Abstract
Polymalic acid (PMA) is a water-soluble polyester produced by Aureobasidium pullulans. In this study, the physiological response of A. pullulans after the addition of vegetable oils was investigated. Soybean oil (SBO) is pivotal for shortening fermentation time and achieving high PMA titer. With the addition of 1% (w/v) SBO, the titer and productivity of PMA was, respectively, increased by 34.2% and 80%. SBO acted as a chemical stimulatory agent rather than a carbon source, the enhancement on PMA production was attributed to the component of fatty acid. SBO induced the dimorphism (yeast-like cells and mycelia) of A. pullulans, in vitro enzyme activities indicated that the TCA oxidative branch for malic acid synthesis might be strengthened, which could generate more ATP for PMA synthesis, and the assay of intracellular energy supply validated this deduction. This study provided a new sight for recognizing the regulatory behavior of SBO in A. pullulans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymalic acid (PMA) is a water-soluble polyester consisting of L-malic acid monomers. As PMA has many side-chain carboxyl groups, this polyester is allowed to bind drugs, antibodies and targeting moieties by chemical modification [1]. Besides, PMA is biocompatible, biodegradable and non-immunogenic, and these excellent features enable it to be widely used in pharmaceutical industries. For example, Polycefins™, a new drug delivery system with tumor targeting ability, has been employed in preclinical studies and is considered as a candidate for anticancer therapy [2]. Moreover, PMA can be easily hydrolyzed to malic acid, which is an acidulant in the food and beverage industry. Compared with citric acid, malic acid is built up slower and persisted longer, making it suited to cover up the aftertaste of artificial sweeteners [3]. Malic acid can be mass produced by Aspergillus flavus, but the intrinsic pathogenicity of this strain prevents its commercial production, and the co-production of other organic acids makes the downstream recovery of malic acid relatively expensive [4]. Malic acid can also be produced by engineered Escherichia coli or Saccharomyces cerevisiae, but this bioprocess is limited by the end-product inhibition due to the strong acidity of malic acid [5]. By contrast, the aforementioned disadvantages do not exist in PMA-producing process; thus, microbial fermentation of PMA followed by acid hydrolysis provides an alternative method for malic acid production.
Currently, the Aureobasidium pullulans species are primarily employed to produce PMA by submerged fermentation, and many efforts have been made to improve PMA production to satisfy the growing market demand. A high concentration of sugar in the medium can be efficiently utilized to enhance PMA yield, and decrease the cost for the downstream recovery of PMA. However, excess sugar also brings high osmotic pressure, which would inhibit cell growth and PMA biosynthesis [6, 7]. To overcome this problem, the supplement of other carbon sources was thereby carried out. Lipids, primarily oils, have antifoaming property as well as nutritional particularity; thus, oils are regarded as an additional carbon source by some microorganisms capable of producing lipase, such as Candida cylindracea, Aspergillus ibericus and Penicillium, etc. [8, 9]. A. pullulans is a cosmopolitan fungus with the ability of producing several valuable products, including pullulan, PMA, liamocins and some degradative enzymes [10]. Previous studies indicated that A. pullulans can secrete lipase, which can hydrolyze oil into glycerol and fatty acids [11]; thus, oil has been utilized as an additional carbon source by A. pullulans. For example, Youssef et al. [12] found that the addition of oil could eliminate the inhibitory effect of excess sucrose on pullulan production, and the simultaneous utilization of oil and sucrose obviously improved pullulan yield. The authors also found when oil was used alone, poor cell growth and pullulan production was observed, indicating that oil should be used together with sugar by A. pullulans. However, Shabtai et al. [13] found that when oil was used as a sole carbon source, a higher cell growth rate and biomass amount was yielded than that of sucrose. Moreover, the A. pullulans cells were restricted to yeast morphology and the formation of pigment was inhibited under this condition, which was beneficial for pullulan production. Hence, oil was used in the first stage and sucrose was used subsequently, and a shortened fermentation period as well as a higher pullulan titer was achieved. The above studies showed contradictory effects of oil on cell growth and product synthesis by A. pullulans. So far, the effect of oil on PMA production is not clear, and it is of great interest to conduct some research on PMA production from oil.
