Abstract
The effect of pH and NaCl on solubility and functional properties of walnut proteins obtained through AOT reverse micelles, enzyme-assisted reverse micelles and aqueous phase extraction methods was investigated and compared. Extraction yield, foaming properties, water holding capacities of protein obtained through enzyme-assisted reverse micelles at pH 2–12 and NaCl concentration 0.1–1 M were significantly higher than those of the AOT reverse micelles and aqueous phase extracted two. The solubility of proteins by AOT reverse micelles and enzyme-assisted reverse micelles at certain pH and NaCl concentration had no significant difference, but was higher that of the aqueous buffer. Oil holding capacity of three proteins was 2.35, 3.96 and 1.08 cm3/g, respectively. At pH 6–12 and NaCl concentration 0.1–1 M, the emulsifying activity of protein from AOT reverse micelles was higher than those of other two methods, while the emulsifying stability of protein from enzyme-assisted reverse micelles was the highest. The functional properties of walnut proteins were affected by extraction methods. It indicated that the walnut protein might be potentially applied in food industry as a food ingredient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protein has the higher nutritional value, and can affect the physiochemical and sensorial properties of food. Vegetable protein resources are very wide, and inexpensive, such as legumes, nuts and grains.
Although walnut, as a nut, has been widely consumed, the protein-based ingredients can be not made good use in food industry (Cofrades et al. 2008). The oil and protein content in walnut kernel was about 52–64 and 18-24%, respectively (Sze-Tao and Sathe 2000). But the protein in walnut meal obtained after extraction walnut oil can reach around 45%, which contains 18 amino acids, including 8 human essential amino acids, the protein digestibility is about 87.2%. The walnut protein chiefly consists of 6.8% albumin, 17.6% globulin, 5.3% prolamin and 70.1% glutelin (Sze-Tao and Sathe 2000). The above results suggested that walnut protein can provides several nutritional and health benefits (FDA 2011). However, many studies focusing on the physicochemical and antioxidant properties of walnut oil have been reported (Downs et al. 2016; Gharibzahedi et al. 2013; Labuckas et al. 2014), only a few investigations are found on the physicochemical and functional characteristics of walnut protein, which may become interesting for specific applications in several segments of the food industry (Cofrades et al. 2008; Labuckas et al. 2014).
Some plant proteins owning to desirably functional properties have widely applied in food products. The effects of processing parameters on functional properties of water-soluble proteins form different plant sources have been reported, such as soybean (Wang et al. 2008), rice (Zhao et al. 2012), Ginkgo biloba seeds (Deng et al. 2011), peanut (Liu et al. 2012) and pea seed (Adebiyi and Aluko 2011) et al. These studies were aimed at effective application of plant protein sources to formulation of new food products. However, the functional investigation of walnut protein is rare (Chen et al. 2012).
The process conditions in different extraction methods can affect the quantity, quality and functional properties of walnut proteins. There is a paucity of information regarding protein recovery and quality from walnut meal flour by different extraction methods including reverse micelles, enzyme assisted reverse micelles and walnut flour defatted by aqueous phase extraction.
In this study, walnut proteins were prepared from reverse micelles, enzyme assisted reverse micelles and aqueous phase extraction. The effect of different pH and salt concentration on the functional properties of walnut protein were investigated, in order to evaluate the potentiality of walnut protein source in food product formulation.
Materials and methods
Materials
Dry walnut (Xinxin No. 2) was collected by Xinjiang Academy of Forestry Sciences (Xinjiang, China). The oil in walnut seed was removed by pressing, then the defatted walnut meal flour obtained was ground and sieved through a 100 mesh screen. The walnut cake contained 46.96% protein, 5.98% humidity, 15.87% oil and 3.58% ash, respectively (AOAC 2000). Bis (2-ethylhexyl) sodium sulfosuccinate (AOT) and alcalase was bought of Sigma Chemical Co. (St. Louis, MO, USA, purity >98%). All other chemical reagents used were of analytical grade.
