Abstract
The metabolically engineered plant pathogen Ustilago maydis MB215 Δcyp3 Petefria1 has been cultivated to produce more than 80 g/L itaconate in 16 L scale pH and temperature controlled fermentation, in fed-batch mode with two successive feedings. The effect of pH as well as successive rounds of feeding has been quantified via elemental balances. Volumetric itaconic acid productivity gradually decreased with successive glucose feedings with increasing itaconic titers, with nearly constant product yield. Extracellular pH was decreased from 6 down to 3.5 and the fermentation was characterized in specific uptake, production, and growth rates. Notable is that the biomass composition changes significantly from growth phase to itaconic acid production phase, carbon content increases from 42% to around 62%. Despite the gradual decrease in itaconic acid levels with decreasing pH (nearly 50% decrease in itaconic acid at pH 3.5, compared to pH 6), significant itaconate production is still observed at pH 4 (around 63 g/L). Biomass yield remained nearly constant until pH 4. Taken together, these results strongly illustrate the potential of engineered Ustilago maydis in itaconate production at commercial levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organic (weak) acids are ubiquitous in various area, ranging from biobased materials, biofuels, and medical to food applications. So far, various organic acids have been and are still being produced, e.g. citric, propionic, succinic, and adipic in both academic, and industrial studies [1,2,3].
To produce the weak organic acids, a variety of industrial workhorses both eukaryotic and prokaryotic are being used. Saccharomyces cerevisiae, enjoying the available know-how on its physiology, genome, fermentation characteristics, and recombinant DNA technology has been used to produce succinic acid [4,5,6,7]. Various other bacterial or fungal hosts (e.g. Mannheimia succinoproducens, Aspergillus niger) are also used for production of organic acids [8,9,10,11,12]. While the available genetic toolbox renders known industrial hosts (S. cerevisiae, E. coli) favorable for tailor-made production, new hosts are still highly valuable, not only to enable economically attractive production, but also to allow the discovery of potentially novel production and/or secretion pathways, thereby improving our understanding on such systems [13, 14].
Itaconic acid (IA, Methylenesuccinic acid) is a commercially important unsaturated dicarbonic organic acid, with interesting features and applications in a range of industries, in particular bio-based materials [15, 16]. It can easily be incorporated into polymers and may serve as a substitute for petrochemical-based acrylic or methacrylic acid with applications in polymer industry, papermaking, and waste water treatment [17].
Conventionally, Aspergillus terreus is used to produce itaconic acid. However, the filamentous morphology of Aspergillus species presents challenges in particular during production caused by decreased oxygen transfer, sensitivity to hydromechanical stress, and sensitivity to mg-range medium impurities [18, 19]. Alternative wild-type production hosts have been identified, e.g., Pseudozyma, Candida and Ustilago strains [20,21,22].
Ustilago strains have been shown to be a good candidates for itaconic acid production. They grow unicellularly in their haploid form (yeast-like growth), eliminating the disadvantages of filamentous fungi and making them useful for production using lignocellulosic carbon sources [17, 23]. Ustilago maydis is a Basidiomycete fungal pathogen of maize and teosinte and has been recently used to produce a variety of organic acids, glycolipids, and polyols, with several favorable characteristics such as high-stress resistance and insensitivity to medium impurities [19, 23,24,25,26,27]. Its genome (~ 20 Mb) has been recently published ([26], NCBI accession nr: GCF_000328475.2) and is larger than that of S. cerevisiae.
Itaconic organic acid production by Ustilago is affected by both the strain and culture conditions. Cultivation conditions such as temperature, pH, growth-limiting nutrients, and aeration significantly affect the product range and productivity of the organic acids. Generally, a nitrogen limitation is used to efficiently induce itaconic acid production in Ustilaginaceae [19]. Due to the activity of the promoters of itaconate cluster which are induced by nitrogen depletion, itaconate is produced along with malate and succinate after nitrogen depletion [28, 29]. Secondary growth continues also after nitrogen depletion, characterized by intracellular lipid formation leading to swollen cells and the use of intracellular nitrogen pools [17]. Under nitrogen limitation, cell composition changes and hydrocarbons accumulate, leading to higher C/N ratio with larger cell sizes [17, 19, 30]. Osmolarity causes delayed exponential growth phase and decreased maximum oxygen transfer rate, so decreased growth in high osmolarity might accelerate IA secretion [19]. Finally, pH stress causes metabolic shift from acid production to polyol and glycolipid production, so careful pH control and the selection nature of acid used as buffer are essential [19, 31].