In addition to being a carbon source, oil might also work similar to a surfactant. Oil is structurally similar to surfactant owing to its abundant content of fatty acids, which are hydrophobic moieties acting as “tails” of surfactant. Surfactants are amphiphilic molecules which consist of hydrophilic and hydrophobic moieties, and this structure confers some unique properties such as lowering surface and interfacial tension of liquids and forming micelles between two different phases [14]. Polyethylene glycol sorbitan monooleate, known as Tween 80, is a non-ionic surfactant containing a mono-unsaturated fatty acid. In previous studies, Tween 80 has been proved to be an effective surfactant for PMA production. Tu et al. [15] found that Tween 80 was beneficial for the co-production of PMA and pullulans, the cell morphology was changed, and the transcription levels of key genes involved in PMA and pullulan biosynthesis were upregulated after the addition of Tween 80. Further studies showed that Tween 80 increased the cell membrane permeability, and the key pathway of PMA synthesis along with energy metabolism was enhanced [16]. Considering the structurally similarity of oil and Tween 80, do they have the same action mechanism on PMA production?
In this study, vegetable oil was introduced into PMA fermentation process, and the effect of oil addition manner on PMA production was investigated. Then one question arises: which role dose vegetable oil play in PMA production? a carbon source or a chemical stimulatory agent? The underlying mechanism was studied with regard to the cell morphology, intracellular energy supply and key enzyme activities involved in vital pathways of PMA production.
Materials and methods
Microorganisms and culture methods
A. pullulans HA-4D (CGMCC No.7.208) was maintained on potato dextrose agar (PDA) slants at 4 °C. For seed culture, a loop of A. pullulans HA-4D cells was inoculated into a 250 mL flask containing 50 mL of the seed medium, which was composed of (g/L): glucose 80, Yeast extract 1, NaNO3 1, KH2PO4 0.1, MgSO4 0.1, KCl 0.5 and CaCO3 20. The seed culture was aerobically conducted at 25 °C, 200 rpm for 48 h. The fermentation medium included (g/L): glucose 100, NaNO3 2, KH2PO4 0.1, MgSO4 0.1, KCl 0.5 and CaCO3 30 [6]. All reagents were of analytical grade and were purchased from Macklin Reagent (China). All vegetable oils were provided by Professor Liu Xiaoyan, Huaiyin Normal University. The fatty acid composition of these oils was determined in a previous study [17].
Fermentation in shake-flask and stirred-tank fermentor
For shake-flask fermentation, the above seed culture (10%, v/v) was inoculated into a 250 mL shake-flask containing 50 mL of fermentation medium, and the shake-flask fermentation was operated at 25 °C, 200 rpm for 8 days [6]. The one-stage and two-stage fermentation were, respectively, conducted in shake-flasks. For one-stage fermentation, both glucose and vegetable oils were simultaneously added into the medium at the beginning of fermentation. Then, the dosage of oil was varied from 5 to 30 g/L, and the effect of oil component (glycerol and fatty acid) on PMA production was investigated. For two-stage fermentation, oil was used as a sole carbon source in the first stage and glucose was used subsequently.
Batch fermentation was carried out in a 5-L stirred-tank fermentor (FS-02 series, Winpact, USA). 300 mL of the seed culture was inoculated into 2.7 L of the fermentation medium-containing 10 g/L soybean oil, the fermentation was performed at 25 °C, 400 rpm, and the aeration rate was 1 vvm [6].
Analytical methods
The analysis of PMA production was carried out according to our previous study [6]. Briefly, the culture broth was centrifuged and the supernatant was mixed with an equal volume of 2 mol/L H2SO4, the mixture was heated at 90 °C for 9 h and the hydrolyzed PMA was determined by HPLC (Agilent 1260, USA) equipped with a Spursil C18-EP column. The mobile phase was 25 mmol/L KH2PO4 (pH 2.5)/methanol (9:1, v/v) with a flow rate of 0.5 mL min−1 at 30 °C. The residual glucose in the supernatant was determined enzymatically by a biosensor (SBA-40C, Shangdong Science Academy, China). For the measurement of dry cell weight (DCW), excess CaCO3 in the culture broth was eliminated by adding 1 mol/L HCl, the broth was centrifuged, and the precipitate was collected, washed, and dried to a constant weight. The residual soybean oil in the medium was determined by a solvent-extraction method [18], 2 mL culture broth was mixed with 5 mL n-hexane, the mixture was vigorously shaken for 2 min and then centrifuged at 4000 rpm for 10 min, and the upper layer was removed and dried at 80 °C for 3 h to determine the extracted oil weight. The cell morphology was observed under optical microscope (Olympus CX31, Japan) with crystal violet staining.