AOT reverse micelle extraction
The walnut cake flour and organic phases were mixed, which was called forward extraction experiments. The organic phase was prepared from known quantities of surfactant AOT, hexane and phosphate buffer containing KCl (Liu et al. 2014). For all the experimental runs, the ratio of solid phase to organic phase was 1:20 (w/v), and extracted by stirring for 30 min at 45 °C. Phase separation was carried out in a laboratory centrifuge at 6, 000 rpm for 20 min. The organic supernatant solution was used for the backward extraction.
Backward extraction was carried out by mixing the forward extraction solution with an equal volume of new aqueous phase containing 1 M KCl phosphate buffer with pH 9.0, followed by stirring for 1.5 h at room temperature, then centrifuging at 4, 000 rpm for 20 min and separation of the two phases. The protein solution was dialyzed for 24 h at 4 °C and freeze-dried in vacuum at −55 °C. Then, the walnut protein product was obtained.
Enzyme assisted AOT reverse micelle extraction
The extraction process was the same as above. But in process of forward extraction, 3.00% alcalase was added.
Aqueous phase extraction
The defatted walnut cake flour was dispersed in 50 mM phosphate buffer solution (1:20, w/w) (pH 8.0), stirred at room temperature for 2 h (Chen et al. 2012). The suspension was centrifuged at 6000 rpm at 20 °C for 30 min. Finally, the protein solution was extensively dialyzed against water at 4 °C for 24 h and lyophilized at −55 °C.
Sodium dodecyl sulphate poly-acrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE of walnut proteins was performed according to the method of Laemmli (1970). Protein samples (10 mg/ml) were dissolved in 1 ml of protein extraction buffer pH 6.8, containing 10 mM Tris-HCl, 2.5% SDS, 2% β-mercaptoethanol, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.01% bromophenol blue and 2% glycerol. The sample mixtures were boiled for 5 min, then samples (10 μl) were loaded into 12.5% resolving gel and 4.5% stacking gel, respectively with electrophoresis at 110 V and a constant current of 10 mA. The gel was stained overnight with 2.5% Coomassaie Brilliant Blue R 250 in water-methanol-acetic acid (4:5:1, v/v/v) and destained using with water-methanol-acetic acid (10:4:1, v/v/v).
Protein solubility analysis
The ratio of protein sample to deionized water 1/100 (w/v) was mixed. The suspension was adjusted to the desired pH (3–12) using 1 M HCl or 1 M NaOH, and did not contain NaCl. The mixtures were magnetically stirred for 1 h at 30 °C, and then centrifuged at 5, 000 rpm for 10 min at 4 °C. After the protein solution was appropriately diluted, the protein content of each supernatant was measured by Kjeldahl method. The nitrogen solubility index (NSI) (%) was as following (Lawal et al. 2005):
where W1 was the amount of protein in the supernatant (g), W0 was the amount of protein in the sample (g).
In addition, the effects of NaCl concentration (0–1 M) on protein solubility at pH 7.0 were also investigated.
Emulsifying properties
Effects of pH (2–12) and salt concentration (0–1 M) on emulsifying capacity (EC) and emulsion stability (ES) of the walnut proteins were examined. 500 mg of protein samples were dissolved in 100 ml phosphate buffer (10 mM, pH 7.0). Then, 24 ml the protein dispersion and 8 ml soybean oil were mixed by a homogenizer (PT-10-35GT, BUCHI Labortechnik AG, Switzerland) at 10, 000 rpm for 3 min at 25 °C. At 0 and 10 min after the homogenization, 0.05 ml of the obtained emulsion was took at 0.5 cm from the bottom of the container and mixed with 5 ml 0.1% SDS (w/v). Absorbance value of the mixture solution (A0) was recorded at 500 nm, and the blank solution was 0.1% SDS without protein. EA was calculated as the multiply A0 by 100. After the protein solution was heated at 80 °C for 30 min, and 0.05 ml of protein emulsion was took at 0.5 cm from the bottom of the container and mixed with 5 ml 0.1% SDS (w/v) after homogenization, which showed the EC80 at 80 °C, was determined at 500 nm. Each protein concentrate was analyzed triplicate. ES was calculated as following formula (Lawal et al. 2005).
