Abstract
On the basis of determining isoelectric point of algae residue protein obtained from Scenedesmus dimorphus, this study investigated the effects of pH values, ratio of liquid to solid, extraction temperature and time on protein extraction rates, and assessed the nutritional value of protein extracted from microalgae residues. The results from orthogonal experiments revealed the optimum conditions for extracting proteins from algal residues (pH: 12; liquid-to-solid ratio: 40 mL/g; extraction temperature: 45 ℃; extraction time: 140 min). It was observed that under the optimal conditions, the protein extraction rate was 40.13%. Essential amino acids account for 44.3% of the proteins obtained from algal residues. The content of anti-nutritional factors in algal residues was significantly reduced, and the digestibility of algal residue proteins was higher than the digestibility of algal powder. This indicated that algal residues could be an ideal source of proteins for humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are classified as photosynthetic microorganisms, and these are characterized by high photon-to-biomass conversion efficiency. They also exhibit strong environmental adaptability and can utilize light energy to efficiently fix carbon dioxide and synthesize organic substances such as protein, polysaccharides, lipids, and pigments [1]. These properties increase the application prospects of microalgae, and they find their applications in various industries such as food, medicine, and fine chemicals [2,3,4,5]. Researchers have focused on identifying alternate energy sources as the content of crude oil, coal, and other fossil fuels are continuously decreasing over the years. In this regard, microalgae have attracted immense attention as they can be potentially used as raw materials in the field of biodiesel production [6, 7]. Microalgae contain a large amount of unsaturated fatty acids in their lipids as they fall under the category of submerged plants that grow under anoxic conditions, and these are important sources of edible oil [8]. Unlike the traditional sources of vegetable oils (such as maize and soy crops), microalgae can be cultivated using seawater, alkaline water, or semi-alkaline water, in saline-alkali land or mudflats. Moreover, they do not compete with food crops for arable land and freshwater [9]. Therefore, the mass cultivation of microalgae is an effective way to obtain biological resources that can help reduce carbon emissions.
The growth of the algae-based industry can be attributed to the abundance of microalgal residues present in nature. Algal residues are generated during the process of oil extraction, and these account for 70% of the microalgal biomass. The primary constituents of the algal residues include protein, carbohydrates, and oils [10]. The high protein content (> 50%) has given microalgae residues great application potential in the development of protein-based foods. Whole-cell proteins, protein concentrates, protein isolates, protein hydrolysates, and bioactive peptides can be obtained from microalgal proteins by tuning the processing method [11, 12]. Microalgae-based proteins can also provide interesting technological functionalities for the food industry. It is noteworthy that the emulsifying ability and stability of proteins extracted from Chlorella vulgaris are comparable to the emulsifying ability and stability of commercial emulsifiers [13]. The full utilization of microalgal residues that are rich in nutrients can potentially help reduce the wastage of resources while minimizing the production cost of algae-based biodiesel or edible oils [14, 15]. To the best of our knowledge, there are no reports on the high-value development and commercial application of algal residues.
Currently, various techniques, such as the alkali-extraction and acid-precipitation [16], enzyme decomposition [17], reverse micellar extraction [18], salt-soluble protein extraction [19], ultrasonic protein dissolution promotion [20], organic solvent extraction, and membrane-based separation [21, 22], are used to extract proteins from plant tissues. The method of alkali-extraction and acid-precipitation is the most widely used protein extraction method. Though the applicability of this method is limited by the long extraction time and the consumption of a large amount of energy, this method is easy to operate and control. The method is cost-effective, and high extraction efficiency can be achieved. Thus, this method is suitable for mass production of edible oil. This method remains the mainstream industrial method for the extraction of proteins from vegetation [23]. Protein extraction under conditions of alkaline solubilization and acid-precipitation proceeds via the breakage of hydrogen bonds present between protein molecules under highly alkaline conditions. This increases the surface charge of protein molecules, resulting in an increase in the solubility of proteins. When the pH of the protein solubilizing solution is adjusted to the isoelectric point of the protein, the protein molecules aggregate and precipitate as the surface hydration and electric double layers are damaged under these conditions. This method has been used to separate high-quality protein from tea leaves, seeds of kiwi fruits, rice bran, sporophore of Coprinus comatus, and other plant parts [24,25,26,27].
