Abstract
Biosurfactants stand for highly useful and promising compounds. They basically serve for a variety of applications in multiple industries and aspects of human life. Therefore, it is highly required to improve their production yield especially through the development of new and more efficient fermentation processes. In this aim, batch and fed-batch were studied and compared in terms of their effective biosurfactant production by Bacillus subtilis SPB1. Experiments of fed-batch fermentations were carried out through three different glucose feeding strategies, namely the pulsed, the constant Donespeed and the exponential feeding. The comparison between different fermentation processes revealed that fed-batch process proved to be a more efficient cultivation strategy than the batch process in terms of cell biomass, biosurfactant production and productivity. Among the three different feeding strategies, the exponential feeding process achieved the highest fermentation results of final biosurfactant concentration. The latter increased more than twofolds compared to batch fermentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term ‘‘biosurfactant’’ corresponds to an isolated or a non-isolated compound obtained from microbial cells to affect surfaces and interfaces and to reduce needed efforts to overcome surface tension. This process allows one system to disperse into another [1]. Relying upon their molecular weight, microbial surfactants can be classified into high molecular weight including polysaccharides and lipoproteins and low molecular weight involving glycolipids and lipopeptides. The second group is more extensively used than the first one owing to its high surface tension reduction potential. It displays an emulsifying capacity that can be applied in the bioremediation of hydrophobic compounds within numerous environments. Biosurfactants have whetted the widest interest in view of their higher level of degradability and their production from renewable sources. Indeed, these upsides cannot be seen in synthetic surfactants [2,3,4]. They often exhibit specific biological activities and are involved in cell–cell interactions such as in sensing, biofilm formation and cellular differentiation [3, 5]. Additionally, biosurfactant can function at extreme pH, temperature and salt concentration [3]. Thanks to their low toxicity, good biodegradability and specific bioactivity, biosurfactants present a powerful potential for practical applications, in particular in the fields of cosmetics, healthcare and food industries as well as the biomedical and pharmaceutic areas [6,7,8]. However, their use in certain applications depends on the production and purification costs for specific activities. Currently, biosurfactants are still unable to economically compete with the chemically synthesized surfactants in the market, referring to the high production costs.
From this perspective, elaborating biosurfactant production where the cost of raw materials and the processing become minimal seems to be of an urgent industrial concern. Provided that this requirement is met and the price of the biosurfactant becomes lower than that of chemical ones, the biosurfactants will have a chance to be used at a large scale for the industrial applications. In terms of the development of any fermentation process, two main areas are basically considered, namely those associated with the strain selection and development program [1, 9, 10] and those associated with the process development such as the improvement at the level of microbial medium and culture conditions. Therefore, different strategies were adopted to enhance the production yields and to decrease their cost including the development of recombinant and over-productive strains [11, 12], the use of low-cost or economic substrates [13, 14], media formulation and the optimization of nutritional and physic-chemical conditions during the fermentation [15, 16], strain immobilization [15] and the incorporation of different fermentation processes [1, 15, 17]. The last-mentioned strategy resting upon modification of the fermentation process is considered as the most prominent one for increasing biosurfactant production by microorganisms. Another area associated with the process development involves fermentation technique and the mode of reactor operation [1, 18, 19].
Common fermentation methods invested in biosurfactant research are batch, fed-batch and continuous batch [20, 21]. In batch process fermentation, media and inoculum are integrated simultaneously to the culture medium before the beginning of cultivation. At the end of the process, the product is only discharged from the fermenter [20, 21]. However, in the continuous process, all the nutrients are continuously added to the fermenter and the components of the culture medium are removed from the fermenter at the same time to maintain a constant culture volume [20]. Biosurfactant production studies investing this type of fermentation have been scarcely reported referring to the problems in terms of controlling substrate availability. These difficulties are assigned to the addition of new media that need to reach a fixed volume while maintaining output cultures in a constant cell physiology phase [21]. On the other side, during a fed-batch process, new media are inserted regularly, without removing the culture fluid present in the fermenter [20, 21], there by entailing a gradual increase in the culture volume. In this type of system, the regular addition of nutrients prevents nutrient depletion until a product close to the maximum yield is obtained [21, 22]. Notably, as reported by Eslami et al. [20], two main types of feeding strategies existed; the feedback control mechanism and no feedback control mechanism.