Several recent works describe the selection and engineering of itaconic acid hyper-producing Ustilago strains. U. maydis MB215 (= DSM 17144) was selected as best production strain out of 68 Ustilaginaceae [17]. The itaconate production pathway and associated genes were characterized in this strain [32]. This knowledge was applied to enhance yield, titer, and rate through the overexpression of the transcriptional regulator ria1 and the deletion of the itaconate oxidase cyp3 [32, 33], enabling the production of up to 63 g/L itaconate at benchtop scale. Further optimization targets include the MttA mitochondrial transporter from A. terreus [34], and the control of morphology and pathogenicity through the deletion of the regulator gene fuz7, especially in the alternative host Ustilago cynodontis, which is more pH-tolerant than U. maydis [35].
An efficient, robust producer strain (high specific production rate, resilient to stress, with minimal by-products) is undoubtedly central for an economically viable fermentative production [36]. Yet, once the promising host has been obtained and tested in bench-top scale, a fermentation, and possibly downstream strategy, suitable for that host’s needs, must be adopted [37,38,39]. Considering this, the aim of this work was to design and implement a fermentation strategy to produce around 80 g/L itaconic acid using previously obtained metabolically engineered U. maydis MB215 Δcyp3 Petefria1 [32] cells using glucose as the carbon source. The fermentation characteristics of the cells have been quantified by yield, final titer, and the so-called qRates, i.e. specific substrate consumption, itaconate production, growth rates as well as specific oxygen uptake (OUR) and carbon dioxide production (CPR) rates. Related to that, the elemental balances for carbon, nitrogen as well as degree of reduction are used to assess the quality of the fermentation process.
Materials and methods
Strains
Wild-type U. maydis MB215 (DSM 17144) used in this study was obtained from DSMZ (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures) culture collection; recombinant strain U. maydis MB215 ∆cyp3 Petef ria1 was constructed by Geiser et al. [32, 40]. The strains were stored at – 80 ℃ in 80% (v/v) glycerol stocks. Stock cultures were grown on YEPS medium consisting of 10 g yeast extract, 20 g peptone, and 20 g sucrose per liter and 20 g agar for solid media.
Growth, production, and feeding media
Pre-cultivation
Cells, from glycerol stocks, were activated on YEPS agar plates for 24 h at 30 ℃. For the preparation of the bioreactor inoculum, a two-stage liquid precultivation procedure was used. In the first stage, cells were inoculated to YEPS liquid medium incubated in shake-flasks at 30 ℃ for 24 h. Afterwards, Modified Tabuchi Medium (MTM) [23], consisting of 30 g/L glucose, 1.6 g/L NH4Cl, 0.5 g/L KH2PO4, 0.41 g/L MgSO4·7H2O, 1 g/L yeast extract, 0.14 g/L FeSO4·7H2O was inoculated with 50 mL YEPS grown cells for a final volume of 300 mL in each shake-flask. After 24 h at 30 0C, precultures were inoculated to the bioreactor to achieve an initial OD600 of 1.0.
Cultivation at 16 L Bioreactor
Bioreactor scale fermentations were performed using a production medium containing approximately 190 g/L glucose, 4 g/L NH4Cl, 1 g/L yeast extract, 0.2 g/L MgSO4·7H2O, 0.01 FeSO4·7H2O, 0.5 g/L KH2PO4, 1 mL/L vitamin solution, and 1 mL/L trace element solution [32]. The vitamin solution containing 0.05 g/L D-biotin, 1 g/L D-calcium panthotenate, 1 g/L nicotinic acid, 25 g/L myo-inositol, 1 g/L thiamine hydrochloride, 1 g/L pyridoxol hydrochloride, 0.2 g/L para-aminobenzoic acid, and trace element solution containing 1.5 g/L EDTA, 0.45 g/L ZnSO4·7H2O, 0.10 g/L MnCl2·4H2O, 0.03 g/L CoCl2·6H2O, 0.03 g/L CuSO4·5H2O, 0.04 g/L Na2MoO4·2H2O, 0.45 g/L CaCl2·2H2O, 0.3 g/L FeSO4·7H2O, 0.10 g/L H3BO3, and 0.01 g/L KI were used as previously described by Geiser et al. [17]. Glucose additions were made by a sterile stock glucose solution with a concentration of 700 g/L. All chemicals were purchased from Merck (Germany), Sigma-Aldrich (USA), VWR BDH Chemicals (USA) and BD Difco (USA).