Scenedesmus dimorphus is an important oil-producing microalgal species, the lipid content in which is ≥ 50% under certain inducing culture conditions. The lipid content in this species is significantly higher than the lipid content of most of the commercialized species of algae [28, 29]. It is known that S. dimorphus is also rich in proteins. We extracted lipids from S. dimorphus using a mixed solvent system consisting of ethanol and n-hexane, as high oil extraction rates and high retention rates for proteins and total sugar can be achieved post oil extraction using this solvent system [30, 31]. The composition of the algal residues obtained post oil extraction was analyzed, and the algal residue protein was prepared following the method of alkali-extraction and acid-precipitation. We also investigated the factors influencing the protein extraction rate, optimized the process conditions, and assessed the nutritional value of the extracted proteins. The results reported herein can potentially provide a reference for the high-value utilization of microalgal residues.
Materials and methods
Algal species culture
The species of Scenedesmus dimorphus was obtained from Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences. And it was cultured in the homemade 300 L airlift photobioreactor using BG11 medium [32] in the laboratory under the following conditions: temperature of 25 °C, light intensity of 100 μmmol/(m2·s), 24 h continuous light, gas content of 0.1 vvm, and 2% CO2 volume ratio in the gas [30].
Preparation and analyzing the fractions of algal residues
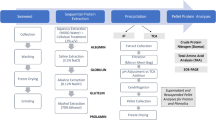
The solution of algae cultured for 7 d was collected and centrifuged at 5000 r/min for 10 min. The microalgal biomass obtained after removing the liquid supernatant was washed with distilled water, following which it was freeze-dried to obtain the algal powder. The powder was wall-broken under ultrasonic conditions using a mixed solvent system (ethanol:n-hexane = 3:2, v/v). The process of extraction was continued for 4 h, following which the extract was centrifuged at 5000 r/min for 5 min, and the organic solvent was removed. Subsequently, the precipitate was dried in an oven at 60 °C to obtain the algal residues [30].
Technical flow process involved with the extraction of algal residue proteins
The process of protein extraction from algal residues has been outlined in this section. First, the algal residues were isolated based on the liquid-to-solid ratio, and the isolated extracts were leached using an alkaline solution. Next, the residues were centrifuged at 5000 r/min for 5 min to obtain the liquid supernatant, and the pH of the supernatant was adjusted to the isoelectric point of the protein using dilute sulfuric acid. The process of precipitation was allowed to proceed for 1 h. Following this, the supernatant was centrifuged at 5000 r/min for 5 min and subsequently rinsed and precipitated twice using steamed water. The sample was freeze-dried to obtain the algal residue protein. The protein extraction process is illustrated in Fig. 1, and the protein extraction rate (%) is calculated using Eq. (1) as follows:
Determination of the isoelectric point of the algal residue protein
The supernatant obtained following the extraction of the microalgal residue protein was divided into several portions. The pH of the isolated portions was adjusted in the range of 3.6–5.0. The supernatant was allowed to set for 1 h, following which it was centrifuged at 5000 r/min for 5 min. The protein contents in the supernatant (before and after acid deposition) were determined to calculate the protein residual rate. The pH value corresponding to the minimum protein residual rate is the isoelectric point of the algal residue protein. The protein residual rate (%) is calculated as follows:
Assessing the nutritional value of the algal residue protein
The composition and the content of the amino acids in algal residue protein were determined using an automatic amino acid analyzer. According to the standard amino acid scoring pattern and the amino acid pattern of the whole egg protein [33, 34], the amino acid score (SA) and essential amino acid index (IEA) were calculated as follows:
where P1, P2, P3, …and Pn denote the amino acid contents in the algal residues (%), P1E, P2E, P3E, … and PnE denote the contents of the essential amino acids in the whole egg protein (%), and n denotes the number of essential amino acid varieties analyzed.
Literature reports were analyzed, and the chemical scores (SC) of the amino acids were calculated as follows:
where MAX denotes the content of a certain essential amino acid in the algal residue protein, MAe denotes the total content of the essential amino acids in the microalgal residue protein, MEX denotes the essential amino acid in the standard egg white, and MEe denotes the total amount of the essential amino acid in the standard egg white. The closer the SC value is to 100, the closer the composition of the amino acid is to the composition of the standard protein, and the higher the nutritional value of the protein [35].
Biological value (SB) and nutrition index (IN) were calculated as follows [36]:
where IEA denotes the essential amino acid index, and Pp denotes the protein content (%).