The fed-batch culture has been extensively used for the enhancement in terms of the production of diverse primary and secondary microbial metabolites, proteins and other biopolymers compared to the batch fermentation [1, 23]. Numerous studies reported the use of fed-batch fermentation for the production of biosurfactant from different microorganisms. For instance, Zambry et al. [23] handled the production of lipopeptide biosurfactant in batch and fed-batch Streptomyces sp. PBD-410L cultures growing on palm oil. Moreover, Hajfarajollah et al. [17] tackled various fed-batch strategies for lipopeptide biosurfactant production by Aneurinibacillus thermoaerophilus HAK01. Yao et al. [24] addressed the production of a lipopeptide biosurfactant in Bacillus amyloliquefaciens fmb50 by controlling the foam overflow rate of a fed-batch culture. In another work, Zhu et al. [25] examined a pH–stat process for the production of rhamnolipid biosurfactant by P. aeruginosa. Fed-batch cultivation of Pseudomonas aerugenosa with a pulsed feeding strategy using multiple substrates (nitrogen phosphorus and carbon sources) was also investigated by Ghomi et al. [10].
As portrayed in our previous studies, B. subtilis SPB1 generated a mixture of lipopeptides biosurfactant belonging to surfactin, iturin and fengycin isoforms as identified by LC- MS analysis [26]. The SPB1 biosurfactants exhibit a broad spectrum of actions, including antimicrobial activity against microorganisms with multidrug resistant profiles [27], antifungal activity toward phytopathogenic fungi [28], insecticidal activity [29,30,31], antioxidant activity [32] and antidiabetic as well as antilipidemic properties in alloxan-induced diabetic rats [33,34,35] with a reduced toxicity toward living cells [36]. This is indicative of their potential application in biomedical, pharmaceutical and agricultures fields. Moreover, the lipopeptide reduces the surface tension of water along with emulsifying, solubilizing and mobilizing activities [11, 37, 38]. They display the ability to stimulate hydrocarbons biodegradation [39] and colors removal [40,41,42] allowing their application in the environmental field. Additionally, when incorporated in the different formula of dough, baked products and cookies, they brush up their textural and sensorial properties [38, 43, 44]. Furthermore, as corroborated by Bouassida et al. [45, 46], SPB1 lipopeptide was incorporated in toothpaste and detergent formula and proved its efficiency. Having this broad spectrum of applications, we attempted to further enhance SPB1 biosurfactant production. As demonstrated above, different strategies were adopted and published by our team group. First, SPB1 biosurfactant was refined by an adequate control of aeration [47]. Second, it was produced on various agro-industrial residues [48,49,50,51]. In this respect, the optimization of the medium composition and the physic-chemical conditions of the fermentation by the experimental planning methodology followed by the response surface methodology was performed [51, 52]. Submerged and Solid State Fermentations were undertaken. Third, investing mutant strain with better productivity was explored by Bouassida et al. [11]. Basically, the study of different fermentation strategies can be an optimal choice to improve biosurfactant production on glucose based medium. Within this framework and to accomplish high productivity and yield of the product, we adopted a suitable feeding strategy. Experiments of batch and fed-batch processes using different glucose feeding strategies, including pulsed feeding, constant speed feeding and exponential feeding were investigated and compared for the efficient production of microbial surfactants by B. subtilis SPB1. In each process, different initial glucose concentrations were invested to assess the impact of this source of carbon on biomass and biosurfactant production.
Materials and methods
Microorganisms and medium
The microorganism handled in this research work was Bacillus subtilis SPB1 (HQ392822), which was isolated from a Tunisian soil contaminated by hydrocarbon soil as clarified by Ghribi and Chaabouni [47].The basal medium used for cell growth and biosurfactant production was of the following composition (g/L): yeast extract as organic nitrogen sources (5 g/L), ammonium sulfate as inorganic nitrogen source (an adequate concentration was added to maintain a constant C/N ratio of 7 [47]), K2HPO4 (1.5 g/L), KH2PO4 (0.5 g/L), MgSO4 (0.5 g/L), KCl (0.1 g/L) at pH 7 supplemented with different glucose concentrations.
Inoculum preparation and culture conditions
The inoculums was prepared as follows: one isolated colony was dispensed in LB medium and incubated overnight at 37 °C in a rotatory shaker at 150 rpm until absorbance around 3, measured spectrophotometrically at 600 nm, was reached. The culture was placed, in 500 ml Erlemeyer flasks containing 100 ml of basal medium, for 48 h at 37 °C and 150 rpm. Samples were collected at time-defined intervals and subjugated to analysis for determination of biomass production and changes in biosurfactants concentration. All experiments were conducted in duplicates.
Fermentation procedure
To elucidate the effect of the fermentation process on SPB1biosurfactants production yield, we tried one batch process and three different fed batch systems. For each process, three different fermentation runs were carried out to test conversion efficiency of different glucose amounts into cell biomass and biosurfactant productions. In each fermentation run, 1000 ml Erlenmeyer flasks were inoculated with the calculated initial biomass concentration and allowed to work for almost one minute to disperse bacterial cells. Subsequently, fermentation process was initiated and different glucose concentrations were fed an on hourly basis and over 12 h.