Intracellular NADH/NAD+ ratio and ATP level
The intracellular NADH/NAD+ ratio was determined as described previously, which was based on alcohol dehydrogenase cycling reaction [19]. For the analysis of intracellular ATP, an ATP Assay Kit (Beyotime, China) was used on the basis of luciferase–luciferin reaction.
Enzyme activities
A. pullulans HA-4D cells were harvested by centrifugation and washed twice by physiological saline, and then, the cells were suspended in Tris–HCl buffer (100 mM, pH7.0) and sonicated at 400 W for 15 min by 2-s working pulses and 3-s cooling intervals. The cell debris was removed and the crude extract was used for enzyme activity assay. The pyruvate carboxylase (PYC) activity was assayed according to our previous study [6], and the isocitrate lyase (ICL), malate synthase (MLS), isocitrate dehydrogenase (IDH), and fumarase (FUM) activities were determined as described previously [20]. The protein content of the crude extract was measured by Bradford method with bovine serum albumin as a standard.
Results and discussion
Effect of soybean oil on PMA fermentation in shake-flasks
Our previous study indicated that the optimum initial glucose concentration for PMA production by A. pullulans HA-4D is 100 g/L [6]; thus, 100 g/L glucose plus different oily substrates were used to verify if the addition of oil is beneficial for PMA production (Fig. 1a). Compared to the control, soybean, olive and corn oil showed positive effects, whereas rapeseed and sunflower oil did not result in any obvious effects on PMA production. Waste cooking oil is an organic waste slurry produced from cooking, it is a low-cost oily substrate consisting of edible vegetable matter. Unfortunately, PMA concentration was decreased with the supplement of waste cooking oil, which was probably due to the inhibitors generated during the thermolytic, oxidative and hydrolytic reactions from frying. The highest PMA concentration of 41.5 g/L was obtained when soybean oil (SBO) was employed, and the biomass of A. pullulans HA-4D was also increased by 38.0%. The next step was to determine the optimum concentration of SBO for PMA biosynthesis. As shown in Fig. 1b, SBOs varying from 5 to 10 g/L stimulated PMA production and cell growth, and the addition of 10 g/L SBO gave the highest PMA titer of 42.3 g/L, along with the highest DCW of 23.6 g/L. When the concentration of SBO was more than 10 g/L, negative effects occurred to PMA production. It is worthy to note that when SBO was added without glucose, both cell growth and PMA synthesis were quite poor, indicating that SBO was an inferior carbon source. When SBO and glucose were used simultaneously, the time profiles of their consumption are shown in Fig. 1c, glucose was first utilized and a lagged SBO utilization was observed, and SBO utilization rate was increased when glucose was nearly exhausted. At the end of the fermentation, the residual glucose and SBO concentration is 4.2 g/L and 3.1 g/L, respectively. The presence of glucose inhibited the utilization of SBO in the early stage of fermentation, but SBO was partially hydrolyzed by A. pullulans HA-4D in the later stage of fermentation. It is well known that the main component of SBO is triglyceride, which consists of glycerol and fatty acid. Previous study showed that the main fatty acid composition of SBO is linoleic acid (C18:2, 51.2%), oleic acid (C18:1, 22.3%), stearic acid (C18:0, 5.7%) and palmitic acid (C16:0, 12.4%) [17]. To elucidate which component is responsible for the enhanced PMA production, the aforementioned fatty acids and glycerol were supplemented into the medium. It turned out that the addition of fatty acids, rather than glycerol, led to the enhancement of PMA production. The final PMA titer was almost the same no matter if glycerol was added. Of the tested fatty acids, oleic acid (C18:1) led to the highest PMA concentration of 42.9 g/L.
Effect of glucose/oil mixture on PMA production. a PMA production with the addition of different oils. b PMA production with the addition of 5–30 g/L soybean oil. c Time course of glucose and soybean oil consumption. d Effect of soybean oil component on PMA production. C18:2, linoleic acid; C18:1, oleic acid; C18:0, stearic acid; C16:0, palmitic acid. The fermentation conditions: 10% (v/v) A. pullulans HA-4D seed culture was inoculated into a 250 mL shake-flask containing 50 mL of fermentation medium, oils were initially added together with 100 g/L glucose, and the shake-flask fermentation was conducted at 25 °C, 200 rpm for 8 days. The fermentation with 100 g/L glucose served as control. Each column in the figures was calculated with three parallel experiments. **P < 0.01; *P < 0.05
In addition to being used with sugar simultaneously, oil was also used as a sole carbon source in a previous study, and the authors claimed that the A. pullulans cells were restricted to yeast forms under this condition, which is regarded to be a productive status for pullulan or PMA synthesis [13]. To validate the feasibility of this method, a two-stage fermentation strategy in which SBO and glucose were separately used was employed in this study. As shown in Fig. 2, SBOs varying from 10 to 30 g/L were utilized in the first stage, then glucose was supplemented into the medium, and the total carbon source concentration was 100 g/L. However, the two-stage fermentation strategy did not bring the hoped-for improvement on PMA production. As the dosage of SBO increased, both PMA and DCW titer were decreased. The highest PMA concentration was only 30.8 g/L (oil/glucose = 10/90), which was decreased by 27.7% than that of oil/glucose mixture. Therefore, the two-stage fermentation strategy is not feasible for PMA production by A. pullulans HA-4D.