The ratio coefficient (RC) and the score of ratio coefficient (SRC) were calculated as follows [37]:
where R = MAX/MAs denotes the amino acid ratio, MAs denotes the amino acid content based on the FAO/WHO amino acid scoring pattern, \(\mathop R\limits^-\) denotes the mean of R, and Re denotes the relative standard deviation of RC.
Analysis of the anti-nutritional factors
The method reported by Rabaina et al. was followed to determine the trypsin inhibitor activity [38]. Algal powder (50 mg; or algal residue) was fully suspended in a solution of NaOH (5 mL; 0.01 mol/L) to determine the inhibitor activity. The pH was adjusted to 9.5 with HCl solution, and the sample was sonicated in an ice bath at 600 W for 10 min. The sample was allowed to stand overnight at 4 °C, following which it was centrifuged at 8000 r/min for 20 min. The supernatant was used for analysis. Trypsin (2 mL) and water (1 mL) were added to 1 mL of the supernatant. The obtained mixture was preheated in a water bath at 37 ℃. The BAPA substrate (5 mL) was preheated to 37 ℃, and the reaction was terminated after 10 min by adding 1 mL of 30% glacial acetic. The sample was centrifuged at 4000 r/min for 3 min to obtain the supernatant, and the absorbance was recorded at 410 nm. The activity of the trypsin inhibitor (TIM, TUI/g) was calculated according as follows:
where AN is the absorbance of the control, AT is the absorbance of the test group, V is the total volume of the reaction system (mL), t is the reaction time (min), and m is the mass of the algal powder (or algal residue) (g).
The tannin content in the algal powder was determined following the Folin–Denis method [39]. Algal powder (or algal residue; 100 mg) was mixed with water (4 mL), and the mixture was sonicated for 10 min. Following this, the mixture was heated for 30 min using a boiling water bath. The volume was fixed to 5 mL after cooling, and the sample was centrifuged at 8000 r/min for 5 min. The supernatant (0.5 mL) was isolated, and water (10 mL) was added. The Folin–Denis reagent (1.5 mL) was added to the mixture, and the mixture was allowed to stand for 1 min. Following this, a solution of saturated sodium carbonate (2.0 mL) was added to the mixture, and the volume of the solution was made up to 50 mL using water. The reaction was allowed to proceed for 1.5 h at 55 °C, and the absorbance was measured at 760 nm. The tannin content was calculated by analyzing the standard curve, which was generated using different concentrations of tannic acid solutions.
Results
Nutritional composition of algal residues
After the oil extraction of S. dimorphus residues using ethanol/n-hexane mixed solvent, the nutrition composition of algal residues was determined. The protein content, the carbohydrates, the lipid content, the pigment content and the ash content in the microalgae residues were 41.05%, 25.17%, 4.41%, 0.78% and 9.60%, respectively. In the algae residues, calcium, potassium and sulfur were the most dominant mineral elements, with their contents at 13.49 ‰, 5.71 ‰ and 5.49 ‰, respectively, while other mineral elements are small in proportion, which was consistent with the findings of Yang et al. on the composition of algal residues after oil extraction [40].
Optimization of the algal residue protein extraction process
When the algal residue protein is extracted following the alkali-extraction and acid-precipitation method, the maximum extent of protein precipitation in the extracting solution can be realized by adjusting the pH of the alkali-extracting solution to the isoelectric point of the protein. Analysis of Fig. 2 reveals that the extraction rates of the residual proteins were influenced by the pH of the solution. The lowest protein residual rate was obtained, and the maximum extent of protein precipitation was achieved at a pH value of 4.4. Therefore, it can be concluded that the isoelectric point of the protein obtained from the residues of S. dimorphus (obtained post oil extraction) is attained when the pH is approximately 4.4.
Algal residues (1 g) were used to conduct leaching experiments at 40 °C. The samples were leached for 100 min, and the liquid-to-solid ratio was set at 20 mL/g. The pH of the extracting solution was adjusted in the range of 8–13 using a solution of NaOH. The protein extraction rate was determined, and the results are presented in Fig. 3A. The pH of the extracting solution significantly affected the protein extraction rates. It was observed that the protein extraction rates increased with an increase in the pH values. The most significant change in the protein extraction rate (an increase from 7.1 to 23.8%) was observed when the pH values varied between 10 and 12. A plateau was reached when the pH value exceeded 12. It is noteworthy that when the pH of the solution was 12.5, the extracting solution had a slight odor which intensified as the pH increased. It can be inferred that the optimal pH value for protein extraction under the protein extraction conditions mentioned previously is 12.