Fermentation conditions
Fermentations were conducted in 16-L stainless steel stirred tank bioreactor (NLF22, Bioengineering, Switzerland) with a cooler at the air outlet to avoid evaporation and having rushton 6-blade impellers. Initial working volume was used as 8 L where temperature was controlled at 30 0C. During fermentations, dissolved oxygen levels were maintained above 30% by controlling the stirrer speed between 700–900 rpm while the aeration rate was fixed at 3 L/min where 0.3–0.4 vvm was achieved. pH was adjusted by automatic addition of 10 M NaOH (Merck, Germany) to pH 6 for the development of the feeding strategy and the wild-type productions, whereas for the investigation of the effects of pH, pH 3.5, 4, 5, and 6 were used.
Analytical methods
Quantification of cell dry weight and cell composition
At selected time intervals, fermentation broth was centrifuged at 10,000 rpm for 10 min to separate cells from the fermentation medium. Cell dry weights were measured by drying to constant weight with an electrical moisture analyzer (MA 150Q, Sartorius, Germany) at 105 °C. Cell C, H, N contents were analyzed by Dumas method using LECO Truspec CHN system (LECO, USA); ash contents were determined by a laboratory chamber furnace (CWF 1200, Carbolit Gero, UK) with a method of heating the dried cell sample to 600 ℃ for 16–18 h until constant weight achieved.
Quantification of sugars, organic acids, nitrogen, and phoshate
For the quantification of the sugars, organic acids and nitrogen in the media, supernatant samples were filtered through a 0.45-µm filter (Minisart RC, Sartorius, Germany). Itaconic acid, glucose and other acid concentrations were analyzed by HPLC (1100 Series, Agilent Technologies, USA). Total nitrogen and ammonia concentrations were determined by Kjeldahl and distillation-titration methods, respectively [41]. Phosphate contents were analys***ed by a colorimetric method using LCK 349 Phosphate Kit produced by HACH LANGE, Germany. Osmotic pressure was determined by an automatic cryoscopic osmometer (OSMOMAT 030, Gonotec, Germany). Dissolved CO2 concentrations were estimated via alkalinity measurements as CaCO3 equivalent using acidimetric-titration method in which the samples were titrated with 0.1 N H2SO4 to pH:4.0. Due to the presence of high levels of itaconic (and other organic) acid(s), this alkalinity measurement needs to be corrected. Focusing on the itaconic acid, CaCO3 alkalinity is total alkalinity minus the alkalinity due to itaconic acid as follows:
where \(\pi_{pH = 4}\) is the fraction of undissociated itaconic acid at pH 4 equal to 0.4, and second term is the ratio of molecular weights of CaCO3 to itaconic acid, corrected for their valence, equal to 0.77. In the above equation, all entities are in g/L.
Results
Comparison of wild-type and mutant strains for itaconic acid production fermentation
U. maydis MB215 WT and the Δcyp3 Petefria1 strain were cultivated in fed-batch mode, to compare the growth and itaconic acid production performance. Overall, following the Total Kjehldahl Nitrogen (TKN) decrease in the first 48 h, the biomass levels stabilizes around 25–30 g/L, and the glucose fed is used for acid production in both WT and mutant strain. The glucose consumption profile is found to be similar for both strains. As for organic acid production, WT strain produces mixed organic acids, while the mutant strain produce itaconic acid without detectable byproducts (Fig. 1). In HPLC profile of the supernatant, a peak that does not match the available organic acid standards (succinic, malic, itaconic, fumaric, citric acids) is obtained and following Geiser et al. [32] the peak is attributed to 2-hydroxyparaconate and values should be taken as rough estimates only.