Foam properties
1 g of samples was dispersed in 100 ml 10 mM phosphate buffer (pH 7.0). The mixture was adjusted to the desired pH (2–12) without containing NaCl or NaCl concentration (0–1 M) at pH 7.0, which was blended using a homogenizer (PT-10-35GT, BUCHI Labortechnik AG, Switzerland) at 12, 000 rpm for 2 min at room temperature. The blend was poured into 100 ml graduated cylinders, and measured. The volumes of foam (V0 and Vt) were immediately recorded at 0 time and 20 min, respectively. FC and FS were calculated as follows (Lawal et al. 2005):
Water holding capacity
Changes of water holding capacity (WHC) at different pH (2–12) without containing NaCl and NaCl concentration (0–1 M) at pH 7.0 were studied. The walnut protein powder (0.5 g) was mixed with 10 ml distilled water in a pre-weighed 50 ml centrifuge tube by magnetic stirring with occasional vortex agitations for 10 min at room temperature. The samples were placed for 30 min at room temperature before centrifuging for 15 min at 4, 000 rpm. The centrifuge tube containing sediment was weighed. WHC (grams of water per gram of protein) was expressed as follows (Lawal et al. 2005):
where W0 was the weight of the dry sample (g), W1 was the weight of the tube plus the dry sample (g), and W2 was the weight of the tube plus the sediment (g).
Oil holding capacity
0.5 g of protein powder (W0) was mixed with 5 ml (V1) of soybean oil in pre-weighed 15 ml centrifuge tubes. Before centrifuging at 4000 rpm for 15 min, the mixtures were placed for 30 min at room temperature. The clear supernatants were carefully poured into a 10 ml graduated cylinder, and recorded the volumes (V2). The OHC (cm3 of oil trapped per gram protein) was calculated as follows (Lawal et al. 2005):
Statistical analysis
All experiments were conducted in triplicate, and results were presented as means ± standard deviations (SD). The data subjected to analysis variance (ANOVA) was conducted using Duncan’s multiple range tests. Tukey test (p < 0.05) was used to compare the means of the different variables.
Results and discussion
Protein content
The protein extraction yield obtained through conventional aqueous phase, reverse micelles and enzyme-assisted reverse micelles were 38.63% ± 1.5, 55.40±1.42 and 67.29 ± 1.33%, respectively, the protein contents were 71.29 ± 2.56, 86.60 ± 2.13 and 92.58 ± 2.07%, respectively. The protein extraction yield and content from conventional aqueous phase extraction was the lowest, while from enzyme-assisted reverse micelles was the highest. The protein extraction yield and content obtained through three methods was significantly different (P < 0.05), the order from high to low was enzyme-assisted AOT reverse micelles > AOT reverse micelles > aqueous extraction. The results indicated that the protein purity from AOT reverse micelles and aqueous extraction was significantly different (P < 0.05), and also suggested that AOT reverse micelle could improve significantly the extraction efficiency of protein from walnut meal, especially enzyme-assisted reverse micelle extraction. The reasons possibly were as following: (1) the enzyme can hydrate the large protein molecules, the smaller protein molecules were more likely to dissolve in reverse micelles; (2) there was the strong interaction among protein molecules, AOT, hexane and interior water of reverse micelles, which could affect the extraction yield of protein (Zhao et al. 2008). In addition, it was supposed that the protein could be purified during enzyme assisted and AOT reverse micelle processing (Gaikaiwari et al. 2012).
SDS-PAGE analysis of proteins
SDS-PAGE profiles of walnut proteins from different extraction methods are presented in Fig. 1. Proteins showed a number of polypeptide subunits of molecular weight (Mw) between 66.2 and 14 kDa. The electrophoresis profiles of walnut proteins were consistent with earlier report of Mao and Hua (2012). The polypeptide subunits of 45 and 14.4 kDa were most prominent in three proteins (Fig. 1). Be comparing with three proteins, the subunit numbers of proteins from aqueous extraction were the least, while enzyme-assisted reverse micelles was the most. The bands of walnut proteins separated by reverse micelles and enzyme-assisted reverse micelles exhibited a similar intensity in all formulations. This observation strongly suggested that the reverse micelles or enzyme-assisted reverse micelles would be help to improve the subunits of walnut proteins.