The algal residues were leached using the extracting solution at a pH of 12, and the extraction process was allowed to proceed for 100 min at 40 °C. The liquid-to-solid ratio was adjusted in the range of 10–55 mL/g to investigate the effect of the ratio on the protein extraction rate. The protein extraction rate increased gradually when the liquid-to-solid ratio increased from 10 to 35 mL/g, and the rate decreased gradually when the liquid-to-solid ratio was greater than 35 mL/g (Fig. 3B). Therefore, a liquid/solid ratio of 35 mL/g is considered to be the optimal ratio to conduct the extraction process under the protein extraction conditions mentioned previously.
When the algae residues were leached with a pH value of 12 and a liquid/solid ratio of 35 mL/g for 100 min, the extraction temperature was adjusted to 30 °C ~ 55 °C to investigate its effects on protein extraction rate. As shown in Fig. 3C, the protein extraction rate increased with an increase in the temperature within a certain range. The maximum extraction rate of 38.38% was achieved when the extraction temperature was 45 °C. Beyond this temperature, the rate of extraction decreased slightly, and the decrease could be potentially attributed to the denaturation of proteins occurring under conditions of extremely high temperatures [34]. Therefore, it could be inferred that the optimal extraction temperature was 45 °C when the other conditions were kept fixed (as described previously).
Following this, the extraction time was adjusted in the range of 40–180 min to investigate the effects of time on the protein extraction rate. The algal residues were leached using the extracting solution (pH: 12). The liquid/solid ratio was maintained at 35 mL/g, and the process was conducted at 45 °C. As shown in Fig. 3D, the extraction rate increased gradually with an increase in the extraction time. It was observed that the rate of increase decreased when the extraction time exceeded 140 min. The protein extraction rate increased from 23.1 to 23.7% when the extraction time increased from 140 to 180 min. Thus, a significant increase in the extraction rate could not be achieved under these conditions. Therefore, it can be inferred that the optimal extraction time was 140 min.
Based on the results of the single-factor experiments, a three-level orthogonal array experiment was conducted to study the four factors (pH value of the extracting solution, liquid/solid ratio, extraction temperature, and extraction time) affecting the extraction rate of the algal residue protein. The results are presented in Table 1. The factors influencing the extraction rate are labeled A, B, C, and D. It was observed that the pH value of the extracting solution exerted the maximum influence. The trend in the degree of influence has been presented: pH of the extracting solution > liquid/solid ratio > extraction temperature > extraction time. The results obtained by conducting the three-level orthogonal array experiment reveal that the optimal combination of experimental factors is A3B3C2D2 (pH value: 12; liquid/solid ratio: 40 mL/g; extraction temperature: 45 ℃; extraction time: 140 min). The algal proteins were extracted multiple times under these experimental conditions, and the average protein extraction rate was calculated to be 40.13%. The results reveal the feasibility of conducting the studies under the optimized process conditions.
Nutritional assessment of algal residue protein
As shown in Table 2, 18 amino acids, including 8 essential amino acids, were detected in the protein extracted from the residues of S. dimorphus. The amino acid and chemical scores were analyzed to assess the quality of the extracted protein. The closer the scores are to 100, the closer the composition of the protein is to the composition of the standard protein in terms of the essential amino acids and the higher the nutritional value. The SA and SC values recorded for the protein obtained from the S. dimorphus residues are presented in Table 3. The results indicate that the sulfur-containing amino acids, methionine and cystine, are the first limiting amino acids present in the algal residue protein. The biological value (SB), nutrition index (IN), and the score corresponding to the ratio coefficient of the amino acid (SRC) were 77.94, 35.84, and 74.21, respectively.
The trypsin inhibitor activity was recorded to be 255 TUI/g for the algal powder. The value was recorded before oil extraction. The trypsin inhibitor activity decreased to 70 TUI/g post oil extraction. The oil extraction process was conducted using the ethanol/n-hexane mixed solvent system. The value was only 27.4% of the value recorded for the original algal powder sample. The content of tannin, another anti-nutritional factor presents in microalgae, changed significantly before and after oil extraction (from 1.95 to 0.88%). The retention rate was as low as 45% (Fig. 4).