a The schematic diagram of a two-stage fermentation strategy. b PMA production with a two-stage fermentation strategy. SBO soybean oil, Glc glucose. The fermentation was divided into two stages: in stage I, 10, 20, and 30 g/L soybean oil was used as a sole carbon source, the culture was maintained for 24, 36 and 48 h, respectively; in stage II, 90, 80 and 70 g/L glucose was supplemented into the medium, and the shake-flask fermentation was conducted at 25 °C, 200 rpm for 8 days. The fermentation with 100 g/L glucose served as control. **P < 0.01; *P < 0.05
The use of vegetable oil as a carbon source is usually studied in the light of lipase production. Oil can be used as a sole carbon source instead of sugar by some fungi, including Yarrowia lipolytica, Aspergillus, etc. [9, 21]. In the case of the fungus A. pullulans, vegetable oil has been employed for pullulan production, and oil acted as either a sole carbon source or an additional carbon source to sugar [12, 13]. Our study showed that vegetable oil was an inferior carbon source for cell growth and PMA synthesis; thus, the simultaneous utilization of oil and sugar was indispensable. In the original plan of this study, oil was regarded as an additional carbon source, the purpose of adding oil was to eliminate the inhibitory effect of excess sugar on PMA production. However, our further study proved that when oil was hydrolyzed into glycerol and fatty acids by A. pullulans, the enhancement of PMA production was attributed to the fatty acids, and SBO was acting as a chemical stimulatory agent rather than a carbon source. In fact, previous studies also showed that when glycerol was used for PMA production, both cell growth and PMA synthesis were quite poor, indicating that glycerol does not contribute to the enhanced PMA synthesis [22, 23]. We also tested the feasibility of PMA production using fatty acids as a sole carbon source, and it turned out that no PMA production was observed (data not shown). In conclusion, SBO was acting as a chemical stimulatory agent for PMA production, and the underling mechanism of SBO acting on PMA biosynthesis was investigated in the subsequent sections.
Effect of soybean oil on PMA fermentation in a 5-L fermentor
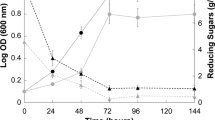
Based on the results of the shake-flasks, the addition of 10 g/L SBO was carried out in a 5-L stirred-tank fermentor. As shown in Fig. 3, the supplement of SBO was pivotal for enhancing cell growth and sugar consumption, and PMA production was also significantly improved. Compared to the control, the fermentation period was shorten from 96 to 72 h; as a result, the consumption rate of glucose was increased from 1.04 to 1.39 g/L h. On the other hand, the final titer of PMA and DCW was 45.5 g/L and 25.9 g/L, which was increased by 34.2 and 13.6%, respectively. The maximal concentration of PMA and DCW in 5-L fermentor was comparable to those of shake-flasks, but the fermentation period of 5-L fermentor was obviously shortened, which was probably attributed to the better transfer of oxygen and mass in the fermentor. The aforementioned results indicated that the increased uptake of carbon source was distributed to both cell growth and PMA biosynthesis; thus, the addition of SBO was a feasible approach to produce high amount of PMA. Furthermore, SBO significantly promoted cell growth and PMA production, and similar phenomenon was also observed with the addition of Tween 80 [15]. Considering that both SBO and Tween 80 contain abundant content of fatty acids, do they have the same acting mechanism on PMA production? Thus the underling mechanism of SBO acting on PMA biosynthesis was investigated in the subsequent experiments.