Comparison of the fermentation and itaconic acid production profile for the wild-type (blue lines in all plots) and Δcyp3 Petefria1 mutant (black lines in all plots) strains of Ustilago maydis. a glucose, biomass (filled circles), and TKN levels (squares), b itaconic acid, c organic acids produced other than itaconic acid. The mutant has no significant acid production other than itaconic acid. Shown are representative data from single fermentations (color figure online)
Fermentation strategy: feeding time, number of feeding cycles
The following experiments were performed with U. maydis MB215 Δcyp3 Petefria1 cells to test itaconic acid production in 16 L bioreactor in fed-batch mode. Based on the results of preliminary experiments in batch mode with various glucose concentrations, no significant substrate inhibition was observed as the initial glucose uptake rate was the same for the substrate range studied (50–200 g/L, data not shown). Following this, pulse feeding was adopted. Figure 2 represents the results of a typical fed-batch itaconic acid fermentation with five feeding cycles.
Glucose was pulse-fed into the fermenter when, residual glucose concentration was around 5–10 g/L as consuming the remaining glucose would result in extended fermentation time and repeated feeding also dilutes the product, being collectively undesired features. The specific glucose consumption rate changes minimally in the first two cycles, while in the third cycle, all three specific q-rates (growth, glucose consumption and itaconate production) decrease significantly while the yield was nearly constant. Based on this data, the base case scenario is set to two feeding cycles, corresponding to approximately 48th and 96th hours for the first and second pulse feeds.
For the base case scenario, U. maydis MB215 Δcyp3 Petefria1 cultivated at pH 6, with two pulse feeding cycles, the fermentation profile including the offline and online data is given in Fig. 3. Overall, by using the described fermentation strategy, a commercially relevant itaconic acid level (80–90 g/L) is reached within 150 h with two feeding cycles. Furthermore, the strain produces no other significant organic acids. Biomass is produced in the first 50 h (in line with TKN measurements), later sustaining constant level around 21–22 g/L. As for the offgas data, the CO2 level gradually increases due to intensive growth at the beginning followed by a decrease during itaconate production at the second stage after 48 h (Fig. 3). Overall, the substrate is only used for production of biomass, itaconate, and cellular maintenance, with the carbon balance being closed with a gap of less than 10%.
Fermentation data on fed-batch cultivation of U. maydis MB215 Δcyp3 Petefria1 for the base case scenario (pH 6, feeding with two successive substrate pulses) as a glucose (circles), itaconic acid (squares), biomass (diamonds) in g/L, and TKN (filled circles) in g/L and b off-gas data as CO2 and O2 fractions of the offgas, the upper panel plots the working volume of fermentor (L), indicating each feeding time
Changes in the elemental composition of biomass:
Itaconic acid fermentation with U. maydis consists of two distinct stages: (1) biomass growth and itaconic production and (2) itaconic acid production alone. Throughout the fermentation, the morphology of the cells changes. At the initial stage of the fermentation, the cells are yeast-like, while at a later stage, they elongate and transform into longer filaments. Additionally, the TKN measurements over time yielded on average a lower nitrogen content than what can be expected from an average biomass composition (CH1.65O0.54N0.14 [42]). This is apparent as a gap between TKN and biomass measurements, which further resulted in a gap in carbon and degrees of reduction balances. To understand this, we quantified C, N, and H content of U. maydis biomass samples taken from different stages of the fermentation (Fig. 4). When the cells cease secondary growth, exhibited by relatively constant CDW measurements (IA production only, later than 48th hour) the carbon content of the cells increased by 47% (from 0.42 to 0.62), while the nitrogen content decreased by 28% (from 0.053 to 0.038). Such a decrease in nitrogen content of biomass has been previously pointed by von Stockar and Liu, where algea, typically storing oils, contain considerably less nitrogen than average yeast [42, 43]. In our current work, the reducing power of biomass, calculated from the determined elemental composition, increases. The increase is due to increased carbon percentage and this increase points to lipid accumulation, in line with previous studies with Ustilago maydis under nitrogen starvation conditions [44,45,46].
Effect of pH on itaconic acid production under successive pulse-feeding
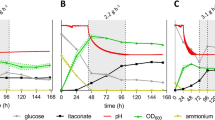
To observe the impact of cultivation at low pH on growth and itaconic acid production, cells were cultivated at pH 6, 5, and 4 and the fermentation is monitored both in its growth and itaconic production as well as corresponding rates (Fig. 5). The carbon- and degree of reduction balances are also reported (Fig. 6a). Overall, the recoveries are within accepted ranges, i.e. within 10–15% margin. Remaining carbon and degree of reduction gap is attributed to visually observed glycolipids secreted to fermentation medium, also reported in the literature [44, 45].
