laryngopharyngeal sensitivity in patients with COPD [43].
5.4 Gastroesophageal Reflux Disease (GERD) and COPD Exacerbations
GERD is a common comorbidity of COPD, with a prevalence of 10–29% in the Western population and 5–14% among Japanese adults [44]. Terada, Muro et al. [45] first reported that GERD symptoms are an important factor associated with COPD exacerbation. Later, large-scale cohort studies verified that GERD is an independent predictor of frequent exacerbations [46,47,48]. In the Copenhagen City Heart Study, in which 1259 patients with COPD were enrolled, the association between GERD and COPD exacerbation was found only in those individuals who did not use acid-inhibitory treatment regularly [47]. Interestingly, another cohort study with 4483 participants [48] showed that the use of a proton-pump inhibitor (PPI) was associated with frequent exacerbations but did not meaningfully influence the GERD–exacerbation association. A recent cohort study with 638 participants revealed that therapy with PPIs for GERD did not reduce the risk for severe exacerbations in COPD [49]. These results may suggest that regurgitation itself, rather than acid, causes COPD exacerbations. Patients with COPD who had experienced exacerbations in the previous year had prolonged latencies from water injection into the pharynx to the onset of the swallowing reflex compared to patients without exacerbations [3]. A more recent prospective study showed that a prolonged latency to initiate the swallowing reflex predisposed patients with COPD to exacerbations [4]. They suggested that abnormal swallowing reflexes in COPD might be affected by the comorbidity of GERD and cause bacterial colonization. Alternatively, impairment of swallowing function may contribute to the manifestation of GERD symptoms by lowering of upper airway clearance and microaspiration. Tsuzuki et al. [5] showed that RSST is also a useful test for predicting exacerbations in patients with COPD.
6 Coordination of Swallowing and Breathing in Aging and Diseases
6.1 Discoordination Between Swallowing and Breathing May Predict Aspiration, Exacerbation, and Prognosis of Diseases
I-SW and SW-I patterns increase with aging [29] and in patients with stroke [50], head and neck cancer after treatment [51], Parkinson’s disease [52], or COPD [53]. I-SW-pattern swallows in combination with a delayed onset of the swallowing reflex may cause aspiration before the swallow, particularly in the case of liquid swallows. On the other hand, SW-I-pattern swallows may cause aspiration after the swallow when food residue is in the pharynx after the first swallowing reflex. Healthy subjects rarely aspirate even on such occasions; however, SW-I-pattern swallows and the shortening of the duration of deglutition apnea are correlated with laryngeal penetration and aspiration in patients with Parkinson’s disease [54]. Further, frequent I-SW and/or SW-I patterns (>25% occurrence rate) are associated with COPD exacerbation [55]. Therefore, identifying such subjects with a high I-SW/SW-I rate and treating them to reduce the I-SW/SW-I rate may prevent exacerbations of these diseases.
6.2 Coordination of Swallowing and Breathing in Patients with Obstructive Sleep Apnea Syndrome (OSAS)
CPAP prolongs the latency and reduces the frequency of swallows in healthy subjects [56]. Animal experiments suggest that these effects are mediated by bronchopulmonary receptors rather than upper airway receptors [57]. In patients with OSAS, swallows occur in periods of microarousal, and swallows with the SW-I pattern are increased compared with healthy subjects [14]. CPAP restores the frequency of SW-I-pattern swallows to the normal level in patients with OSAS [14], and the frequency and duration of GERD symptoms are also reduced [58].
7 Factors Affecting Coordination Between Swallowing and Breathing
7.1 Coordination of Swallowing and Breathing During Anesthesia
As described in Sect. 4, in conscious subjects, swallows preferentially occur during the early–middle phase of expiration, when the lung volume falls within a specific range. However, in unconscious subjects, e.g., during anesthesia, such a preference has not been observed [59]. It is well known that anesthesia attenuates airway defensive reflexes and heightens the risk of aspiration. During anesthesia, when swallows occur during inspiration or at the expiratory-to-inspiratory phase transition, coughing and apnea are often evoked due to laryngeal stimulation, resulting in a risk of aspiration [60]. Further, anesthesia affects the coordination of swallowing and breathing. Sevoflurane and propofol decrease the frequency of spontaneous swallows and increase the frequency of I-SW and SW-I pattern swallows [61].