Time course of PMA production a, cell growth b and residual sugar c with the addition of soybean oil in 5-L stirred-tank fermentor. SBO, soybean oil. The batch fermentation conditions: 300 mL of A. pullulans seed culture was inoculated into a 5-L stirred-tank fermentor (FS-02 series, Winpact, USA) containing 2.7 L of fermentation medium, 10 g/L soybean oil were initially added together with 100 g/L glucose, the fermentation was performed at 25 °C, 400 rpm, and the aeration rate was 1 vvm
The underling mechanism of SBO acting on PMA biosynthesis
SBO induced variations of cell morphology
To further elucidate the mechanism of SBO acting on PMA production, the variations of cell morphology in shake-flask fermentation were first observed (Fig. 4). When glucose was used as a sole carbon source, the cells presented yeast-like form at the beginning of fermentation. Then, the size of cells became larger and the swollen cells were dominant in the culture at 48 h; this morphological feature was maintained to the end of fermentation. By contrast, the addition the SBO led to obvious influence on cell morphology. The cell morphology between 0 and 48 h was similar to that of control, but both swollen cells and mycelia were appeared at 96 h, the mycelia were gradually broken, and unicells were formed at 168 h. In conclusion, the addition of SBO resulted in the enhancement of PMA biosynthesis; in response to that, the stimulation of mycelial growth by A. pullulans HA-4D cells was simultaneously observed.
The morphological parameters, such as cell size, cell shape and accumulation of pigments, etc., can indicate the state of the cell. Linkage between cell morphology and metabolic activity is of great importance, since it can help us to quickly assess the performance of the culture. The A. pullulans has a complex polymorphic life cycle, and the variations of cell morphology follow the subsequent path: yeast-like form, young blastospore, swollen blastospore and mycelium [24]. The morphological feature of A. pullulans is influenced by process variables, such as pH, nitrogen source and metal ions. For example, the addition of zinc ion caused the transformation of yeast-like cells into mycelial form by A. pullulans QM3092, and the mycelial accounted for 90% of the total biomass when 7.6 μmol/L zinc ion was added [25]. In this study, the cell morphology of the control group was sole yeast-like cells, whereas SBO induced the dimorphism (yeast-like cells and mycelia) of A. pullulans. It seemed that the supplement of SBO accelerated the metabolism and promoted differentiation of A. pullulans cells. Currently, the linkage between PMA biosynthesis and A. pullulans cell morphology is not clear yet, but considerable efforts have been made to elucidate the relationship between pullulan production and A. pullulans cell morphology. Generally, the researchers agreed that the yeast-like or swollen cells are the main producer of pullulan, but they argued on the pullulan-producing ability of mycelia of A. pullulans. Simon et al. [26] and Campbell et al. [27] believed that the swollen cells are responsible for producing pullulan, and the mycelia do not play a role in exopolysaccharide biosynthesis. However, Catley [28] founded that the blastospores are the major producer of pullulan, the mycelia are equally capable of producing pullulan with a lower efficiency, and McNeil et al. [29] observed that pullulan production was optimal when both swollen cells and mycelia were present in the culture. Our study is in good agreement with the latter viewpoint, compared with the morphology of sole yeast-like cells, and the dimorphism (yeast-like cells and mycelia) of A. pullulans was more beneficial for PMA biosynthesis.
SBO induced variations of key enzymes and energy supply
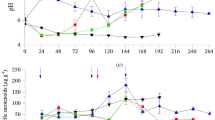
Because malic acid is the only monomer of PMA, the biosynthesis of malic acid is closely related to PMA production. So far, three metabolic pathways for malic acid biosynthesis have been identified, including TCA oxidative branch, TCA reductive branch and glyoxylate shunt (Fig. 5a). The changes of key enzyme activities might result in metabolic flux redistribution; thus, the key enzymes involved in biosynthetic pathway of malic acid were assayed. Compared to the control, the activities of IDH and FUM were increased by 34.3% and 16.8% at 48 h, respectively, whereas no significantly changes on the activities of PYC, ICL and MLS were observed. When the time of the fermentation reached 72 h, the activities of IDH and FUM were still higher than those of control, whereas the ICL activity was slightly lower, and PYC and MLS activities were comparable to control group. These results indicated that SBO might induce a strengthened TCA oxidative branch for malic acid synthesis.
