Abstract
Itaconic acid is mainly produced with the filamentous fungi Aspergillus terreus. An increase in the pH during the production phase of the cultivation resulted in an increase in the itaconic acid concentration. The pH was raised by a single pH shift ranging from pH 4 to 6 or by a pH control to pH 3. Different lyes can be used for the pH shift, but ammonia solution has proven to be the best, because here the productivity does not drop after the pH shift. The highest itaconic acid concentration of 146 g/L was reached when a pH control to pH 3 was started after 2.1 days of cultivation. This is an increase of 68 % to the cultivation without pH control. When this technique was combined with previously found optimizations, a final itaconic acid concentration of 129 g/L was reached after 4.7 days of cultivation, resulting in a productivity of 1.15 g/L/h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
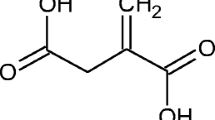
Itaconic acid is one of the most interesting chemicals derived from biomass. It can be used as a platform chemical for the chemical industry. The use of itaconic acid in various applications, which are summarized in Willke and Vorlop (2001) and Okabe et al. (2009), can help the transition from fossil fuels to renewable resources. The fermentative process was first discovered by Kinoshita using Aspergillus itaconicus (Kinoshita 1932). In the following years, Aspergillus terreus was discovered to be a better producer. Chemical processes for itaconic acid production exist, but they cannot compete with the fermentative production route (Tate 1981).
The fermentation process has been established since the 1960s. There has been a lot of research since, but no large increase in the final itaconic acid concentration could be reached. This and the low productivity is a big disadvantage of the process. These problems need to be solved to make the process economical.
The influence of the pH on the cultivation of A. terreus was investigated several times, but with inconsistent results. The initial pH used in cultivations ranges from pH 2.0 (Park et al. 1993; Yahiro et al. 1995) to pH 5.9 (Gyamerah 1995). Studies examining the effect of the initial pH came to different results: depending on the used conditions and strains, the optimal initial pH ranged from pH 2.5 (Kautola 1990) to pH 3.1 (Rychtera and Wase 1981). The same applies for the pH control. While some authors found the highest product concentrations when the pH was kept at pH 2.8 (Riscaldati et al. 2000), others found the best product formation at pH 2.1 to 2.2 (Lockwood and Reeves 1945; Rychtera and Wase 1981).
The goals of our study were to examine the influence of the pH on the itaconic acid production with A. terreus and to achieve a higher product concentration. Also, the productivity was increased by combining different previously found optimizations and the one from this study.
Material and methods
Organism
The organism used was a self-screened filamentous fungus. It was identified as A. terreus and is stored at the DSMZ as DSM-23081.
For long-term storage, a glycerol stock culture was prepared. Spore suspension obtained from the surface culture was mixed in equal parts with pure glycerol, shock frozen in liquid nitrogen, and stored at −80 °C.
Inoculum production
Conidia were used for the cultivations, which were derived from two different cultivation methods: surface cultivation and submerged cultivation. If not stated otherwise, conidia obtained from surface cultivation were used.
Spores from a frozen culture were plated on Czapek-Dox-Agar (Merck), incubated for 7 days at 30 °C, and harvested with a NaCl solution (0.9 % w/v).
A cultivation in the 1.5 L bioreactor was performed for the production of spore suspension from submerged cultivation. After 3 days of cultivation, a single pH shift to pH 5 was performed, using NaOH as lye. After an additional 2 days of cultivation, the formed spores were separated from the mycelia by a sieve with a mesh width of 80 μm. The spore suspension was centrifuged at 10,000g for 10 min and washed with 0.9 % NaCl (w/v) solution twice.
The concentration of the spore suspensions was determined with a Thoma counting chamber at a Zeiss microscope (Axioplan, Carl Zeiss AG, Germany).
Media composition
If not mentioned otherwise, the following medium was used: 180 g/L glucose, 0.1 g/L KH2PO4, 3 g/L NH4NO3, 1 g/L MgSO4·7H2O, 5 g/L CaCl2∙2H2O, 1.67 mg/L FeCl3∙6H2O, 8 mg/L ZnSO4∙7H2O, and 15 mg/L CuSO4∙5H2O. For the optimized medium, the KH2PO4 concentration was increased to 0.8 g/L. A separate stock solution was made for every media component and the pH was adjusted to pH 3.1 with 0.5 M H2SO4; the separate stock solutions were autoclaved. The pH of the CaCl2 solution was not adjusted, and the FeCl3 solution was sterile filtered. The sterile solutions were combined aseptically. Glucose was added if necessary in solid form in portions of 35 g/L during the cultivations without previous sterilization.