Discussion
Protein extraction from algal residue
The algal residues obtained from S. dimorphus post oil extraction (using the ethanol/n-hexane mixed solvent system), were still rich in proteins and carbohydrates. The protein content in algal residues was higher than the protein content in other vegetations (Table 4). This indicates that algal proteins can be potentially used to develop protein-based foods. After determining the isoelectric point of the protein, we conducted a single-factor experiment to optimize the extraction process of algal residue proteins. The experimental results revealed that proteins could not be efficiently extracted and separated under extreme experimental conditions. Deamination, decarboxylation, and breakage of peptide bonds were observed when the level of alkalinity of the extracting solution was significantly high. This resulted in the transformation of amino acids into other compounds (toxic and non-toxic), and reduced the degree of edibility of the protein [41]. Although a high liquid-to-solid ratio promotes the process of protein extraction, a significantly high liquid-to-solid ratio can strengthen the hydration layer present in the protein molecules. This results in insufficient protein precipitation during the alkali-extraction and acid-precipitation processes [41].
The alkali-extraction and acid-precipitation processes are followed to extract soluble proteins from raw materials. The algal residues are dissolved in an alkaline solution, following which the pH of the solution is adjusted with sulfuric acid to reach the isoelectric point. The precipitated protein is cleaned, sterilized, and spray-dried for developing protein-based food items [16, 23]. The optimized process conditions were obtained by conducting orthogonal experiments, and the protein extraction rate recorded under these conditions was 40.13%. This rate was higher than the rates recorded when Sporophore, Coprinus comatus, hemp seed, or purple perilla were used as the raw materials [43,44,45]. It was also observed that the rate was comparable to the protein extraction rate recorded when walnut was used as the raw material (43.15%) [46]. The rate was lower than the rates recorded when peanut meals were used as raw materials under high-temperature conditions (64.2%) [47]. It was observed that the efficiency of the protein extraction process varied significantly when different raw materials were used. The difference in efficiency can be attributed to the differences in the nature of the raw materials or the differences in the pre-treatment processes. It has been observed that homogenization-based, ultrasound-based, and cellulase-based pre-treatment methods (used for extracting proteins from peanut meals under high-temperature conditions) help improve the protein extraction rate. Thus, it can be inferred that the protein content in algal residues is higher than the protein content in other vegetations used as raw materials. Hence, algal residues can be developed into protein-based food items.
Nutritional assessment
The nutritional value of proteins depends on the types, quantity, and proportion of essential amino acids present in them. The ratio of essential amino acids to total amino acids (EAA/TAA ratio), the ratio of essential amino acids to non-essential amino acids (EAA/NEAA ratio), and the essential amino acid index (IEA) of algal residue protein are higher than those recorded for Chlorella pyrenoidosa, whey protein concentrate, and soy protein isolate cultured under heterotrophic conditions (Table 5). The values were higher than the reference values provided by WHO/FAO [48, 49]. This indicates that the nutritional value of the S. dimorphus residues subjected to oil extraction conditions remains high. Sulfur-containing amino acids are the limiting amino acids in algal residue proteins. It is noteworthy that the nutritional value of the protein powder obtained from algal residues can be improved by compounding algal residue proteins and food products rich in sulfur-containing amino acids (such as corn gluten meals rich in methionine or other animal proteins) with food items.
Anti-nutritional factors are substances that can adversely affect the processes of digestion, absorption, and utilization of nutrients. The typical anti-nutritional factors in food ingredients include trypsin inhibitors and tannins [54]. Trypsin inhibitors and tannins, the two anti-nutritional factors present in S. dimorphus [55], were reduced following the process of oil extraction (using organic solvents). The reduction of anti-nutritional factors under these conditions can be attributed to two factors. Trypsin inhibitors are protein-based substances, the structure of which changes in the presence of organic solvents. This results in a decrease in their activity. Some of these anti-nutritional factors can be solubilized by organic solvents, which also results in a decrease in activity. The results reported herein confirm that the digestibility of algal residue proteins is better than the digestibility of raw algal powder.