In vitro enzyme activities suggested that the TCA oxidative branch for malic acid synthesis might be strengthened. If so, the intracellular energy supply should be enhanced, since the TCA oxidative branch generates NADH, FADH2 and GTP. To validate this speculation, the effect of SBO on the intracellular nucleotides associated with energy metabolism was investigated. As shown in Fig. 6, the NADH/NAD+ level was maintained at a relative high level at 0–36 h, which could provide sufficient energy for cell growth at this stage, and then, the ratio of NADH/NAD+ was decreased along with rapid PMA biosynthesis. Compared to the control, a higher NADH/NAD+ ratio was achieved after the addition of SBO, suggesting that the intracellular NADH level is high, whereas NAD+ level was low; thus, SBO promoted the transformation from NADH to NAD+ to generate ATP. The intracellular ATP level was also determined, and the ATP level was indeed higher than control group. Previous study showed that in the bio-polymerization process of PMA, the malic acid monomer is activated by the adenylation domain of PMA synthetase, malic acid was transformed to malyl-AMP, and ATP is required for this activation process [30]. Hence, the biosynthesis of PMA is regulated by intracellular ATP level, and a high ATP level is beneficial for PMA synthesis. The above results indicated that the addition of SBO increased the driving force for energy supply, which would certainly contribute to the enhanced PMA production.
The synthesis of malic acid in A. pullulans involves three pathways: (1) the TCA oxidative pathway in the mitochondria; (2) the TCA reductive branch in the cytoplasm, in which PYC converts pyruvate to oxaloacetate, and malate dehydrogenase converts oxaloacetate to malate; (3) the glyoxylate shunt, in which ICL converts isocitrate to glyoxylate, and MLS converts glyoxylate to malate [31]. Previous studies showed that the culture stress could change the carbon metabolic fluxes in the above three pathways. For example, the addition of ethanol brought about enhanced PMA yield but inhibited cell growth, and the expression levels and enzyme activities of ICL and MLS were significantly improved, indicating that glyoxylate shunt strengthened carbon flux for PMA synthesis [32]. Similar result was reported by Zeng et al. [19], the addition of sodium malonate resulted in the increased enzyme activities of ICL and MLS, whereas the activities of IDH and FUM were inhibited, and thus, the glyoxylate shunt was more active than the TCA oxidative pathway when sodium malonate was supplemented. Different from the above studies, strengthening the TCA reductive branch also led to improved PMA synthesis. For instance, Feng et al. [33] reported that when sucrose was used as a sole carbon source by A. pullulans CCTCC M2012223, the TCA reductive branch was identified as a main pathway for malic acid synthesis; thus, an engineered strain was constructed by overexpressing PYC gene, and PMA titer was increased by 15% consequently. It should be noted that the theoretical yield of malic acid in the TCA oxidative branch, TCA reductive branch and glyoxylate shunt is 1.0, 2.0 and 1.3 mol/mol glucose, respectively. The latter two pathways can generate malic acid with a higher yield; thus, these two pathways have been selected as the targets for constructing engineered strains [32, 33]. However, Wang et al. [30] reported that malic acid for PMA biosynthesis came mainly from the TCA oxidative branch in Aureobasidium melanogenum, and the present study indicated that strengthening the TCA oxidative branch was also helpful for enhanced PMA synthesis, which was probably due to that the TCA reductive branch and glyoxylate shunt is ATP neutral, whereas the TCA oxidative branch is ATP positive.
Furthermore, it should be noted that SBO is structurally similar to Tween 80, since they both contain lots of fatty acids. Tween 80 is a non-ionic surfactant which is widely used in biotechnology processes to improve product synthesis. The proposed mechanisms of Tween 80 improving product formation include increasing cell membrane permeability, changing key enzyme activities and stimulating the secretion of product [34]. Previous studies showed that Tween 80 can stimulate PMA production, the common aspects of SBO and Tween 80 acting on PMA production can be summarized as follows: (i) the consumption rate of sugar was increased; as a result, both cell growth and PMA synthesis were improved; (ii) the intracellular energy supply was improved, including a higher NADH/NAD+ ratio and a higher intracellular ATP level [15]. Although both SBO and Tween 80 acted as a chemical stimulatory agent for PMA production, there were some differences as well: (i) the effects on cell morphology are different, and A. pullulans cells became looser and bigger after adding Tween 80, but the unicells were predominate and no mycelia were observed. By contrast, the addition of SBO led to the transformation of swollen cells into mycelia; (ii) the glyoxylate shunt pathway was strengthened after adding Tween 80, whereas the TCA oxidative branch was strengthened with the addition of SBO. The molecular mechanism underlying the linkage between SBO and the TCA oxidative branch remains to be elucidated.