Solubility measurement
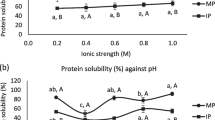
Solubility is an important parameter in protein functional properties, which is clearly related with emulsion and foam properties etc. (Yin et al. 2008). However, walnut protein is generally difficult to dissolve in water, it is attributed to containing mostly glutelin (about 70%) with a rigid globular structure with excessive intra and intermolecular disulfide bonds, hydrophobic interactions (Sze-Tao and Sathe 2000). So the walnut protein was not widely applied in food industry as functional ingredient. The pH solubility profile of walnut protein obtained through three extraction methods is visualized in Fig. 2a, which shows the typical U shaped profile. The NSI of walnut proteins significantly decreased across the pH range from 4 to 2, but increased protein solubility at higher pH (pH 4–12).The minimum NSI of three proteins obtained through AOT reverse micelles, enzyme-assisted AOT reverse micelles and aqueous extraction was recorded at pH 4.0 (Sze-Tao and Sathe 2000), and maximum solubility at pH 12. Similar pH value was also reported for minimum solubility of water-soluble proteins from kidney bean (Shevkani et al. 2015) and pea seed (Adebiyi and Aluko 2011). The reasons were that the protein aggregates were formed with the low electrostatic repulsive forces enhancing in isoelectric region, which resulted in the decrease of protein solubility (Mao and Hua 2012). The resulted indicated the pH had a significant effect on the solubility of walnut protein. Figure 2 a shows that AOT reverse micelles and enzyme-assisted AOT reverse micelles significantly increased the protein solubility (p < 0.05). The results suggested that the reverse micelle or enzyme-assisted treatment during food processing could induced the increase of charged molecules buried interior proteins, due to protein conformation change (Zhao et al. 2008).
Figure 2b shows the effects of salt concentration on the NSI of the walnut protein from three extraction methods. The NST of three proteins obtained through AOT reverse micelles, enzyme-assisted AOT reverse micelles and aqueous extraction in salt concentration from 0 to 0.1 M gradually decreased. With the increasing of the NaCl concentration from 0.1 to 1 M, the solubility of proteins considerably increased, which was due to salting-in effect (Deng et al. 2011). The different conformational characteristics of proteins could cause various solubility in salt solution (Vuong et al. 2016). Maximum solubility of three proteins was found at 1.0 M NaCl solution, which was 46.68, 48.92 and 39.56%, respectively. The results were agreed with the investigation Mao and Hua (2012). In the range of 0–1 M, the NSI of protein obtained through enzyme-assisted reverse micelles was significantly higher other proteins (p < 0.05). It was attributed that the different extraction methods resulted in the difference in protein conformational characteristics, which would cause various solubility in salt solution.
Emulsifying properties
The effects of pH on EC and ES of walnut protein from three methods are showed in Fig. 3a, b). The lowest EC and ES of three proteins were founded in the isoelectric region (pH 4). Under strong acidic (pH 2) or alkaline conditions (pH 12), the emulsifying properties were strongly improved. The highest EC and ES were achieved at pH 12. The reason was that the partial denaturation of protein occurred under extreme acidic or alkaline pH, which could affect the emulsifying properties of a protein, and was relative with its hydrophilic-lipophilic balance as well as net charge (Ragab et al. 2004).
As shown Fig. 3a, b, when pH value was 2, the EC obtained through AOT reverse micelles, enzyme-assisted reverse micelle and aqueous extraction was 60.36, 45.00 and 46.67%, respectively; the ES was 48.10, 60.00 and 41.59%, respectively. At pH 12, the EC reached the highest, and was 81.82, 61.43 and 70.67%, respectively; the ES also was the highest, and was 65.17, 77.55 and 58.22%, respectively. However, these were poor in the pI (pH 4.0). The results were supported by those observed earlier for kidney bean and field pea protein isolates (Shevkani et al. 2015).