The results reveal that the S. dimorphus residue subjected to conditions of oil extraction is protein rich. However, the content of anti-nutritional factors is low in these residues. The algal residue protein extracted following the alkali-extraction and acid-precipitation method is rich in various types of amino acids. The extracted protein is found to be nutritionally balanced. Thus, it can be an ideal protein source for humans. Algal residue proteins present broad application prospects. The development of algal residue-based processed and refined food can help in the proper utilization of microalgae biomass. This, in turn, can help minimize the wastage of protein sources. The production costs of algae-based biodiesel or edible oil can also be reduced.
Conclusion
After oil extraction using the ethanol/n-hexane (3:2, v/v) mixed solvent, it was determined that the protein content and the polysaccharide content in the S. dimorphus residues were 41.05% and 25.17%, respectively. Single factor experiments and orthogonal array experiments were conducted, and the results revealed that the protein extraction rate achieved under the optimal extraction conditions (pH of the extracting solution: 12; liquid/solid ratio: 40 mL/g; extraction temperature: 45 °C; Extraction time: 140 min) was 40.13%. Essential amino acids account for 44.3% of the total amino acids in the extracted protein. The essential amino acid index (IEA) was higher than the reference value provided by WHO/FAO. Following the process of oil extraction, the trypsin inhibitor activity recorded for the algal residue decreased to 70 TUI/g, and the tannin retention rate recorded for the algal powder was as low as 45%. The results reported herein reveal that the protein extracted from the residues of S. dimorphus can be potentially used as an ideal protein source for human beings.
Availability of data and materials
The datasets used and analyzed for the current study are available from the corresponding author upon reasonable request.
References
Hu R, Cao Y, Chen X, Zhan J, Luo G, Hao Ngo H, Zhang S (2022) Progress on microalgae biomass production from wastewater phycoremediation: metabolic mechanism, response behavior, improvement strategy and principle. Chem Eng J. https://doi.org/10.1016/j.cej.2022.137187
Bature A, Melville L, Rahman KM, Aulak P (2022) Microalgae as feed ingredients and a potential source of competitive advantage in livestock production: a review. Livest Sci 259:104907
Kumar R, Hegde AS, Sharma K, Parmar P, Srivatsan V (2022) Microalgae as a sustainable source of edible proteins and bioactive peptides—current trends and future prospects. Food Res Int 157:111338
Bhattacharya T, Rather GA, Akter R, Kabir MT, Rauf A, Rahman MH (2021) Nutraceuticals and bio-inspired materials from microalgae and their future perspectives. Curr Top Med Chem 21:1037–1051
Madadi R, Maljaee H, Serafim LS, Ventura SPM (2021) Microalgae as contributors to produce biopolymers. Mar Drugs 19:8
Muhammad G, Alam MA, Mofijur M, Jahirul MI, Lv YK, Xiong WL, Ong HC, Xu JL (2021) Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew Sust Energ Rev. https://doi.org/10.1016/j.rser.2020.110209
G. F. Mota, I. G. de Sousa, A. L. B. de Oliveira, A. L. G. Cavalcante, K. D. Moreira, F. T. T. Cavalcante, J. E. D. Souza, I. R. D. Falcao, T. G. Rocha, R. B. R. Valerio, S. C. F. de Carvalho, F. S. Neto, J. D. Serpa, R. K. C. de Lima, M. C. M. de Souza and J. C. S. dos Santos (2022) Biodiesel production from microalgae using lipase-based catalysts: Current challenges and prospects. Algal Res 62.