In the conventional batch cultivation, the culture broth was used to inoculate the studied media starting with an initial optical density of 0.15. Three fermentation runs involving respectively 22 g/L, 35 g/L and 47 g/L of glucose were conducted. All fed-batch fermentations were initiated as a batch culture with initial glucose concentrations of 10, 20 and 30 g/L in runs 1, 2 and 3 respectively. Next, substrate was pumped into the medium with three different strategies during the first 12 h of fermentation. During the first process, constant glucose concentrations of 10, 20 and 30 g/L, respectively, were kept in the medium. The second fed-batch fermentation process was undertaken through supplying glucose every 2 h with a constant feed rate for each run. Finally, the third one was fed exponentially.
First fed batch strategy
During this process, successive feeds of glucose concentrations were added at different times to keep a constant glucose concentration (10, 20 or 30 g/L, respectively) in each run. After 12 h, the amount of glucose added in each run was 12, 15 and 17 g/L, respectively (Table 1).
Second fed batch strategy
The second fed-batch fermentation process was carried out through supplying a constant feed rate of glucose concentration. For each run, the feed rate was kept constant for 12 h as depicted in Table 1. For instance, for run 1 we started the culture with 10 g/L, then we added 2 g/L every 2 h to achieve a total concentration of glucose of 22 g/L.
Third fed batch strategy
The possibility of utilizing an exponential fed-batch process for production of biosurfactants by B. subtilis was investigated. Supply of predetermined fermentative substrate (glucose) on an hourly basis was maintained through the use of the equation developed by Amin and Al-Zahrani [53] (Eq. 1):
Where S indicates the hourly added glucose; X0 refers to the initial bacterial biomass (seed cells); µ denotes the specific growth rate and t expresses time.
Using the estimated specific growth rate (0.377 h−1), the initial bacterial biomass (X0) required to convert a certain amount of glucose into biomass within 12 h was determined.
For instance, to specify the initial amount of biomass required to convert 22 g of glucose into bacterial biomass and biosurfactant, the above-equation was used (Eq. 2):
Similarly, the initial biomass amounts for the other fermentation runs were measured. The produced data are plotted in Table 2. Afterwards, the obtained value for initial bacterial cells (X0) was used to calculate the hourly added portions of glucose by substituting “t” for 1 within the first hour, for 2 within the second hour and so on as illustrated in Table 3.
Lipopeptide extraction for production quantification
The lipopeptide was extracted as described by Mnif et al. [54]. The protocol consists of three cycles of acid precipitation-neutralization after elimination of the cell pellet. First, the cell-free supernatants obtained from the SSF and SmF were acidified to pH 2.0 by adding 6 N HCl and incubated overnight at 4 °C for the precipitation of the most BioS products. After that, the BioS were then collected by centrifugation at 10,000 rpm and 4 °C during 10 min and washed twice with acid distilled water (pH = 2) to eliminate any impurities. To determine the production yield, which is expressed as the amount of crude BioS obtained per g of dry substrate, the lipopeptide pellet was desiccated at 105 °C until constant weight and evaluated by gravimetric method [31].
Emulsification activity
To measure the emulsification activity, in the culture supernatant recuperated by centrifugation at 10000 rpm and 4 °C for 20 min to remove bacterial cells, we follow the protocol described by Bouassida et al. [11]. A screw-capped tube containing 0.5 ml of cell-free culture broth, 7.5 ml of Tris-Mg (20 mM Tris HCl (pH 7.0) and 10 mM MgSO4), and 0.1 ml of kerosene were prepared. After a vigorous agitation with a vortex (SCI LOGEX MX-S), the tubes were allowed to rest for 1 h. Emulsification activity (EA) was defined as the optical density at 540 nm measured via a spectrophotometer (UV-1800; SHIMADZU, Japan). For each cultural condition; the presented results correspond to the average of three different measurements of three separate experiments.
Biomass determination
The sample was taken at a regular interval and the number of cells was estimated by counting colony forming units (CFU). Appropriate dilutions of culture samples were placed on solid LB medium and incubated at 37 °C overnight [47]. Assays were carried out in triplicates.
Results and discussion
Effect of fermentation process strategy on Bacillus subtilis SPB1 biomass production
In this section, we analyzed the effect of batch and fed-batch fermentation processes on SPB1 biomass production (Fig. 1). Figure 1 reveals that glucose fostered bacterial growth and multiplication of Bacillus subtilis SPB1 cells. Biomass concentrations of 5.41*107 CFU/ml, 5.72*107 CFU/ml and 6.14*107 CFU/ml were achieved after 48 h in batch culture. Additionally, we inferred that the application of fed batch strategy increased biomass production with the most pronounced effect displayed with the constant feeding rate strategy. In fact, during the later fermentation system, biomass production rose more than threefold compared to the one obtained in batch system when using the same glucose concentrations. It seems that the process of fed-batch fermentation was more efficient in reducing growth inhibition triggered by high substrate concentrations. Indeed, according to Amin and Al-Zahrani [53], the sequentially added carbon source, was most probably assimilated rapidly during each respective hour to form new cells of Bacillus subtilis. Similarly, another study proved that fed-batch fermentation led to the production of 5.71 g/L biomass of Bacillus pumilus 2IR versus 4.15 g/L in batch system [23].