Conclusions
SBO is pivotal for shortening the duration of fermentation and achieving high PMA titer, and the addition of 10 g/L SBO brought about 45.5 g/L of PMA with a productivity of 0.63 g/L h in batch fermentation. SBO acted as a chemical stimulatory agent rather than a carbon source, and the enhancement on PMA production was attributed to the component of fatty acid in SBO. The supplement of SBO had significant effects on cell morphology, key enzyme activities and intracellular energy supply. After the addition of SBO, the dimorphism (yeast-like cells and mycelia) of A. pullulans was observed, which was a more productive status for PMA synthesis than the sole yeast-like cells. In vitro enzyme activities indicated that the TCA oxidative branch for malic acid synthesis might be strengthened, which could generate more ATP for PMA synthesis, and the assay of intracellular energy supply validated the deduction.
Abbreviations
- PMA:
-
Polymalic acid
- SBO:
-
Soybean oil
- TCA:
-
Tricarboxylic acid
- PYC:
-
Pyruvate carboxylase
- ICL:
-
Isocitrate lyase
- MLS:
-
Malate synthase
- IDH:
-
Isocitrate dehydrogenase
- FUM:
-
Fumarase
References
Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KL, Holler E, Ljubimova JY (2010) Inhibition of brain tumor growth by intravenous poly(β-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc Natl Acad Sci USA 107:18143–18148
Ding H, Ljubimova JY, Holler E, Black KL (2009) Poly(β-malic acid) with pendant leu-leu-leu tripeptide for effective cytoplasmic drug delivery. PCT/US2009/040252, WO/2009/126913.
Chi Z, Wang ZP, Wang GY, Khan I, Chi ZM (2016) Microbial biosynthesis and secretion of L-malic acid and its applications. Crit Rev Biotechnol 36:99–107
Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW (2007) Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677–1692
Zhang X, Wang X, Shanmugam KT, Ingram LO (2011) L-malate production by metabolically engineered Escherichia coli. Appl Environ Microbiol 77:427–434
Xia J, Xu JX, Liu XY, Xu JM, Wang XF, Li XQ (2017) Economic co-production of poly(malic acid) and pullulan from Jerusalem artichoke tuber by Aureobasidium pullulans HA-4D. BMC Biotechnol 17:20
Wei PL, Cheng C, Lin M, Zhou YP, Yang ST (2017) Production of poly(malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: Kinetics and process economics. Bioresource Technol 224:581–589
Kim BS, Hou CT (2006) Production of lipase by high cell density fed-batch culture of Candida cylindracea. Bioproc Biosyst Eng 29:59–64
Abrunhosa L, Oliveira F, Dantas D, Goncalves C, Belo I (2013) Lipase production by Aspergillus ibericus using olive mill wastewater. Bioproc Biosyst Eng 36:285–291
Chi ZM, Wang F, Chi Z, Yue LX, Liu GL, Zhang T (2009) Bioproducts from Aureobasidium pullulans, a biotechnologically important yeast. Appl Microbiol Biotechnol 82:793–804
Leathers TD, Rich JO, Anderson AM, Manitchotpisit P (2013) Lipase production by diverse phylogenetic clades of Aureobasidium pullulans. Biotechnol Lett 35:1701–1706
Youssef F, Biliaderis CG, Roukas T (1998) Enhancement of pullulan production by Aureobasidium pullulans in batch culture using olive oil and sucrose as carbon sources. Appl Biochem Biotechnol 74:13–30
Shabtai Y, Mukmenev I (1995) Enhanced production of pigment-free pullulan by a morphogenetically arrested Aureobasidium pullulans (ATCC 42023) in a two-stage fermentation with shift from soy bean oil to sucrose. Appl Microbiol Biotechnol 43:595–603
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresource Technol 232:389–397
Tu GW, Wang YK, Ji YC, Zou X (2015) The effect of Tween 80 on the polymalic acid and pullulan production by Aureobasidium pullulans CCTCC M2012223. World J Microbiol Biotechnol 31:219–226
Yin HS, Gao C, Ye K, Zhao TB, Sun AY, Qiao CS (2019) Evaluation of surfactant effect on β-poly(L-malic acid) production by Aureobasidium pullulans. Biotechnol Biotech Equip 33:954–966
Liu XY, Yu XJ, Lv JS, Xu JX, Xia J, Wu Z, Zhang T, Deng YF (2017) A cost-effective process for the coproduction of erythritol and lipase with Yarrowia lipolytica M53 from waste cooking oil. Food Bioprod Process 103:86–94