These values showed that the EC from AOT reverse micelles were significantly higher than those of enzyme-assisted reverse micelles and aqueous buffer extraction (p < 0.05), which the highest ES was from enzyme-assisted reverse micelle. The results were supported by the investigation of Chove et al. (2001), they found that the EC with the increase of protein content reduced, while the ES increased. Similar results were reported for sunflower meal products (Lin et al. 1974) and cashew nut (Ogunwolu et al. 2009). It was supposed that the increase of protein solubility could improve the mutual function between oil and water phase. So the EC decreased and ES increased (Lawal et al 2005). Being comparison with EC and ES of three proteins, the effect of pH on the EC and ES was significantly different (p < 0.05), it might be due to the different chemical component, solubility and surface hydrophobicity of three proteins (Zhao et al. 2012).
Figure 3c, d show the effect of salt concentration on the EC and ES of three proteins. As the salt concentration increased from 0 to 0.4 M, the EC and ES of proteins gradually increased. While as further increase in salt concentration (from 0.4 to 1.0 M), both EC and ES decreased (Fig. 3c, d). The result was supported by the investigation of Chobert et al. (1987). The reasons were as following: the electrostatic repulsion between droplets was reduced by electrostatic shielding; high salt concentration could change the water structure, which would change the strength of hydrophobic interactions between nonpolar groups; low salt concentration might help to formation of charged layers around oil droplets, which would result in mutual repulsion between dispersed droplets in oil-in-water emulsion (Ragab et al. 2004; Yuliana et al. 2014).
When the salt concentration was 0.4 M, the EC of proteins obtained through AOT reverse micelle extraction, enzyme-assisted reverse micelle and aqueous extraction was the maximum, and 83.33, 66.67 and 75.00%, respectively. The EA of walnut protein obtained through AOT reverse micelles were significantly higher than that of other two extraction methods (p < 0.05). At the salt concentration 0.4 M, the ES of proteins obtained through AOT reverse micelle, enzyme-assisted reverse micelle and aqueous buffer also was the maximum, and 52.45, 63.12 and 41.22%, respectively. The ES of walnut protein obtained through enzyme-assisted reverse micelle extraction were significantly higher than that of other two extraction methods (p < 0.05). The results were agreed with the finding of previous studies on emulsifying stability and nitrogen solubility (Hung and Zayas 1991; Yuliana et al. 2014). Some reports thought that many factors could influence the protein ES, such as pH, net charge, interfacial tension, viscosity and protein conformation (Hung and Zayas 1991; Yuliana et al. 2014).
These results also indicated that the extraction parameters could affect the EC and ES of proteins, especially enzyme-assisted reverse micelles. The differences in EC and ES of walnut protein using three extraction methods might be due to the differences of solubility and molecular structure in proteins (Mao and Hua 2012; Zhao et al. 2012). Some reports have proved that reverse micelle and/or enzyme can change the structure and functional properties (Zhao et al. 2008).
Foaming properties
The effects of pH value on the foaming capacities and foam stabilities of protein samples using three extraction methods are presented in Fig. 4a, b. At pH 4.0, the FC and FS of three proteins was the lowest, which was supported by the investigation of Mundi and Aluko (2012). They found that low solubility of protein resulted in low FC and FS in the isoelectric region (Mundi and Aluko 2012). When pH value was 12, the FC of three proteins obtained through AOT reverse micelles, enzyme-assisted reverse micelles and aqueous extraction reached the maximum, FC values were 52.62, 60.48 and 46.35%, respectively, while FS values reached the maximum at pH 10, were 49.62, 52.68 and 40.48%, respectively. The results indicated that the pH value could affect the FC and FS. In addition, the FC and FS were significant difference between three proteins (p < 0.05). Comparing to protein obtained through AOT reverse micelles and aqueous extraction methods, walnut protein using enzyme-assisted reverse micelles had the highest FC and FS (p <0.05). The reason was that proteins with high concentration and solubility could increase the viscosity and improve the formation of protein film with strong cohesion (Belitz et al. 2009).