Effect of fermentation process strategy on Bacillus subtilis SPB1 biosurfactant production
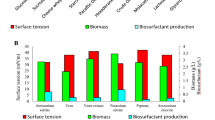
To optimize the fed-batch process for the biosurfactant production by Bacillus subtilis SPB1, different feeding strategies were compared and the results were displayed in Fig. 2. As exhibited in Fig. 2, the increase in glucose concentration entailed an increase in SPB1 biosurfactant production of more than 53%, 13%, 44% and 15% from run 1 to run 3 during the conventional batch, the first, the second and the third fed batch processes, respectively. These results go in good accordance with those reported by Amin and Al-Zahrani [53]. In fact, they emphasized that the highest concentration of biosurfactant produced by Bacillus subtilis was obtained with the fermentation run supplied with the highest amount of carbon source.
We noticed also that biosurfactant production was obviously more significant when using FB fermentation process than when using the conventional batch fermentation system. The exponential feeding method promoted the most important biosurfactant production of 400 mg/L (corresponding to 0.12 when measuring the emulsifying activity at a DO of 540 nm) during run 3. These results go in good agreement with those found by Amin and Al-Zahrani [53]. They asserted that during the exponential fed batch process, surfactin production by Bacillus subtilis increased more than sevenfolds compared to that obtained during conventional batch fermentation. However, Eswari [55] argued that out of 3 fed batch strategies, constant glucose fed batch system supplied the highest rhamnolipid concentration from Pseudomonas aeruginosa.
Effect of fermentation process strategy on Bacillus subtilis SPB1 biosurfactant production yield
It was reported that fed-batch fermentation was considered as highly efficient in terms of maintaining higher biosurfactant production yield [56,57,58,59]. Grounded on statistical calculations, Ghomi et al. [10] also revealed that, fed batch runs were better than batch in terms of rhamnolipid production. This result is depicted in Fig. 3 indicating that the exponential feeding system provided the highest biosurfactant production yield of 15.74 mg/g, which is more than threefold higher than the one obtained in batch process. This was confirmed even with increasing glucose concentration. As shown in Fig. 3, the optimal glucose concentration of 22 g/L gives the best biosurfactant production yield.
As recognized, batch fermentation has been widely used in multiple fermentation systems handling process development such as screening and optimization of medium composition as well as other physical culture conditions. However, the overall batch productivity may be lower referring to its long operating time, taking into account the time for cleaning, reloading and sterilization between batches. Additionally, this mode of fermentation is a closed system, which displays, as a matter of fact, certain shortcomings including the use of high initial substrate concentrations and the accumulation of undesired by-products [23]. It is noteworthy that an inhibitory effect can be accounted for in terms of the catabolism repression triggered by high initial glucose concentration. Glucose is a rapidly metabolizable carbon-energy that grows in the intracellular ATP concentration, yielding the repression of enzyme biosynthesis as well as the slower metabolization of energy source [60]. Amin and Al-Zahrani [53] managed to successfully avoid the catabolic repression which impacts the formation of certain metabolic products occurring at high glucose concentrations. Maldex-15 used as a carbon source in addition to other nutrients were supplied adequately to the growing cells of Bacillus at their specific growth rate maximizing cell growth and surfactin formation, yield and productivity. Therefore, by controlling nutrient supply, the fed-batch fermentation served to prevent or reduce substrate-associated growth inhibition [53].
Basically, cells and products remain in the fermenter until the end of the operation during fed-batch cultivation while one or more nutrients are supplied to the fermenter. When designing a fed-batch fermentation, the most intriguing variable which needs to be defined is the feeding profile. The control-system development for feeding strategy is not straightforward, which is due to: (1) the lack of accurate models describing cell growth and product formation; (2) the nonlinear nature of the bioprocess; (3) the slow process response, and (4) deficiency of reliable on-line sensors. It is to be noted that there are various useful methods which were described to determine and optimize the feeding profile [56].
Microbial surfactants which are secondary metabolites are generated during the stationary phase and usually need a few days for one production cycle in batch fermentation. Extension of production cycle and maintenance of high productivity at a late stage are important to improve biosurfactant production efficiency [7].