Fawcett CA, Senhorinho GNA, Laamanen CA, Scott JA (2022) Microalgae as an alternative to oil crops for edible oils and animal feed. Algal Res 64:102663
Kong W, Shen B, Lyu H, Kong J, Ma J, Wang Z, Feng S (2021) Review on carbon dioxide fixation coupled with nutrients removal from wastewater by microalgae. J Clean Prod 292:125975
Ehimen EA, Sun ZF, Carrington CG, Birch EJ, Eaton-Rye JJ (2011) Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Appl Energy 88:3454–3463
Soto-Sierra L, Stoykova P, Nikolov ZL (2018) Extraction and fractionation of microalgae-based protein products. Algal Res 36:175–192
Chen C, Tang T, Shi Q, Zhou Z, Fan J (2022) The potential and challenge of microalgae as promising future food sources. Trends Food Sci Technol 126:99–112
Ursu A-V, Marcati A, Sayd T, Sante-Lhoutellier V, Djelveh G, Michaud P (2014) Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Biores Technol 157:134–139
Huang ZG, Zhang J, Pan MM, Hao YH, Hu RC, Xiao WB, Li G, Lyu T (2022) Valorisation of microalgae residues after lipid extraction: pyrolysis characteristics for biofuel production. Biochem Eng J 179:108330
Torres A, Padrino S, Brito A, Diaz L (2021) Biogas production from anaerobic digestion of solid microalgae residues generated on different processes of microalgae-to-biofuel production. Biomass Convers Bior. https://doi.org/10.1007/s13399-021-01898-9
Wu D, Wu C, Ma W, Wang Z, Yu C, Du M (2019) Effects of ultrasound treatment on the physicochemical and emulsifying properties of proteins from scallops (Chlamys farreri). Food Hydrocolloids 89:707–714
Cheng Y, Liu Y, Wu J, Ofori Donkor P, Li T, Ma H (2017) Improving the enzymolysis efficiency of potato protein by simultaneous dual-frequency energy-gathered ultrasound pretreatment: thermodynamics and kinetics. Ultrason Sonochem 37:351–359
Leser ME, Marcozzi G, Caselli M, Luisi PL (1992) Protein extraction by reverse micelles. In: Sekine T (ed) Process metallurgy. Elsevier, Amsterdam
Niu L, Yuan H, Gong F, Wu X, Wang W (2018) Protein extraction methods shape much of the extracted proteomes. Front Plant Sci 9:802
Esclapez MD, García-Pérez JV, Mulet A, Cárcel JA (2011) Ultrasound-assisted extraction of natural products. Food Eng Rev 3:108
Verostek MF, Lubowski C, Trimble RB (2000) Selective organic precipitation/extraction of released n-glycans following large-scale enzymatic deglycosylation of glycoproteins. Anal Biochem 278:111–122
Saxena A, Tripathi BP, Kumar M, Shahi VK (2009) Membrane-based techniques for the separation and purification of proteins: an overview. Adv Coll Interface Sci 145:1–22
Contreras MdM, Lama-Muñoz A, Manuel Gutiérrez-Pérez J, Espínola F, Moya M, Castro E (2019) Protein extraction from agri-food residues for integration in biorefinery: potential techniques and current status. Biores Technol 280:459–477
Deng H, Sun Z, Cao Y, Fan Z, Cheng N-C, Wang Z, Gao Z, Yang H (2014) Extraction optimization and functional properties of proteins from kiwi fruit(Actinidia chinensis planch) seeds. International Journal of Food Properties 17:7
Zhang C, Bozileva E, van der Klis F, Dong Y, Sanders JPM, Bruins ME (2016) Integration of galacturonic acid extraction with alkaline protein extraction from green tea leaf residue. Ind Crops Prod 89:95–102
Wang C, Li D, Xu F, Hao T, Zhang M (2014) Comparison of two methods for the extraction of fractionated rice bran protein. J Chem 2014:546345
Wang H, Ng TB (2002) Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochemistry 61:1–6
Yang LB, Ren L, Tan XB, Chu HQ, Chen JB, Zhang YL, Zhou XF (2020) Removal of ofloxacin with biofuel production by oleaginous microalgae Scenedesmus obliquus. Biores Technol 315:123738
Jiang Y, Zhang W, Wang J, Chen Y, Shen S, Liu T (2013) Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Biores Technol 128:359–364
Li C, Xin M, Sun Z (2021) Selection of extraction solvents for edible oils from microalgae and improvement of the oxidative stability. J Biosci Bioeng 132:365–371
Huang Y, Zhang D, Xue S, Wang M, Cong W (2016) The potential of microalgae lipids for edible oil production. Appl Biochem Biotechnol 180:438–451
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Marinangeli CPF, House JD (2017) Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr Rev 75:658–667
Schaafsma G (2000) The protein digestibility-corrected amino acid score. J Nutr 130:1865S-1867S
Bell SJ, Bistrian BR, Ainsley BM, Manji N, Lewis EJ, Joyce C, Blackburn GL (1991) A chemical score to evaluate the protein quality of commercial parenteral and enteral formulas: emphasis on formulas for patients with liver failure. J Am Diet Assoc 91:586–589