Figure 4c, d show the effect of salt concentration on FC and FS of three proteins. As the salt concentration increased from 0 to 0.6 M, the FC of proteins obtained through AOT reverse micelles, enzyme-assisted reverse micelles and aqueous buffer increased by 61, 75 and 52%, respectively, while FS increased by 48, 55 and 38%, respectively. When the salt concentration was higher than 0.6 M, both FC and FS reduced. The result was agreed with the investigation of Deng et al. (2011), who found that high salt concentration could increase protein aggregation and lead to the decrease of foaming properties. In the range of salt concentration from 0 to 1.0 M, the FC and FS of proteins obtained through enzyme assisted reverse micelles were significantly higher than other two proteins (p <0.05), which was due to the different protein contents (Deng et al. 2011). Proteins with poor solubility might be attributed to high content of disulphide bonds, which resulted in lower foam properties (Agboola et al. 2005). It indicated that enzyme-assisted and AOT reverse micelles significantly impacted the FC and FS. The good foaming properties of proteins would be used for foam stabilization in food industry, such as baked goods, sweets and soft drinks (Kinsella and Melachouris 1976).
Water holding capacity (WHC) measurement
As shown Fig. 5, the WHC of three proteins decreased with the increase of pH from 2 to 4, and then increased when the pH rose from 4 to 12. At pH 4, the WHC was the lowest, which the protein solubility was the minimum. It was supposed that the interaction between protein molecules at isoelectric region was improved, while the interaction between proteins and water decreased. Thus the poor WHC would be shown (Deng et al, 2011). When the pH was above 4, WHC increased significantly. It suggested that the interaction among proteins decreased (Yuliana et al. 2014). By comparing with the WHC of proteins from three extraction methods, WHC value for walnut protein obtained through enzyme-assisted reverse micelles was significantly higher (p < 0.05) than that of reverse micelles and aqueous buffer. Some references reported that the enzymatic hydrolysis could lead to the hydrophobic amino acids exposed, which improved the protein binding capacity with water (Guan et al. 2007).The WHC of proteins from aqueous buffer was the lowest (Fig. 5a). For lower WHC values, it was likely attributed to the structural changes of proteins and weak intermolecular interaction of walnut proteins with water molecules (Ghumman et al. 2016; Withana-Gamage et al. 2011).
Figure 5b shows the effect of NaCl concentration on protein WHC. When the NaCl concentration was increased from 0 to 0.6 M, WHC of proteins obtained through enzyme-assisted reverse micelles, AOT reverse micelles and aqueous buffer increased from 2.87 to 5.12, 2.21 to 4.87 and 2.01 to 4.03 g/g, respectively. Then WHC decreased moderately with further increase of NaCl concentration. At low salt concentration, hydrated salt ions could combine with charged protein molecules, and reduce the water molecule around proteins (Lawal et al. 2005). However, at high salt concentration, the interaction between water and salt ion was improved, which led to the dehydration of the protein and reduction in WHC (Lawal et al. 2005). As shown Fig. 5, the WHC of walnut protein from enzyme-assisted reverse micelles was significantly higher (p < 0.05) than that of other two extraction methods. However, WHC of protein from aqueous buffer was the lowest. The reason was that the enzyme function in reverse micelles could make more hydrophobic amide acids exposed, which led to improving the protein binding capacity with water (Guan et al. 2007). It indicated the extraction methods could change WHC as well as other functional properties of proteins (Zhao et al. 2015; Fabian and Ju 2011). High WHC of proteins can help to reduce moisture loss and maintain freshness of processing foods, such as baked foods and meat batters.
Oil holding capacity (OHC)
The OHC of walnut protein from enzyme-assisted reverse micelles was 3.96 cm3/g, which was much higher than that of AOT reverse micelles (2.35 cm3/g) and aqueous buffer (1.08 cm3/g). OHC of protein from enzyme-assisted reverse micelles was significantly high than other two extraction methods. It was probably that the enzyme in reverse micelles could hydrolyze walnut proteins, which made hydrophobic amide acids exposed, and more nonpolar side chains appeared to be able to bind more aliphatic hydrocarbon chains. At the same time, higher protein content showed the higher OHC (Campbell et al. 1992). Difference OHC of walnut proteins obtained through three extraction methods were likely due to the changes of structure and/or physical-chemical properties, for example, second structure, lipophilic groups and degeneration of proteins (Deng et al. 2011). It suggested that walnut protein with high OHC could be potentially used in different food products, which improved flavor of food and retained structural interactions, palpability and extended shelf life (Deng et al. 2011).