Fed-batch operation is one of the most effective methods invested to achieve high cell densities, productivity, and yields of the desired products. Generally, controlling the substrate concentration within an optimal range is the main challenge in the fed-batch fermentation to avoid limiting and inhibiting concentration levels. As a result, the substrate feeding strategy is crucial for successfully obtaining high cell density cultures [61]. Several feeding strategies, such as (a) pH–stat mode, (b) constant feeding rate strategy and (c) DO-stat mode [17], were set forward to improve biosurfactant productivity and yield.
In the current study, batch and three fed-batch fermentation processes by Bacillus subtilis SPB1 were investigated and compared for the effective biosurfactant production. The fed batch process yielded optimal and maximum biosurfactant concentration, which goes in good line with the findings obtained by other researchers. In fact, Fooladi et al. [1] unveiled that the lipopeptide biosurfactant concentration improved by 8.2% from batch to that of fed-batch fermentation of Bacillus pumilus in the 5-L bioreactor. Moreover, Bazsefidpar et al. [56] revealed that the lipopeptide biosurfactant by Aneurinibacillus thermoaerophilus was increased from 4.9 g/L in batch mode to 11.2 g/L in combined feeding strategy, which shows 128% increase in lipopeptide production. Another study identified a significant increase in lipopeptide biosurfactant production in batch (3.74 g/L) versus fed-batch (5.32 g/L) systems by genus Streptomyces [23]. In addition, our results are consistent with the finding reported by He et al. [57] who found that the production of biosurfactant in a fed-batch type fermenter (150 g/L) was better than the one obtained using a batch system under the same conditions. For this reason, they recommended the use of the fed-batch type at the industrial scale thanks to low economic costs. Moreover, a significant improvement (30%) in the lipopeptide-type biosurfactant, surfactin, was observed when employing a fed-batch strategy compared to the batch fermentation of B subtilis DSM 10 T [62]. Similarly, Jin et al. [63] reported the enhancement of iturin A production by a novel two-stage glucose-feeding strategy with a stepwise decrease in feeding rate by B. subtilis 3–10. Using this strategy, an optimum iturin A concentration of about 1.12 g/L was obtained, which was twofold higher than that of batch culture [63].
Conclusion
To conclude, to reduce the cost of production and increase productivity, an outstanding fermentation strategy stands for a crucial factor to be optimized. The knowledge about metabolic pathway can help select the best type of fermentation. In fed-batch fermentation method, the substrate inhibition is controlled. Therefore, developing fed-batch fermentation can effectively enhance the biosurfactant yield as a kinetics model for substrate utilization shows. In addition, in fed batch cultivation, the impact of nutrient concentration on yield and productivity is more powerful than that of batch fermentation process. The type of feeding strategy also depends on the bacterial strains and the desired metabolites.
Availability of data and materials
The data sets supporting the conclusion of this article are included in the article.
References
Fooladi T, Abdeshahian P, Moazami N, Soudi MR, Kadier A, Yusoff WMW, Hamid A (2018) Enhanced biosurfactant production by Bacillus pumilus 2IR in fed-batch fermentation using 5-L bioreactor. Iran J Sci Technol Trans A: Sci 42:1111–1123
Cruz JM, Hughes C, Quilty B, Montagnolli RN, Bidoia E (2018) Agricultural feedstock supplemented with manganese for biosurfactant production by Bacillus subtilis. Waste Biomass Valoriz 9:613–618
Kumar PS, Ngueagni P (2021) A review on new aspects of lipopeptide biosurfactant: types, production, properties and its application in the bioremediation process. J Hazard Mater 407:124827
Naughton P, Marchant R, Naughton V, Banat I (2019) Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J Appl Microbiol 127:12–28
Patel S, Homaei A, Patil S, Daverey A (2019) Microbial biosurfactants for oil spill remediation: pitfalls and potentials. Appl Microbiol Biotechnol 103:27–37
Liu K, Sun Y, Cao M, Wang J, Lu JR, Xu H (2020) Rational design, properties, and applications of biosurfactants: a short review of recent advances. Cur Opin Col Inter Sci 45:57–67
Xu N, Liu S, Xu L, Zhou J, Xin F, Zhang W, Qian X, Li M, Dong W, Jiang M (2020) Enhanced rhamnolipids production using a novel bioreactor system based on integrated foam-control and repeated fed-batch fermentation strategy. Biotechnol Biof 13:1–10
Sharma S, Verma R, Dhull S, Maiti SK, Pandey L (2021) Biodegradation of waste cooking oil and simultaneous production of rhamnolipid biosurfactant by Pseudomonas aeruginosa P7815 in batch and fed-batch bioreactor. Bioprocess Biosys Eng 45(2):309-319