Cultivation
The medium was inoculated with a spore suspension to a spore concentration of 1 × 106 spores/mL.
The cultivation in round 96-well microtiter plates (Sarstedt, Germany) was performed as previously described (Hevekerl et al. 2014). The filling volume was 100 μL and the plate was sealed with a lid and Parafilm (Pechiney Plastic Packaging Company, USA). Due to evaporation, the two outer rows of wells were filled with water and not used for analysis. The plates were incubated at 950 rpm and 33 °C with a shaking diameter of 2.5 mm (Kisker, Germany). For every sample point, one of the single parallel microtiter plates was used.
Cultivations in the 400 mL parallel bioreactor system (DASGIP, Germany) were performed at 400 rpm and a filling volume of 200 mL. Cultivations in the custom-made 1.5 L parallel bioreactor system were performed at 470 rpm and a filling volume of 1 L.
In both bioreactor systems, the online signals of dissolved oxygen, pH, and redox were monitored by the DASGIP Control software (DASGIP, Germany). The cultivation was started at 100 % dissolved oxygen and was controlled at 25 % dissolved oxygen by the variation of the air flow rate. Antifoam (Ucolup N-115, Brenntag Mühlheim/Ruhr, Germany) was added after 1 day of cultivation to inhibit foaming.
The pH was left free to change in the standard cultivations. When performing a single pH shift the pH was manually adjusted by the addition of NaOH (5 M), KOH (5 M), or ammonia solution (6.25 %). For pH regulation, the pH was monitored and adjusted by the DASGIP Control software.
Analytical methods
The sample was centrifuged at 21,000g for 25 min at 20 °C. The supernatant was kept for further analysis. The pellet was washed twice with deionized water and dried at 105 °C for at least 48 h and weighed to determine the cell dry weight. During microtiter plate cultivation, the whole content of a well was used for sampling and centrifuged as above.
The analysis of glucose and organic acid concentrations was performed by high-performance liquid chromatography (HPLC). A Shimadzu (Shimadzu Corp., Japan) HPLC equipped with a HPX87H column (BioRad, Germany) was used at a flow rate of 0.6 mL/min, 40 °C, and 5 mM H2SO4 as mobile phase. The detection was performed using a refractive index detector and UV detector at 210 nm.
The determination of the phosphate, nitrate, and ammonium concentrations was performed with an ICS-1000 ion chromatography (Dionex, USA). For the detection of cations, an IonPac NG1 precolumn (4 × 35 mm), an IonPac CS12 column (4 × 250 mm), 8 mM H2SO4 as mobile phase with a flow of 1 mL/min, and a Cation Self-Regenerating Suppressor (4 mm) were used. For the detection of anions, an IonPac AS11-HC precolumn (4 × 50 mm), an IonPac AS11-HC column (4 × 250 mm), 25 mM NaOH as mobile phase with a flow of 1 mL/min, and an Anion Self-Regenerating Suppressor (4 mm) were used. The columns had room temperature and the signals were detected by a conductivity detector.
Microscopy
For microscopic analysis, the cells were examined with a phase-contrast microscope (Axioplan, Carl Zeiss AG, Germany). The pictures were processed with the software analySIS pro (5.1, Olympus Soft Imaging Solutions GmbH).
Determination of solubility
The solubility of itaconic acid was determined by a saturated solution. For this purpose, cell-free fermentation broth from the end of a standard cultivation was used. Itaconic acid was added in solid form and stirred until an equilibrium occurred. The pH was raised to different values by the addition of ammonia solution (25 %), and the temperature was kept constant at 33 °C. Samples were taken and immediately centrifuged for 30 s at 20,817g to remove the undissolved crystals and diluted for analysis.
Results
Influence of initial pH
In the literature, the initial pH values for the itaconic acid production with A. terreus are varying immensely. They range from pH 2 (Park et al. 1993; Yahiro et al. 1995) up to pH 5.9 (Gyamerah 1995).
To determine whether the initial pH had an influence on the production in the used medium and strain, it was varied from pH 1.9 to 4.9 (Fig. 1). The cultivations with the initial pH values from pH 2.9 to 4.9 showed similar behavior. After 2 days of cultivation, the pH had dropped to pH 2.1 and the itaconic acid production had started. At the lower pH values of 1.9 and 2.4, the growth and product formation were delayed. This was especially so at pH 1.9. Here, the spores germinated after 2 days of cultivation, which was about twice the time the higher pH values needed. The itaconic acid concentrations of pH 2.4 to 4.9 were very close after 7 days of cultivation. They ranged from 66 to 68 g/L itaconic acid. Despite the late germination, the culture with the initial pH of 1.9 showed a normal itaconic acid production and reached 60 g/L after 7 days of cultivation.