The effect of extracellular pH on the growth and itaconic acid production of U. maydis MB215 Δcyp3 Petefria1. Left panel describes the measured concentrations of glucose, biomass, and itaconic acid while the right panel depicts the calculated biomass specific substrate consumption, growth, and itaconic acid production rates. In both panels, the extracellular pH was kept at 6 (black),5 (blue),4 (red), and 3.5 (green). Despite higher specific glucose uptake rate, the cells grow and produce itaconic acid at an approximately same rate, pointing the fact that the excess glucose would be used for maintenance due to weak acid stress. Fermentation were performed in at least duplicates, error bars depict the standard deviation from the mean (color figure online)
Probing the response to weak acid stress in U. maydis. a Carbon and Degrees of Reduction recoveries for the corresponding fermentations at different extracellular pH. The relative contributions of biomass (black), itaconic acid (white), carbon dioxide (blue), and oxygen (red) for each balance is given. b offgas data from U. maydis MB215 Δcyp3 Petefria1 fermentations at different extracellular pH conditions. Colors correspond to Fig. 5 (color figure online)
The general trend is that, as pH decreases, U. maydis cells grow slower and produce less itaconic acid (Fig. 5), in line with several previous reports with different hosts. In the experiments reported here, the biomass level after 48 h decreased around 3.5 gDW/L for pH from 6 to 5, while the biomass dropped more than 10 gDW/L for pH from 6 to 4, the change being statistically significant considering the batch-to-batch difference as well (p = 5.4 10–4).
Overall, as the pH decreases, a decrease in yields of biomass and itaconic acid is observed (Fig. 6a), until pH 4. The cells, upon acid stress, consume more oxygen, and produce more CO2 (higher specific OUR and CPR), which is expected since the cells would need additional energy for maintenance of their pH homeostasis (Fig. 6) [13]. Interestingly, at pH 3.5, growth rate and biomass yield are comparable if not higher than those at pH 6; however the itaconic acid production rates as well as itaconic yields are considerably lower.
Effect of nutrient limitation on itaconic acid production
Generally, organic acids are produced under nitrogen and/or phosphate limitation to control the growth and thereby redirect carbon flow to the desired product. In this respect, itaconic acid production by A. terreus is reported to work optimally under phosphate-limited conditions at sugar concentrations between 100 and 150 g/L [16] though Krull et al. reported similar itaconic acid yields under various phosphate conditions [47]. In contrast, nitrogen limitation is reported to induce itaconate production in U. maydis [28]. Indeed, at the initial stage of the fermentations with U. maydis MB215 Δcyp3 Petefria1 (first 24 h), the cells mainly grow while nitrogen levels decrease, followed by the production phase where mainly itaconic acid is produced (Figs. 1 and 3). Soon after the second glucose feeding, we detected significant amounts of nitrogen and phosphate in the medium, possibly due to protein secretion, which is known to occur in U. maydis cultures with similar conditions [48] (Fig. 7). From thereon, despite no apparent nutrient limitation, the cells continue itaconic acid production in the presence of phosphate and nitrogen, while keeping the biomass level constant. Last, the dissolved CO2 level, measured via the net CaCO3 alkalinity illustrates that, near neutral pH there is enough dissolved CO2 in the medium that the cell may use, e.g. for increased PEP carboxykinase or pyruvate carboxylase activity, two enzymes are typically targeted to increased organic acid production [49, 50].
a–b Dynamics of Nitrogen and Phosphate levels for different extracellular pH levels. c–d Alkalinity and Osmotic pressure as a function of extracellular pH. In both panels, the extracellular pH is kept at 6 (black), 5 (blue), 4 (red), and 3.5 (green). For CaCO3 alkalinity, the experiments at pH 3.5 are omitted as the titration in alkalinity test is performed down to pH 4, beyond which no bicarbonate is present in the medium. Fermentation were performed in at least duplicates, error bars depict the standard deviation from the mean (color figure online)
Discussion
In this work, we reported the production of over 80 g/L itaconic acid, using U. maydis MB215 Δcyp3 Petefria1 in pulse fed-batch mode with two successive feedings, and over 110 g/L with five successive feedings. We compare the obtained fermentation performance with earlier works, whereby A. terreus is used for itaconic acid production at (near-)commercial levels [47, 51, 52] (Table 1). Taken together, significant levels of itaconic acid are produced using this fermentation process in a relatively short fermentation time with high volumetric productivity. Surprisingly, compared to previously published U. maydis MB215 Δcyp3 Petefria1 fermentations at benchtop scale [32], the yield and productivity in the current work is higher, albeit being similar in scale (around tenfold increase 1.3 L in [32] vs. 16 L, this work). The exact reason for this large difference is currently unknown, but may be related either to small differences in the pre-cultivation such as the omission of MES buffer and lower glucose concentration, or to scale-up effects which may lead to local heterogeneities in oxygen supply. Especially the latter may have a significant effect on itaconic acid production, as was previously shown fermentations of the closely related Pseudozyma under a regime of oscillating dissolved oxygen concentration [53].