Chenikher S, Guez JS, Coutte F, Pekpe M, Jacques P, Cassar J (2010) Control of the specific growth rate of Bacillus subtilis for the production of biosurfactant lipopeptides in bioreactors with foam overflow. Process Biochem 45:1800–1807
Ghomi AM, Fazaelipoor MH, Jafari SA, Ataei SA (2012) Comparison between batch and fed-batch production of rhamnolipid by Pseudomonas aeruginosa. Iran J Biotechnol 10(4):263–269
Bouassida M, Ghazala I, Ellouze-Chaabouni S, Ghribi D (2018) Improved biosurfactant production by Bacillus subtilis SPB1 mutant obtained by random mutagenesis and its application in enhanced oil recovery in a sand system. J Microbiol Biotechnol 28:95–104
Hu F, Liu Y, Li S (2019) Rational strain improvement for surfactin production: enhancing the yield and generating novel structures. Microbial Cell Fact 18:1–13
Mnif I, Bouallegue A, Mekki S, Ghribi D (2021) Valorization of date juice by the production of lipopeptide biosurfactants by a Bacillus mojavensis BI2 strain: bioprocess optimization by response surface methodology and study of surface activities. Biop and Biosyst Eng 44:2315–2330
Mnif I, Bouallegue A, Bouassida M, Ghribi D (2022) Surface properties and heavy metals chelation of lipopeptides biosurfactants produced from date flour by Bacillus subtilis ZNI5: optimized production for application in bioremediation. Biop Biosyst Eng 45:31–44
Singh P, Patil Y, Rale V (2019) Biosurfactant production: emerging trends and promising strategies. J Appl Microbiol 126:2–13
Fenibo EO, Douglas SI, Stanley H (2019) A review on microbial surfactants: production, classifications, properties and characterization. J Adv Microbiol 18:1–22
Hajfarajollah H, Mokhtarani B, Tohidi A, Bazsefidpar S, Noghabi K (2019) Overproduction of lipopeptide biosurfactant by Aneurinibacillus thermoaerophilus HAK01 in various fed-batch modes under thermophilic conditions. RSC Adv 9:30419–30427
Oh JS, Kim B-G, Park T (2002) Importance of specific growth rate for subtilisin expression in fed-batch cultivation of Bacillus subtilis spoIIG mutant. Enz Microbial Technol 30:747–751
Sivapathasekaran C, Sen R (2013) Performance evaluation of batch and unsteady state fed-batch reactor operations for the production of a marine microbial surfactant. J Chem Technol Biotechnol 88:719–726
Eslami P, Hajfarajollah H, Bazsefidpar S (2020) Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa. RSC Adv 10:34014–34032
Sari C, Hertadi R, Gozan M, Roslan A (2019) Factors Affecting the Production of Biosurfactants and their Applications in Enhanced Oil Recovery (EOR). A Review. IOP Conference Series: Earth and Environmental Science, p. 012048, IOP Publishing.
Kronemberger FA, Borges CP, Freire D (2010) Fed-batch biosurfactant production in a bioreactor. Int Rev Chem Eng 2:513–518
Zambry NS, Rusly NS, Awang MS, Noh NAM, Yahya A (2021) Production of lipopeptide biosurfactant in batch and fed-batch Streptomyces sp. PBD-410L cultures growing on palm oil. Bioprocess Biosys Eng 44(7):1577-1592
Yao S, Zhao S, Lu Z, Gao Y, Lv F, Bie X (2015) Control of agitation and aeration rates in the production of surfactin in foam overflowing fed-batch culture with industrial fermentation. Revista Argent Microbiol 47:344–349
Zhu L, Yang X, Xue C, Chen Y, Qu L, Lu W (2012) Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Biores Technol 117:208–213
Mnif I, Grau-Campistany A, Coronel-León J, Hammami I, Triki MA, Manresa A, Ghribi D (2016) Purification and identification of Bacillus subtilis SPB1 lipopeptide biosurfactant exhibiting antifungal activity against Rhizoctonia bataticola and Rhizoctonia solani. Environ Sci Pol Res 23:6690–6699
Ghribi D, Abdelkefi-Mesrati L, Mnif I, Kammoun R, Ayadi I, Saadaoui I, Maktouf S, Chaabouni-Ellouze S (2012) Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. J Biomed Biotechnol. 2012:373682
Mnif I, Hammami I, Triki MA, Azabou MC, Ellouze-Chaabouni S, Ghribi D (2015) Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani. Environ Sci Pol Res 22:18137–18147
Ghribi D, Elleuch M, Abdelkefi-Mesrati L, Boukadi H, Ellouze-Chaabouni S (2012) Histopathological effects of Bacillus subtilis SPB1 biosurfactant in the midgut of Ephestia kuehniella (Lepidoptera: Pyralidae) and improvement of its insecticidal efficiency. J Plant Dis Protect 119:24–29
Ghribi D, Elleuch M, Abdelkefi L, Ellouze-Chaabouni S (2012) Evaluation of larvicidal potency of Bacillus subtilis SPB1 biosurfactant against Ephestia kuehniella (Lepidoptera: Pyralidae) larvae and influence of abiotic factors on its insecticidal activity. J Stor Prod Res 48:68–72
Mnif I, Elleuch M, Chaabouni SE, Ghribi D (2013) Bacillus subtilis SPB1 biosurfactant: production optimization and insecticidal activity against the carob moth Ectomyelois ceratoniae. Crop Protect 50:66–72