Because the variation in the initial pH showed no influence in the higher pH values, pH 3.1 was maintained as the initial pH.
pH shift
So far, the highest itaconic acid concentration of 90 g/L was achieved by using an unbuffered medium and no pH control (Kuenz et al. 2012). Those cultivation parameters were adapted to a 400 mL bioreactor system and optimized by the variation of the agitation speed. At the agitation of 400 rpm, the highest itaconic acid concentration of 100 g/L was achieved. The pH dropped from the initial pH 3.1 during the growth phase to pH 1.8 and remained at this value.
We found that a single pH shift during a cultivation of A. terreus led to a considerable increase in the final itaconic acid concentration (Fig. 2). The pH shift to pH 5 was performed after 2.1 days of cultivation using NaOH as lye. At this point in time, the pH had dropped to pH 2.0 and the itaconic acid production had started. The pH shift resulted in a decrease in the maximum productivity to 0.55 g/L/h. This productivity was maintained until day 7 of the cultivation. Here, a short glucose limitation occurred so that additional glucose was added to the fermentation broth in solid form. In all further experiments, the glucose concentration was monitored during the cultivation and glucose was added when necessary so that no additional glucose limitation occurred. The itaconic acid production continued despite the short substrate limitation, so that a final concentration of 131 g/L was reached after 15.9 days of cultivation. To test the influence of the added ions, an equivalent amount of sodium ions in the form of NaCl was added at the same time instead of the pH shift. This did not result in the decrease in the maximum productivity, and the final itaconic acid concentration was 80 g/L.
To test the influence of different lyes on the pH shift, KOH and ammonia solution were used instead of NaOH. The increase in the final itaconic acid concentration was independent of the lye used for the pH shift. The use of KOH as a lye resulted in a similar itaconic acid production with a decrease in the maximal productivity and reached a final itaconic acid concentration of 133 g/L. However, when ammonia solution was used as lye, the maximal productivity remained constant at 1.1 g/L/h, which is similar to the cultivation without pH control. The final itaconic acid concentration reached was 122 g/L.
The pH shift did not only have an effect on the final product concentrations, but also showed influences on the mycelium. After the pH shift, the cell dry mass increased independent of the used lye. The color of the fermentation broth changed for unknown reasons from light yellow over reddish brown to brown. This differs from the cultivation without pH control, where the fermentation broth had a light yellow color throughout the cultivation. Submerged conidiophores were formed after the pH shift using NaOH and KOH as lye, but not with ammonia solution. The cultivation with the submerged conidia as inoculum resulted in the same final itaconic acid concentration, but the spores germinated and produced itaconic acid 0.75 days earlier than the spores from surface culture (data not shown).
Influence of the pH shift on by-product formation
When the pH was not controlled, the by-products had a share of 3.3 % of the formed products. The majority of the by-products (97 %) consisted of malic acid and α-ketoglutaric acid. The other by-products were cis- and trans-aconitate. The concentration of α-ketoglutaric and malic acid increased continuously during the production phase of the cultivation.
As a result of a pH shift, the share of the by-products decreased independently of the lye used (Fig. 3). The α-ketoglutaric acid formation stopped after 6 days of cultivation, and the concentration remained constant. The malic acid formation also stopped after 4 days of cultivation, but only when NaOH and KOH were used as lye. This reduced the share of the by-products to 1.1 and 1.5 %, respectively. The use of ammonia solution resulted in an increase in the malic acid production after the pH shift. The concentration reached a maximum after 5 days and then declined, reaching a lower concentration as without pH control. The amount of by-products formed was 2.1 %, which was lower than in the cultivation without pH control.
Height of the pH shift
The further experiments were performed in 1.5 L bioreactors. The scale-up from 200 mL filling volume in the 400 mL bioreactors to 1 L filling volume in the 1.5 L bioreactors resulted without pH control in a similar behavior with a slight decrease in the final itaconic acid concentration to 87 g/L.
By performing a single pH shift, the final concentration of itaconic acid could be raised considerably. In the previous experiments, the pH was raised to a set value of pH 5. In further experiments, we wanted to determine if the height of the pH shift had an influence on the itaconic acid production.