Conclusion
A product with 67.29% protein yield and 92.58% protein content was successfully obtained through enzyme-assisted reverse micelles. The minimum solubility of walnut proteins from three extraction methods occurred at pI of the protein isolate (pH 4), then increased with increasing pH, but solubility from AOT reverse micelles and enzyme-assisted AOT reverse micelles was higher than aqueous buffer. EC, ES, FC, FS, WHC and OHC of proteins obtained through three extraction methods were investigated. It was concluded that the pH and NaCl concentration adjusted could improve the functional properties of walnut protein. Different extraction methods could affect the functional properties of proteins. Especially the enzyme-assisted reverse micelles would both enhance the protein content and modify the protein structure during extracting, which indicated that the walnut protein might be potentially used in a variety of food products as a food ingredient.
References
Adebiyi AP, Aluko RE (2011) Functional properties of protein fractions obtained from commercial yellow field pea (Pisum sativum L.) seed protein isolate. Food Chem 128:902–908
Agboola S, Ng D, Mills D (2005) Characterisation and functional properties of Australian rice protein isolates. J Cereal Sci 41(3):283–290
AOAC (2000) Official methods of analysis of AOAC International (17th ed.). Washington, DS, USA: Association of Official Analytical Chemists (Methods Ba 4e-93)
Belitz HD, Grosch W, Schieberle P (2009) Food Chemistry. Springer, Berlin
Campbell NF, Shih FF, Marshall WE (1992) Enzymic phosphorylation of soy protein isolate for improved functional properties. J Agric Food Chem 40(3):403–406
Chen N, Yang HM, Sun Y, Niu J, Liu SY (2012) Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 38(2):344–349
Chobert JM, Bertrand-Harb C, Nicolas MG, Gaertner HF, Puigserver AJ (1987) Solubility and emulsifying properties of caseins chemically modified by covalent attachment of l-methionine and L-valine. J Agric Food Chem 35(5):638–644
Chove BE, Grandison AS, Lewis MJ (2001) Emulsifying properties of soy protein isolate factions obtained by isoelectric precipitation. J Sci Food Agric 81(8):759–763
Cofrades S, Serrano A, Ayo J, Carballo J, Jiménez-Colmenero F (2008) Characteristics of meat batters with added native and preheated defatted walnut. Food Chem 107(4):1506–1514
Deng QC, Wang L, Wei F, Xie BJ, Huang FH, Huang W, Shi J, Huang QD, Tian BQ, Xue S (2011) Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem 124(4):1458–1465
Downs ML, Simpson A, Custovic A, Semic-Jusufagic A, Bartra J, Fernandez-Rivas M, Taylor SL, Baumert JL, Clare Mills EN (2016) Insoluble and soluble roasted walnut proteins retain antibody reactivity. Food Chem 194:1013–1021
Fabian C, Ju YH (2011) A review on rice bran protein: its properties and extraction methods. Crit Rev Food Sci Nutr 51:816–827
FDA (2011-12-20) Qualified health claims: letter of enforcement discretion-walnuts and coronary heart disease (Docket No.02p-0292). http://www.cfsan.fda.gov/dms/qhcnuts3.html
Gaikaiwari RP, Wagha SA, Kulkarni BD (2012) Extraction and purification of tannase by reverse micelle system. Sep Purif Technol 89:288–296
Gharibzahedi SMT, Mousavi SM, Hamedi M, Rezaei K, Khodaiyan F (2013) Evaluation of physicochemical properties and antioxidant activities of Persian walnut oil obtained by several extraction methods. Ind Crops Prod 45:133–140
Ghumman A, Kaur A, Singh N (2016) Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocolloid 61:843–850
Guan X, Yao H, Chen Z, Shan L, Zhang M (2007) Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem 101(1):163–170
Hung SC, Zayas JF (1991) Emulsifying capacity and emulsion stability of milkproteins and corn germ protein flour. J Food Sci 56:1216–1223
Kinsella JE, Melachouris N (1976) Functional properties of proteins in foods: a survey. Crit Rev Food Sci Nutr 7:219–280