Kahar P, Tsuge T, Taguchi K, Doi Y (2004) High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym Degrad Stab 83:79–86
Zhang D, Feng XH, Li S, Chen F, Xu H (2012) Effects of oxygen vectors on the synthesis and molecular weight of poly(γ-glutamic acid) and the metabolic characterization of Bacillus subtilis NX-2. Process Biochem 47:2103–2109
Zeng W, Zhang B, Liu Q, Chen GG, Liang ZQ (2019) Analysis of the L-malate biosynthesis pathway involved in poly(β-L-malic acid) production in Aureobasidium melanogenum GXZ-6 by addition of metabolic intermediates and inhibitors. J Microbiol 57:281–287
Pereira AS, Fontes-Sant’Ana GC, Amaral PFF (2019) Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod Process 115:68–77
Zhang HL, Cai J, Dong JQ, Zhang DP, Huang L, Xu ZN, Cen PL (2011) High-level production of poly (β-L-malic acid) with a new isolated Aureobasidium pullulans strain. Appl Microbiol Biotechnol 92:295–303
Ma Y, Wang GY, Liu GY, Wang ZP, Chi ZM (2013) Overproduction of poly(β-malic acid) (PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol 97:8931–8939
Ronen M, Guterman H, Shabtai Y (2002) Monitoring and control of pullulan production using vision sensor. J Biochem Biophys Methods 51:243–249
Reeslev M, Jorgensen BB, Jorgensen OB (1993) Influence of Zn2+ on yeast-mycelium dimorphism and exopolysaccharide production by the fungus Aureobasidium pullulans grown in a defined medium in continuous culture. J Gen Microbiol 139:3065–3070
Simon L, Caye-Vaugien C, Bouchonneau M (1993) Relation between pullulan production, morphology state and growth conditions in Aureobasidium pullulnas: new observations. J Gen Microbiol 139:979–985
Campbell BS, Siddique ABM, McDougall BM, Seviour RJ (2004) Which morphological forms of the fungus Aureobasidium pullulans are responsible for pullulan production? FEMS Microbiol Lett 232:225–228
Catley BJ (1980) The extracellular polysaccharide, pullulan, produced by Aureobasidium pullulans: a relationship between elaboration rate and morphology. J Gen Microbiol 120:265–268
McNeil B, Kristiansen B, Seviour RJ (1989) Polysaccharide production and morphology of Aureobasidium pullulans in continuous culture. Biotechnol Bioeng 33:1210–1212
Wang K, Chi Z, Liu GL, Qi CY, Jiang H, Hu Z, Chi ZM (2020) A novel PMA synthetase is the key enzyme for polymalate biosynthesis and its gene is regulated by a calcium signaling pathway in Aureobasidium melanogenum ATCC62921. Int J Biol Macromol 156:1053–1063
Zou X, Cheng C, Feng J, Song XD, Lin M, Yang ST (2019) Biosynthesis of polymalic acid in fermentation: advances and prospects for industrial application. Crit Rev Biotechnol 39:408–421
Yang J, Yang WW, Feng J, Chen J, Jiang M, Zou X (2018) Enhanced polymalic acid production from the glyoxylate shunt pathway under exogenous alcohol stress. J Biotechnol 275:24–30
Feng J, Yang J, Yang WW, Chen J, Jiang M, Zou X (2018) Metabolome- and genome-scale model analyses for engineering of Aureobasidium pullulans to enhance polymalic acid and malic acid production from sugarcane molasses. Biotechnol Biofuels 11:94
Zhang BB, Cheung PCK (2011) A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuber-regium. Bioresource Technol 102:8323–8326
Acknowledgements
This work was supported by the Natural Science Foundation of China (22078122, 22008083), North of Jiangsu Science & Technology Specific Projects (SZ-HA2019005), Major Program of National Natural Science Foundation of Jiangsu Province (19KJA150010), and Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (20KJA416004)
Author information
Authors and Affiliations
Contributions
JX: investigation, methodology, writing-original draft, and editing; SL: investigation and data curation; JJ: visualization and software; ZQ: validation and funding acquisition; XL: investigation and funding acquisition; AH: resources; NX: methodology; JX: conceptualization, supervision, review & editing, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, J., Liu, S., Jiao, J. et al. Evaluation of enhancing effect of soybean oil on polymalic acid production by Aureobasidium pullulans HA-4D. Bioprocess Biosyst Eng 45, 1673–1682 (2022). https://doi.org/10.1007/s00449-022-02772-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02772-2