In analyzing the fermentation data with successive glucose feeds, the glucose consumption as well as itaconate production rates of the U. maydis MB215 Δcyp3 Petefria1 cells in each feeding cycle is important parameters, since they allow to decide whether successive pulse feeding is possible, with the same biomass. We noted that after second glucose feed, despite nearly constant yield, the volumetric productivity decreases, gradually with increasing itaconic titers.
A recent study by Hosseinpour et al. reported 220 g/L IA titer with engineered Ustilago maydis cells when the strain is used in a fermentation with in situ product crystallization with CaCO3 [54]. Such an approach focuses to lift the weak acid inhibition at the expense of solids accumulating in fermentation vessel. Our work focuses on upstream fermentation optimization for IA production, without any additional separation process. The findings can further be combined with further separation techniques, e.g. in situ product removal techniques [55, 56].
The observed change in cell morphology is in line with previous observations [40] with U. maydis cells and is likely due to the stress-induced formation of conjugation tubes [26]. It should be noted that in its haploid form, Ustilago maydis grows like yeast, yet when mating with compatible yeasts, each forms a conjugation tube, to form a pathogenic dikaryotic mycelia, in particular under pH stress [57].
Following this, to understand more the changes in the cell, we quantified the elemental composition of biomass during the itaconic acid fermentation and showed that the carbon content increases by up to 50% during the itaconate production phase, when compared to exponential growth phase, attributed to lipid accumulation.
The desired form of organic acids for further downstream processing is the undissociated acid form, which is predominant at low pH (pKa1 = 3.81, pKa2 = 5.45, Supplementary Figure S1, [58]). This undissociated form generates weak acid stress on the cells, it can diffuse through the membrane into the cell, where it dissociates and causes intracellular acidification [51]. The cytosolic acidification changes the kinetics of enzyme-catalyzed reactions, and, therefore, cell needs to secrete the excess protons, at the expense of ATP in order to maintain pH homeostasis [59]. The effect of extracellular pH on the growth and production of U. maydis cells has been quantitatively analyzed in terms of fermentation profile as well as estimates of maintenance energy requirements.
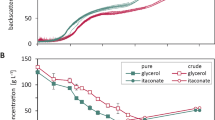
It was thus far often presumed that U. maydis does not produce itaconate below a pH of 5. This presumption was mainly based on wild-type data of shaken cultures with limiting concentrations of MES buffer [17, 35], where wt U. maydis stopped acid production once the pH dropped below 5. In optimized strains of U. maydis MB215, this lower limit of acid production is reduced to as far as 3.5, likely though an increase in the specific production rate. It should be noted that, as observed above for the base case, these scaled up culture produced significantly higher itaconate titers than previously obtained in smaller scale bioreactors. Even with identical cultures conditions, a scale-down to 1.6 L, performed in a different lab, yielded only 11 g/L of itaconate at a pH of 4 (Supplementary Figure S2), compared to 68 g/L at 16 L scale. The reason for this large difference may be the abovementioned scale-up effects on oxygen supply, or subtle differences in medium components, which will be the subject of further study.
An interesting observation was on nitrogen and phosphate levels after the second glucose pulse, while biomass level is observed to be constant. Speculating that the constant level of biomass is the result of continuous growth and lysis, the energy drain particularly in the second half of the fermentation can be attributed to growth-associated maintenance, in addition to the increased non-growth associated maintenance due to weak acid stress. Overall, this work is a stepstone in characterizing fermentation performance of a promising itaconic producing host U. maydis under industrially relevant conditions.