Zouari R, Moalla-Rekik D, Sahnoun Z, Rebai T, Ellouze-Chaabouni S, Ghribi-Aydi D (2016) Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed Pharmacother 84:878–891
Zouari R, Ben Abdallah-Kolsi R, Hamden K, Feki AE, Chaabouni K, Makni-Ayadi F, Sallemi F, Ellouze-Chaabouni S, Ghribi-Aydi D (2015) Assessment of the antidiabetic and antilipidemic properties of bacillus subtilis spb1 biosurfactant in alloxan-induced diabetic rats. Pep Sci 104:764–774
Zouari R, Hamden K, El Feki A, Chaabouni K, Makni-Ayadi F, Sallemi F, Ellouze-Chaabouni S, Ghribi-Aydi D (2017) Evaluation of Bacillus subtilis SPB1 biosurfactant effects on hyperglycemia, angiotensin I-converting enzyme (ACE) activity and kidney function in rats fed on high-fat–high-fructose diet. Arch Physiol Biochem 123:112–120
Zouari R, Hamden K, El Feki A, Chaabouni K, Makni-Ayadi F, Kallel C, Sallemi F, Ellouze-Chaabouni S, Ghribi-Aydi D (2016) Protective and curative effects of Bacillus subtilis SPB1 biosurfactant on high-fat-high-fructose diet induced hyperlipidemia, hypertriglyceridemia and deterioration of liver function in rats. Biomed Pharmacother 84:323–329
Sahnoun R, Mnif I, Fetoui H, Gdoura R, Chaabouni K, Makni-Ayadi F, Kallel C, Ellouze-Chaabouni S, Ghribi D (2014) Evaluation of Bacillus subtilis SPB1 lipopeptide biosurfactant toxicity towards mice. Int J Pept Res Therapeut 20:333–340
Mnif I, Sahnoun R, Ellouze-Chaabouni S, Ghribi D (2014) Evaluation of B. subtilis SPB1 biosurfactants’ potency for diesel-contaminated soil washing: optimization of oil desorption using Taguchi design. Environ Sci Pol Res 21:851–861
Mnif I, Besbes S, Ellouze R, Ellouze-Chaabouni S, Ghribi D (2012) Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Sci Biotechnol 21:1105–1112
Mnif I, Ghribi D (2015) Microbial derived surface active compounds: properties and screening concept. World J Microbiol Biotechnol 31:1001–1020
Mnif I, Fendri R, Ghribi D (2015) Malachite green bioremoval by a newly isolated strain citrobacter sedlakii RI11; enhancement of the treatment by biosurfactant addition. Water Sci Technol 72:1283–1293
Mnif I, Fendri R, Ghribi D (2015) Biosorption of congo red from aqueous solution by Bacillus weihenstephanensis RI12; effect of SPB1 biosurfactant addition on biodecolorization potency. Water Sci Technol 72:865–874
Mnif I, Maktouf S, Fendri R, Kriaa M, Ellouze S, Ghribi D (2016) Improvement of methyl orange dye biotreatment by a novel isolated strain, Aeromonas veronii GRI, by SPB1 biosurfactant addition. Environ Sci Pol Res 23:1742–1754
Zouari R, Besbes S, Ellouze-Chaabouni S, Ghribi-Aydi D (2016) Cookies from composite wheat–sesame peels flours: dough quality and effect of Bacillus subtilis SPB1 biosurfactant addition. Food Chem 194:758–769
Mnif I, Besbes S, Ellouze-Ghorbel R, Ellouze-Chaabouni S, Ghribi D (2013) Improvement of bread dough quality by Bacillus subtilis SPB1 biosurfactant addition: optimized extraction using response surface methodology. J Sci Food Agricul 93:3055–3064
Bouassida M, Fourati N, Ghazala I, Ellouze-Chaabouni S, Ghribi D (2018) Potential application of Bacillus subtilis SPB1 biosurfactants in laundry detergent formulations: compatibility study with detergent ingredients and washing performance. Eng Life Sci 18:70–77
Bouassida M, Fourati N, Krichen F, Zouari R, Ellouz-Chaabouni S, Ghribi D (2017) Potential application of Bacillus subtilis SPB1 lipopeptides in toothpaste formulation. J Adv Res 8:425–433
Ghribi D, Ellouze-Chaabouni S (2011) Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol Res Int. 2011:653654
Mnif I, Ellouze-Chaabouni S, Ghribi D (2013) Economic production of Bacillus subtilis SPB1 biosurfactant using local agro-industrial wastes and its application in enhancing solubility of diesel. J Chem Technol Biotechnol 88:779–787
Zouari R, Ellouze-Chaabouni S, Ghribi-Aydi D (2014) Optimization of Bacillus subtilis SPB1 biosurfactant production under solid-state fermentation using by-products of a traditional olive mill factory. Achiev Life Sci 8:162–169
Zouari R, Ellouze-Chaabouni S, Ghribi D (2015) Use of butter milk and poultry-transforming wastes for enhanced production of bacillus subtilis spb1 biosurfactant in submerged fermentation. J Microbiol Biotechnol Food Sci 4(5):462–466
Ghribi D, Mnif I, Boukedi H, Kammoun R, Ellouze-Chaabouni S (2011) Statistical optimization of low-cost medium for economical production of Bacillus subtilis biosurfactant, a biocontrol agent for the olive moth Prays oleae. Afr J Microbiol Res 5:4927–4936
Mnif I, Chaabouni-Ellouze S, Ghribi D (2012) Optimization of the nutritional parameters for enhanced production of B. subtilis SPB1 biosurfactant in submerged culture using response surface methodology. Biotechnol Res Int.