Moore D, Soeters P (2015) The biological value of protein. Nestle Nutr Inst Workshop Ser 82:39–51
Hu X, Ma J, Qian W, Cao Y, Zhang Y, Liu B, Tang L, Cao W, Zhu Y, Liu L (2022) Effects of low temperature on the amino acid composition of wheat grains. Agronomy 12:1171
Robaina L, Izquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D, Fernández-Palacios H (1995) Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture 130:219–233
Xiao J, Zhang H, Niu L, Wang X, Lu X (2011) Evaluation of detoxification methods on toxic and antinutritional composition and nutritional quality of proteins in jatropha curcas meal. J Agric Food Chem 59:4040–4044
Yang Z, Guo R, Xu X, Fan X, Luo S (2011) Hydrogen and methane production from lipid-extracted microalgal biomass residues. Int J Hydrogen Energy 36:3465–3470
Jarpa-Parra M, Bamdad F, Wang Y, Tian Z, Temelli F, Han J, Chen L (2014) Optimization of lentil protein extraction and the influence of process pH on protein structure and functionality. LWT Food Sci Technol 57:461–469
Wu Q, Liu J, Chen J, Zeng X (2005) Analysis of the nutritional composition of Coprinus comaus. Sci Technol Food Ind 26:77–82
Hadnađev M, Dapčević-Hadnađev T, Lazaridou A, Moschakis T, Michaelidou AM, Popović S, Biliaderis CG (2018) Hempseed meal protein isolates prepared by different isolation techniques Part I physicochemical properties. Food Hydrocoll 79:526–533
Song N, Lee JH, Song KB (2015) Preparation of perilla seed meal protein composite films containing various essential oils and their application in sausage packaging. J Korean Soc Appl Biol Chem 58:83–90
Zhao C, Sun J, Cheng Y, Meng T, Yan Y, Ye X, Chen J (2012) Optimization of extraction process and studies on functional properties of fruit bodies protein of Coprinus Comatus. J Chin Inst Food Sci Technol 12:9–12
Hu H, Fan T, Zhao X, Zhang X, Sun Y, Liu H (2017) Influence of pH and salt concentration on functional properties of walnut protein from different extraction methods. J Food Sci Technol 54:2833–2841
Reddy N, Chen L, Yang Y (2013) Thermoplastic films from peanut proteins extracted from peanut meal. Ind Crops Prod 43:159–164
Zhu S, Wu K (1988) Nutritional evaluation of protein-Ratio coefficient of amino acid. Acta Nutrimenta Sinica 10:23–30
FAO (1991) Protein quality evaluation. Joint FAO/WHO. FAO Food Nutr Pap 51:1–66
Ursu A, Marcati A, Michaud P(2013) Extraction, characterization and technofunctional properties of proteins from Chlorella vulgaris.
Zhang R, Chen J, Zhang X (2018) Extraction of intracellular protein from Chlorella pyrenoidosa using a combination of ethanol soaking, enzyme digest, ultrasonication and homogenization techniques. Bioresour Technol 247:267–272
Banjare IS, Gandhi K, Sao K, Sharma R (2019) Spray-dried whey protein concentrate-iron complex: preparation and physicochemical characterization. Food Technol Biotechnol 57:331–340
Preece KE, Hooshyar N, Zuidam NJ (2017) Whole soybean protein extraction processes: a review. Innov Food Sci Emerg Technol 43:163–172
Sarwar Gilani G, Wu X, Cockell KA (2012) Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr 108:S315–S332
Batista S, Pintado M, Marques A, Abreu H, Silva JL, Jessen F, Tulli F, Valente LP (2020) Use of technological processing of seaweed and microalgae as strategy to improve their apparent digestibility coefficients in European seabass (Dicentrarchus labrax) juveniles. J Appl Phycol 32:3429–3446
Funding
This research was funded by the National Nature Science Foundation grant number 32102819.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.Q.; methodology, S.Z.; validation, S.L., L.Y. and C.Q.; formal analysis, S.Z.; resources, S.L.; data curation, C.Q.; writing—original draft preparation, S.Z.; writing—review and editing, C.Q.; visualization, S.L.; supervision, C.Q.; project administration, C.Q.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
This manuscript does not involve any human participants, human data, human tissue, individual person’s data or animal experiment.
Consent to publish
All authors have read and approved the final version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z., Chi, Q., Sun, L. et al. Protein extraction from microalgae residue and nutritional assessment. Bioprocess Biosyst Eng 45, 1879–1888 (2022). https://doi.org/10.1007/s00449-022-02794-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02794-w