7.2 Coordination of Swallowing and Breathing During Loaded Breathing
The timing of swallows in the respiratory cycle is modified during loaded breathing even in conscious subjects. For example, hypercapnia increases the frequency of I-SW and SW-I swallows in both the conscious and unconscious states [60, 61]. However, hypercapnia decreases the frequency of swallows in the conscious state, whereas it augments the frequency in the unconscious state.
When resistive or elastic loading is applied to the respiratory system, the frequency of swallows during inspiration and at the expiratory-to-inspiratory phase transition increases simultaneously with changes in breathing patterns [62].
7.3 Coordination of Swallowing and Breathing During Continuous Positive Airway Pressure and Assisted Ventilation
A brief (~100 ms) inspiratory flow is often recorded immediately after swallowing. This is not a true inspiration but a negative pressure associated with the relaxation of the pharyngeal constrictor muscle, known as the swallow noninspiratory flow (SNIF) [63]. When a bilevel positive pressure ventilator is used in S (spontaneous) or ST (spontaneous/timed) mode, SNIF may trigger inspiratory support, resulting in an SW-I pattern swallow [64, 65]. Terzi et al. [64] proposed that a device equipped with an off switch is effective to prevent swallowing-induced autotriggering of inspiration. In contrast, continuous positive airway pressure (CPAP) ventilation reduces the frequency of SW-I-pattern swallows in patients with OSAS [14].
Recently, we found that low-pressure (4 cm H2O) CPAP decreases the SW-I frequency, increases the SNIF occurrence, and normalizes the timing of swallowing, suggesting that low-pressure CPAP alleviates the risk of aspiration in patients with COPD [66]. Low-pressure CPAP may affect the lung volume during quiet breathing by relieving the expiratory flow limitation and thereby shift the timing of swallowing toward early expiration. Therefore, although CPAP reduces the frequency of swallows [56], it has a beneficial effect from the viewpoint of airway protection.
Nasal high-flow oxygen therapy (NHF) has recently been widely used for hypoxemic respiratory failure. Since NHF generates low-level positive airway pressure during expiration with the mouth closed (2.7 cm H2O at 35 L/min [67] and 5.5 cm H2O at 40 L/min [68]), it is expected to ameliorate the breathing–swallowing coordination, similar to low-level CPAP. However, in healthy volunteers, swallows during the inspiratory phase and at the expiratory-inspiratory transition tended to increase, although the occurrence rates were not significantly different [69]. On the other hand, NHF shortened the latency for inducing the swallowing reflex [69].
8 Swallowing Rehabilitation to Improve the Coordination Between Swallowing and Breathing
Several methods potentially ameliorate the coordination between swallowing and breathing. The first method is the supraglottic swallow, a maneuver to ensure laryngeal closure during swallowing, in which each subject is instructed to inhale and hold his breath before and during swallowing and exhale after swallowing. By the use of this maneuver, the subject is expected to swallow at a high lung volume and naturally resume his respiration with expiration after swallowing, which would reduce SW-I-pattern swallows. Another method uses biofeedback, where information on the respiratory phase and lung volume is visually presented so that a subject can swallow at an optimal time within the respiratory cycle [70]. Training with this method has improved the swallowing function, such as in the penetration-aspiration score, in patients with head neck cancer.
9 Conclusion
Coordination of swallowing and breathing is regulated by the interaction between central pattern generators of swallowing and breathing within the brainstem. The coordination is impaired in various conditions, e.g., aging, anesthesia, neurological diseases, and respiratory diseases, leading to aspiration and exacerbation of diseases, and it may influence prognosis. Swallowing rehabilitation and low-level CPAP are candidates for therapeutic intervention to normalize the coordination of swallowing and breathing. Studies are needed to elucidate whether such interventions improve the outcome.
References
Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296–302. https://doi.org/10.1111/j.1532-5415.2005.00608.x.
Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–31. https://doi.org/10.1136/thx.2005.040527.
Kobayashi S, Kubo H, Yanai M. Impairment of the swallowing reflex in exacerbations of COPD. Thorax. 2007;62(11):1017. https://doi.org/10.1136/thx.2007.084715.
Terada K, Muro S, Ohara T, Kudo M, Ogawa E, Hoshino Y, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137(2):326–32.
Tsuzuki A, Kagaya H, Takahashi H, Watanabe T, Shioya T, Sakakibara H, et al. Dysphagia causes exacerbations in individuals with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2012;60(8):1580–2. https://doi.org/10.1111/j.1532-5415.2012.04067.x.