References
Piper P et al (2001) Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147(10):2635–2642
Coban HB (2020) Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng 43(4):569–591
Selder L et al (2020) Co-cultures with integrated in situ product removal for lactate-based propionic acid production. Bioprocess Biosyst Eng 2020:1–9
Otero JM et al (2013) Industrial systems biology of Saccharomyces cerevisiae enables novel succinic acid cell factory. PLoS ONE 8(1):e54144
Raab AM et al (2010) Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metab Eng 12(6):518–525
Schuller C et al (2004) Global phenotypic analysis and transcriptional profiling defines the weak acid stress response regulon in Saccharomyces cerevisiae. Mol Biol Cell 15(2):706–720
Wang B et al (2017) High-efficient production of citric acid by Aspergillus niger from high concentration of substrate based on the staged-addition glucoamylase strategy. Bioprocess Biosyst Eng 40(6):891–899
Ikram-ul H et al (2004) Citric acid production by selected mutants of Aspergillus niger from cane molasses. Biores Technol 93(2):125–130
Khurshid S et al (2001) Mutation of Aspergillus niger for hyperproduction of citric acid from black strap molasses. World J Microbiol Biotechnol 17(1):35–37
Liu Y-P et al (2008) Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Biores Technol 99(6):1736–1742
Magnuson JK, Lasure LL (2004) Organic acid production by filamentous fungi, in Advances in fungal biotechnology for industry, agriculture, and medicine. Springer, Boston, pp 307–340
Song H, Lee SY (2006) Production of succinic acid by bacterial fermentation. Enzyme Microbial Technol 39(3):352–361
Wierckx N et al (2019) Metabolic specialization in itaconic acid production: a tale of two fungi. Curr Opin Biotech 62:153
Ju J-H et al (2020) Production of adipic acid by short-and long-chain fatty acid acyl-CoA oxidase engineered in yeast Candida tropicalis. Bioprocess Biosyst Eng 43(1):33–43
Okabe M et al (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84(4):597–606
Willke T, Vorlop KD (2001) Biotechnological production of itaconic acid. Appl Microbiol Biotechnol 56(3–4):289–295
Geiser E et al (2014) Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol Biotechnol 1(1):2
Karaffa L et al (2015) A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl Microbiol Biotechnol 99(19):7937–7944
Klement T et al (2012) Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb Cell Fact 11(1):43
Hossain A et al (2019) Metabolic engineering with ATP-citrate lyase and nitrogen source supplementation improves itaconic acid production in Aspergillus niger. Biotechnol Biofuels 12:233
Levinson WE, Kurtzman CP, Kuo TM (2006) Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microbial Technol 39(4):824–827
Tabuchi T et al (1981) Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric Biol Chem 45(2):475–479
Maassen N et al (2014) Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng Life Sci 14(2):129–134
Feldbrügge M, Kellner R, Schipper K (2013) The biotechnological use and potential of plant pathogenic smut fungi. Appl Microbiol Biotechnol 97(8):3253–3265
Holliday R (1974) Ustilago maydis, in Bacteria, Bacteriophages, and Fungi. Springer, Boston, pp 575–595
Kämper J et al (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444(7115):97
Regestein L et al (2018) From beech wood to itaconic acid: case study on biorefinery process integration. Biotechnol Biofuels 11:279
Zambanini T et al (2017a) Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fungal Biol Biotechnol 4:11
Zambanini T et al (2017b) Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol Biofuels 10(1):131
Aguilar LR et al (2017) Lipid droplets accumulation and other biochemical changes induced in the fungal pathogen Ustilago maydis under nitrogen-starvation. Arch Microbiol 199(8):1195–1209
Rychtera M, Wase DAJ (1981) The growth of Aspergillus terreus and the production of itaconic acid in batch and continuous cultures. The influence of pH. J Chem Technol Biotechnol 31(1):509–521
Geiser E et al (2016a) Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab Eng 38:427–435
Geiser E et al (2018) Evolutionary freedom in the regulation of the conserved itaconate cluster by Ria1 in related Ustilaginaceae. Fungal Biol Biotechnol 5:14
Hosseinpour Tehrani H et al (2019a) The interplay between transport and metabolism in fungal itaconic acid production. Fungal Genet Biol 125:45–52