Labuckas D, Maestri D, Lamarque A (2014) Effect of different oil extraction methods on proximate composition and protein characteristics of walnut (Juglans regia L.) flour. LWT Food Sci Technol 59:794–799
Laemmli UK (1970) Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227:680–685
Lawal OS, Adebowale KO, Ogunsanwo BM, Sosanwo OA, Bankole SA (2005) On the functional properties of globulin and albumin protein fractions and flours of African locust bean (Parkia biglobossa). Food Chem 92:681–691
Lin M, Humbert E, Sosulski F (1974) Certain functional properties of sunflower meal products. J Food Sci 39(2):368–370
Liu Y, Zhao GL, Zhao MM, Ren JY, Yang B (2012) Improvement of functional properties of peanut protein isolate by conjugation with dextran through Maillard reaction. Food Chem 131:901–906
Liu FL, Wang XC, Zhao XY, Hu HF, Chen FL, Sun YL (2014) Surface properties of walnut protein from AOT reverse micelles. Int J Food Sci Technol 49:626–633
Mao X, Hua Y (2012) Composition, structure and functional properties of protein concentrates and isolates produced from walnut (Juglans regia L.). Int J Mol Sci 13:1561–1581
Mundi S, Aluko RE (2012) Physicochemical and functional properties of kidney bean albumin and globulin protein fractions. Food Res Int 48:299–306
Ogunwolu SO, Henshaw FO, Mock HP, Santros A, Awonorin SO (2009) Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chem 115(3):852–858
Ragab DM, Babiker EE, Eltinay AH (2004) Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH an d/or salt concentration. Food Chem 84:207–212
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocolloid 43:679–689
Sze-Tao KWC, Sathe SK (2000) Walnuts (Juglans regia L): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J Sci Food Agric 80:1393–1401
Vuong HTH, Tran NM, Tran TTT, Ton NMN, Le VVM (2016) Effects of pH and salt concentration on functional properties of rambutan (Nephelium lappaceum L.) seed albumin concentrate. Int J Food Sci Technol 51(5):1212–1219
Wang XS, Tang CH, Li BS, Yang XQ, Li L, Ma CY (2008) Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloid 22:560–567
Withana-Gamage TS, Wanasundara JPD, Pietrasik Z, Shand PJ (2011) Physicochemical, thernal and functional characterisation of protein isolates from kbuli and desi chickpean (Cicer arietinum L): A comparative study with soy (Glycine max) and pea (Pisum sativum L). J Sci Food Agric 91(6):1022–1031
Yin S, Tang C, Wen Q, Yang X, Li L (2008) Functional properties and in vitro trypsin digestibility of red kidney bean (Phaseolus vulgaris L.) protein isolate: effect of high-pressure treatment. Food Chem 110(4):938–945
Yuliana M, Truong CT, Huynh LH, Ho QP, Ju YH (2014) Isolation and characterization of protein isolated from defatted cashew nut shell: influence of pH and NaCl on solubility and functional properties. LWT Food Sci Technol 55:621–626
Zhao XY, Chen FS, Xue WT, Lee LT (2008) FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocolloid 22(4):568–575
Zhao Q, Selomulya C, Xiong H, Chen XD, Ruan X, Wang SQ, Xie JH, Peng HL, Sun WJ, Zhou Q (2012) Comparison of functional and structural properties of native and industrial process-modified proteins from long-grain indica rice. J Cereal Sci 56(3):568–575
Zhao XY, Zhu HT, Chen J (2015) Effects of sodium bis(2-ethylhexyl) sulfo succinate(AOT) reverse micelles on physicochemical properties of soy protein. Food Bioprod Process 94:500–506
Acknowledgements
This work was funded by National Natural Science Foundation of China (Grant No. 21406133), Xinjiang Autonomous Region Forestry Science and Technology Special Fund Finance and Shandong Provincial Natural Science Foundation China (ZR2014BP015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, H., Fan, T., Zhao, X. et al. Influence of pH and salt concentration on functional properties of walnut protein from different extraction methods. J Food Sci Technol 54, 2833–2841 (2017). https://doi.org/10.1007/s13197-017-2721-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2721-6