Bautista TG, Sun QJ, Pilowsky PM. The generation of pharyngeal phase of swallow and its coordination with breathing: interaction between the swallow and respiratory central pattern generators. Prog Brain Res. 2014;212:253–75. https://doi.org/10.1016/B978-0-444-63488-7.00013-6.
Wang CM, Shieh WY, Chen JY, Wu YR. Integrated non-invasive measurements reveal swallowing and respiration coordination recovery after unilateral stroke. Neurogastroenterol Motil. 2015;27(10):1398–408. https://doi.org/10.1111/nmo.12634.
Martin BJ, Logemann JA, Shaker R, Dodds WJ. Coordination between respiration and swallowing: respiratory phase relationships and temporal integration. J Appl Physiol (1985). 1994;76(2):714–23.
Gross RD, Atwood CW Jr, Grayhack JP, Shaiman S. Lung volume effects on pharyngeal swallowing physiology. J Appl Physiol (1985). 2003;95(6):2211–7. https://doi.org/10.1152/japplphysiol.00316.2003.
Gross RD, Steinhauer KM, Zajac DJ, Weissler MC. Direct measurement of subglottic air pressure while swallowing. Laryngoscope. 2006;116(5):753–61. https://doi.org/10.1097/01.mlg.0000205168.39446.12.
Crary MA, Sura L, Carnaby G. Validation and demonstration of an isolated acoustic recording technique to estimate spontaneous swallow frequency. Dysphagia. 2013;28(1):86–94. https://doi.org/10.1007/s00455-012-9416-y.
Crary MA, Carnaby GD, Sia I. Spontaneous swallow frequency compared with clinical screening in the identification of dysphagia in acute stroke. J Stroke Cerebrovasc Dis. 2014;23(8):2047–53. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.03.008.
Lichter I, Muir RC. The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol. 1975;38(4):427–32.
Sato K, Umeno H, Chitose S, Nakashima T. Sleep-related deglutition in patients with OSAHS under CPAP therapy. Acta Otolaryngol. 2011;131(2):181–9. https://doi.org/10.3109/00016489.2010.520166.
Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–69.
Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the Decerebrate Cat. J Physiol Lond. 1994;480:309–24.
Sugiyama Y, Shiba K, Mukudai S, Umezaki T, Hisa Y. Activity of respiratory neurons in the rostral medulla during vocalization, swallowing, and coughing in Guinea pigs. Neurosci Res. 2014;80:17–31. https://doi.org/10.1016/j.neures.2013.12.004.
Bautista TG, Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol. 2014;592(Pt 12):2605–23. https://doi.org/10.1113/jphysiol.2014.272468.
Dick TE, Oku Y, Romaniuk JR, Cherniack NS. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol Lond. 1993;465:715–30.
Saito Y, Ezure K, Tanaka I, Osawa M. Activity of neurons in ventrolateral respiratory groups during swallowing in decerebrate rats. Brain and Development. 2003;25(5):338–45. https://doi.org/10.1016/S0387-7604(03)00008-1.
Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G354–60.
Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86. https://doi.org/10.1002/ddrr.12.
Falsetti P, Acciai C, Palilla R, Bosi M, Carpinteri F, Zingarelli A, et al. Oropharyngeal dysphagia after stroke: incidence, diagnosis, and clinical predictors in patients admitted to a neurorehabilitation unit. J Stroke Cerebrovasc Dis. 2009;18(5):329–35. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.01.009.
Masrur S, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Zhao X, et al. Dysphagia screening and hospital-acquired pneumonia in patients with acute ischemic stroke: findings from get with the guidelines—stroke. J Stroke Cerebrovasc Dis. 2013;22(8):e301–9. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.11.013.
Sorensen RT, Rasmussen RS, Overgaard K, Lerche A, Johansen AM, Lindhardt T. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. J Neurosci Nurs. 2013;45(3):139–46. https://doi.org/10.1097/JNN.0b013e31828a412c.
Robbins J, Levin RL. Swallowing after unilateral stroke of the cerebral cortex: preliminary experience. Dysphagia. 1988;3(1):11–7.
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1(1):64–8. https://doi.org/10.1038/264.
Nishino T, Yonezawa T, Honda Y. Effects of swallowing on the pattern of continuous respiration in human adults. Am Rev Respir Dis. 1985;132(6):1219–22. https://doi.org/10.1164/arrd.1985.132.6.1219.
Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, et al. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Phys. 1992;263(5 Pt 1):G750–5.
McFarland DH, Martin-Harris B, Fortin AJ, Humphries K, Hill E, Armeson K. Respiratory-swallowing coordination in normal subjects: lung volume at swallowing initiation. Respir Physiol Neurobiol. 2016;234:89–96. https://doi.org/10.1016/j.resp.2016.09.004.
Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131(9):762–70. https://doi.org/10.1001/archotol.131.9.762.
Yagi N, Oku Y, Nagami S, Yamagata Y, Kayashita J, Ishikawa A, et al. Inappropriate timing of swallow in the respiratory cycle causes breathing-swallowing discoordination. Front Physiol. 2017;8:676. https://doi.org/10.3389/fphys.2017.00676.
Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483(Pt 1):273–88.
Coelho CA. Preliminary findings on the nature of dysphagia in patients with chronic obstructive pulmonary disease. Dysphagia. 1987;2(1):28–31.
Mokhlesi B, Logemann JA, Rademaker AW, Stangl CA, Corbridge TC. Oropharyngeal deglutition in stable COPD. Chest. 2002;121(2):361–9.
Teramoto S, Matsuse T, Fukuchi Y, Ouchi Y. Simple two-step swallowing provocation test for elderly patients with aspiration pneumonia. Lancet. 1999;353(9160):1243. https://doi.org/10.1016/S0140-6736(98)05844-9.
Oguchi K, Saitoh E, Mizuno M, Kusudo S, Tanaka T, Onogi K. The repetitive saliva swallowing test (RSST) as a screening test of functional dysphagia (1) Normal values of RSST. Jpn J Rehabil Med. 2000;37:375–82.
Ohta K, Murata K, Takahashi T, Minatani S, Sako S, Kanada Y. Evaluation of swallowing function by two screening tests in primary COPD. Eur Respir J. 2009;34(1):280–1. https://doi.org/10.1183/09031936.00016909.
Cvejic L, Harding R, Churchward T, Turton A, Finlay P, Massey D, et al. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2011;16(2):269–75. https://doi.org/10.1111/j.1440-1843.2010.01875.x.
Cassiani RA, Santos CM, Baddini-Martinez J, Dantas RO. Oral and pharyngeal bolus transit in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:489–96. https://doi.org/10.2147/COPD.S74945.
Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43(4):537–41.
Stanciu C, Bennett JR. Smoking and gastro-oesophageal reflux. Br Med J. 1972;3(5830):793–5.
Clayton NA, Carnaby-Mann GD, Peters MJ, Ing AJ. The effect of chronic obstructive pulmonary disease on laryngopharyngeal sensitivity. Ear Nose Throat J. 2012;91(9):370, 2, 4 passim.
Kang JY. Systematic review: geographical and ethnic differences in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2004;20(7):705–17. https://doi.org/10.1111/j.1365-2036.2004.02165.x.
Terada K, Muro S, Sato S, Ohara T, Haruna A, Marumo S, et al. Impact of gastro-oesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63(11):951–5. https://doi.org/10.1136/thx.2007.092858.
Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–38. https://doi.org/10.1056/NEJMoa0909883.
Ingebrigtsen TS, Marott JL, Vestbo J, Nordestgaard BG, Hallas J, Lange P. Gastro-esophageal reflux disease and exacerbations in chronic obstructive pulmonary disease. Respirology. 2015;20(1):101–7. https://doi.org/10.1111/resp.12420.
Martinez CH, Okajima Y, Murray S, Washko GR, Martinez FJ, Silverman EK, et al. Impact of self-reported gastroesophageal reflux disease in subjects from COPDGene cohort. Respir Res. 2014;15:62. https://doi.org/10.1186/1465-9921-15-62.
Baumeler L, Papakonstantinou E, Milenkovic B, Lacoma A, Louis R, Aerts JG, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology. 2016;21(5):883–90. https://doi.org/10.1111/resp.12758.
Leslie P, Drinnan MJ, Ford GA, Wilson JA. Swallow respiration patterns in dysphagic patients following acute stroke. Dysphagia. 2002;17(3):202–7. https://doi.org/10.1007/s00455-002-0053-8.
Gillespie MB, Brodsky MB, Day TA, Sharma AK, Lee FS, Martin-Harris B. Laryngeal penetration and aspiration during swallowing after the treatment of advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2005;131(7):615–9. https://doi.org/10.1001/archotol.131.7.615.
Gross RD, Atwood CW Jr, Ross SB, Eichhorn KA, Olszewski JW, Doyle PJ. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia. 2008;23(2):136–45.
Gross RD, Atwood CW Jr, Ross SB, Olszewski JW, Eichhorn KA. The coordination of breathing and swallowing in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(7):559–65.
Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM. Respiratory-swallowing coordination and swallowing safety in patients with Parkinson’s disease. Dysphagia. 2011;26(3):218–24. https://doi.org/10.1007/s00455-010-9289-x.
Nagami S, Oku Y, Yagi N, Sato S, Uozumi R, Morita S, et al. Breathing-swallowing discoordination is associated with frequent exacerbations of COPD. BMJ Open Respir Res. 2017;4(1):e000202. https://doi.org/10.1136/bmjresp-2017-000202.
Nishino T, Sugimori K, Kohchi A, Hiraga K. Nasal constant positive airway pressure inhibits the swallowing reflex. Am Rev Respir Dis. 1989;140(5):1290–3. https://doi.org/10.1164/ajrccm/140.5.1290.
Samson N, Roy B, Ouimet A, Moreau-Bussiere F, Dorion D, Mayer S, et al. Origins of the inhibiting effects of nasal CPAP on nonnutritive swallowing in newborn lambs. J Appl Physiol (1985). 2008;105(4):1083–90. https://doi.org/10.1152/japplphysiol.90494.2008.
Kerr P, Shoenut JP, Millar T, Buckle P, Kryger MH. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101(6):1539–44.
Nishino T, Hiraga K. Coordination of swallowing and respiration in unconscious subjects. J Appl Physiol (1985). 1991;70(3):988–93.
Nishino T, Hasegawa R, Ide T, Isono S. Hypercapnia enhances the development of coughing during continuous infusion of water into the pharynx. Am J Respir Crit Care Med. 1998;157(3 Pt 1):815–21. https://doi.org/10.1164/ajrccm.157.3.9707158.
D’Angelo OM, Diaz-Gil D, Nunn D, Simons JC, Gianatasio C, Mueller N, et al. Anesthesia and increased hypercarbic drive impair the coordination between breathing and swallowing. Anesthesiology. 2014;121(6):1175–83. https://doi.org/10.1097/ALN.0000000000000462.
Kijima M, Isono S, Nishino T. Coordination of swallowing and phases of respiration during added respiratory loads in awake subjects. Am J Respir Crit Care Med. 1999;159(6):1898–902. https://doi.org/10.1164/ajrccm.159.6.9811092.
Brodsky MB, McFarland DH, Michel Y, Orr SB, Martin-Harris B. Significance of nonrespiratory airflow during swallowing. Dysphagia. 2012;27(2):178–84. https://doi.org/10.1007/s00455-011-9350-4.
Terzi N, Normand H, Dumanowski E, Ramakers M, Seguin A, Daubin C, et al. Noninvasive ventilation and breathing-swallowing interplay in chronic obstructive pulmonary disease. Crit Care Med. 2014;42(3):565–73. https://doi.org/10.1097/CCM.0b013e3182a66b4a.
Hori R, Isaka M, Oonishi K, Yabe T, Oku Y. Coordination between respiration and swallowing during non-invasive positive pressure ventilation. Respirology. 2016;21(6):1062–7. https://doi.org/10.1111/resp.12790.
Hori R, Ishida R, Isaka M, et al. Effects of noninvasive ventilation on the coordination between breathing and swallowing in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1485–94.
Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103(6):886–90. https://doi.org/10.1093/bja/aep280.
Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care. 2007;20(4):126–31. https://doi.org/10.1016/j.aucc.2007.08.001.
Sanuki T, Mishima G, Kiriishi K, Watanabe T, Okayasu I, Kawai M, et al. Effect of nasal high-flow oxygen therapy on the swallowing reflex: an in vivo volunteer study. Clin Oral Investig. 2017;21(3):915–20. https://doi.org/10.1007/s00784-016-1822-3.
Martin-Harris B, McFarland D, Hill EG, Strange CB, Focht KL, Wan Z, et al. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil. 2015;96(5):885–93. https://doi.org/10.1016/j.apmr.2014.11.022.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Oku, Y. (2020). Coordination of Swallowing and Breathing: How Is the Respiratory Control System Connected to the Swallowing System?. In: Yamaguchi, K. (eds) Structure-Function Relationships in Various Respiratory Systems. Respiratory Disease Series: Diagnostic Tools and Disease Managements. Springer, Singapore. https://doi.org/10.1007/978-981-15-5596-1_3
Download citation
DOI: https://doi.org/10.1007/978-981-15-5596-1_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5595-4
Online ISBN: 978-981-15-5596-1
eBook Packages: MedicineMedicine (R0)