Hosseinpour Tehrani H et al (2019b) Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Metab Eng 54:293–300
Straathof AJJ et al (2019) Grand research challenges for sustainable industrial biotechnology. Trends Biotechnol 37(10):1042–1050
Gorden J et al (2017) Integrated process development of a reactive extraction concept for itaconic acid and application to a real fermentation broth. Eng Life Sci 17(7):809–816
Kim BS et al (2004a) High cell density fed-batch cultivation of Escherichia coli using exponential feeding combined with pH-stat. Bioprocess Biosyst Eng 26(3):147–150
Huang M et al (2020) Two-stage pH control combined with oxygen-enriched air strategies for the highly efficient production of EPA by Mortierella alpina CCFM698 with fed-batch fermentation. Bioprocess Biosyst Eng 43:1725
Geiser E et al (2016b) Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol 9(1):116–126
Bradstreet R (1965) The Kjeldahl method for organic nitrogen. Academic Press, Cambridge
Von Stockar U, Liu JS (1999) Does microbial life always feed on negative entropy? Thermodynamic analysis of microbial growth. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1412(3):191–211
Ali SM et al (2005) Production of biofertilizers using baker’s yeast effluent and their application to wheat and barley grown in north Sinai deserts: (Produktion von Biodüngern unter Verwendung von Backhefeabwasser und ihre Anwendung zu Weizen-und Gerstenanbau im Norden der Sinai-Wüste). Archiv Agron Soil Sci 51(6):589–604
Hewald S et al (2006) Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus Ustilago maydis. Appl Environ Microbiol 72(8):5469–5477
Juárez-Montiel M et al (2011) Huitlacoche (corn smut), caused by the phytopathogenic fungus Ustilago maydis, as a functional food. Revista iberoamericana de micologia 28(2):69–73
Lemieux R (1953) Biochemistry of the Ustilaginales: VIII. The structures and configurations of the ustilic acids. Can J Chem 31(4):396–417
Krull S et al (2017) Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biotechnol 101(10):4063–4072
Geiser E et al (2013) Identification of an endo-1,4-beta-xylanase of Ustilago maydis. BMC Biotechnol 13:59
Kim P et al (2004b) Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl Environ Microbiol 70(2):1238–1241
Vemuri GN, Eiteman MA, Altman E (2002) Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68(4):1715–1727
Hevekerl A, Kuenz A, Vorlop K-D (2014) Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol 98(24):10005–10012
Kuenz A et al (2012) Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol 96(5):1209–1216
Park YS, Ohta N, Okabe M (1993) Effect of dissolved oxygen concentration and impeller tip speed on itaconic acid production by Aspergillus terreus. Biotech Lett 15(6):583–586
Tehrani HH et al (2019) Integrated strain-and process design enable production of 220 g L− 1 itaconic acid with Ustilago maydis. Biotechnol Biofuels 12(1):1–11
Saboe PO et al (2018) In situ recovery of bio-based carboxylic acids. Green Chem 20(8):1791–1804
Eggert A et al (2019) Integrated in-situ product removal process concept for itaconic acid by reactive extraction, pH-shift back extraction and purification by pH-shift crystallization. Sep Purif Technol 215:463–472
Aréchiga-Carvajal ET, Ruiz-Herrera J (2005) The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot Cell 4(6):999–1008
Bhattacharyya L, Rohrer JS (2012) Applications of ion chromatography for pharmaceutical and biological products. John Wiley & Sons, Hoboken
Mira NP, Teixeira MC, Sรก-Correia I (2010) Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: a genome-wide view. Omics J integr Biol 14(5):525–540
Acknowledgement
This work has been financially supported by TUBITAK (The Scientific and Technological Research Council of Turkey), within International Industrial R&D Projects Grant Programme (Teydeb 1509—Project number: 9150147). The work of NW, HHT and JB was funded by the German Federal Ministry of Food and Agriculture (BMEL), through the Specialist agency renewable raw materials e. V. (FNR) (FKZ 22030515). The study was part of the TTRAFFIC project under the ERA-IB-2 6th Call (Project number: ERA-IB-15-011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demir, H.T., Bezirci, E., Becker, J. et al. High level production of itaconic acid at low pH by Ustilago maydis with fed-batch fermentation. Bioprocess Biosyst Eng 44, 749–758 (2021). https://doi.org/10.1007/s00449-020-02483-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02483-6