Amin G, Al-Zahrani O (2014) A novel approach for surfactin production by locally isolated Bacillus subtilis with commercial potentials. International Conference on Advances in Bio-Informatics, Bio-Technology and Environmental Engineering - ABBE
Mnif I, Rajhi H, Bouallegue A, Trabelsi N, Ghribi D (2022) Characterization of lipopeptides biosurfactants produced by a newly isolated strain Bacillus subtilis ZNI5: potential environmental application. J Polym Environ 30:2378–2391
Eswari J (2020) Production of rhamnolipid biosurfactant from fed batch culture by Pseudomonas aeruginosa using multiple substrates. Cur Nutrit Food Sci 16:928–933
Bazsefidpar S, Mokhtarani B, Panahi R, Hajfarajollah H (2019) Overproduction of rhamnolipid by fed-batch cultivation of Pseudomonas aeruginosa in a lab-scale fermenter under tight DO control. Biodegrad 30:59–69
He N, Wu T, Jiang J, Long X, Shao B, Meng Q (2017) Toward high-efficiency production of biosurfactant rhamnolipids using sequential fed-batch fermentation based on a fill-and-draw strategy. Col Surf B: Biointerf 157:317–324
Heyd M, Franzreb M, Berensmeier S (2011) Continuous rhamnolipid production with integrated product removal by foam fractionation and magnetic separation of immobilized Pseudomonas aeruginosa. Biotechnol Progress 27:706–716
Chen S-Y, Wei Y-H, Chang J (2007) Repeated pH-stat fed-batch fermentation for rhamnolipid production with indigenous Pseudomonas aeruginosa S2. Appl Microbiol Biotechnol 76:67–74
Qu L, Ren L-J, Sun G-N, Ji X-J, Nie Z-K, Huang H (2013) Batch, fed-batch and repeated fed-batch fermentation processes of the marine thraustochytrid Schizochytrium sp. for producing docosahexaenoic acid. Biopr Biosyst Eng 36:1905–1912
Mozumder MSI, De Wever H, Volcke EI, Garcia-Gonzalez L (2014) A robust fed-batch feeding strategy independent of the carbon source for optimal polyhydroxybutyrate production. Process Biochem 49:365–373
Willenbacher J, Yeremchuk W, Mohr T, Syldatk C, Hausmann R (2015) Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 5:1–9
Jin H, Li K, Niu Y, Guo M, Hu C, Chen S, Huang F (2015) Continuous enhancement of iturin a production by Bacillus subtilis with a stepwise two-stage glucose feeding strategy. BMC Biotechnol 15:53
Acknowledgements
This work was supported by grants from the Tunisian Ministry of Higher Education, Scientific Research and Technology. It is part of a research project on Biosurfactant Production, Characterization and Application.
Funding
Funding for this research work was granted by the Ministry of Higher Education and Research of Tunisia.
Author information
Authors and Affiliations
Contributions
All authors directly participated in the planning, execution, or analysis of this study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors read the final manuscript and approved its submission to Bioprocess and Biosystems Engineering.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouassida, M., Mnif, I. & Ghribi, D. Enhanced biosurfactant production by Bacillus subtilis SPB1 using developed fed-batch fermentation: effects of glucose levels and feeding systems. Bioprocess Biosyst Eng 46, 555–563 (2023). https://doi.org/10.1007/s00449-022-02839-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02839-0