The pH was shifted after 2.1 days of cultivations to a pH ranging from pH 3 to pH 6 using ammonia solution as lye (Fig. 4). The pH shift to pH 3 behaved similarly to the cultivation without pH control. The pH decreased after the pH shift to pH 2.0, no change in color appeared, and the final itaconic acid concentration was 84 g/L. The situation was different at the higher pH values. The pH shift to pH 4 and pH 5 raised the final itaconic acid concentration to 102 and 103 g/L, and the color of the fermentation broth changed from light yellow to reddish brown. The pH shift to pH 6 resulted in a slightly higher final itaconic acid concentration of 110 g/L. Here, the color of the fermentation broth changed directly after the pH shift to violet and then slowly to a light brown and a reddish brown. After the pH shifts to pH 4 to 6, the final pH values lay between pH 2.4 and pH 2.7.
Solubility of itaconic acid in fermentation broth
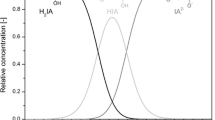
The pH shift to different pH values showed only slight influences between the pH of 4 and 6. The reason for the increase in the itaconic acid concentration could be the raised pH at the end of the cultivations. Due to its dissociation behavior, the solubility of itaconic acid is strongly dependent on the pH value. To find out in what amount the pH influences the solubility of itaconic acid under the conditions used, the solubility was determined in the fermentation broth.
At a pH of 1.95, the solubility of itaconic acid in the fermentation broth was 122 g/L. The solubility increased exponentially with rising pH. After the pH shifts, the pH had final values between pH 2.5 and pH 3. This raised the maximal solubility of itaconic acid between 131 and 166 g/L.
pH control
A pH control to pH 3 from the beginning of the cultivation had a negative influence on the itaconic acid production (Fig. 5). The itaconic acid formation started at the same time as in the cultivation without pH control, but had a very low maximal productivity of 0.3 g/L/h. Therefore, only 17.1 g/L itaconic acid was formed after 4.9 days of cultivation. The formed cell dry weight reached 12.6 g/L and was therefore nearly twice as high as without pH control.
On the other hand, when the pH control to pH 3 was activated after the start of the product formation (2.1 days), the cultivation achieved a similar maximal productivity as without pH control. The final concentration of 146.1 g/L itaconic acid was reached after 12.6 days of cultivation. This was an increase of 33 % in the itaconic acid formation in comparison to the pH shift to pH 6 and an increase of 68 % in comparison to the cultivation without pH control. The yield was 0.59 (w/w). The formation of the cell dry weight was not as high as after the singular pH shift and reached a maximum of 8 g/L. However, the color of the fermentation broth changed from light yellow to red violet like after the pH shift.
Temperature
The temperature was varied from 31 to 37 °C. The cultivation was performed in microtiter plates and analyzed after 7 days of cultivation. The highest itaconic acid concentration of 76 g/L was achieved at 35 °C. The product concentrations at 33 and 37 °C were quite similar, being 66 and 65 g/L itaconic acid, respectively. The lowest concentration of 55 g/L itaconic acid was found at 31 °C.
Cultivation under optimized conditions
The different optimizations presented in this paper and from previous publications were combined. An optimized medum was used, where the KH2PO4 concentration was raised to 0.8 g/L (Hevekerl et al. 2014), which led to an increase in the maximum productivity. For inoculation, we used spores, which were obtained from submerged sporulation. These spores germinate faster than the spores obtained by surface cultivation. The pH was controlled at the pH of 3 after the start of the product formation using ammonia solution as lye, and the temperature was raised to 35 °C.
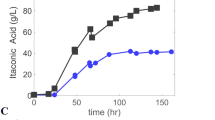
Under the optimized conditions, the growth started after 5 h of cultivation, which was visible in the drop of the pH (Fig. 6). Therefore, the production of itaconic acid started after 1 day of cultivation. In the standard cultivation without pH control, the itaconic acid formation did not begin until 1.7 days of cultivation. The cell dry weight increased until day 3 of the cultivation, forming twice as much as in the standard cultivation without pH control. The formed pellets were uniform with a diameter of 80 μm. The color of the fermentation broth did not change after the initiation of the pH control; it remained light yellow. No conidia formation was observed. During the growth, the phosphate concentration decreased evenly and was not limited at any time. The total nitrate consumption was 3 g/L. This was much higher than in the standard cultivation without pH control, where 1.3 g/L was consumed. The nitrate concentration was monitored during the cultivation, and 0.8 g/L nitrate was added in the form of NH4NO3 to avoid limitation.
Cultivation of A. terreus under the combined optimized conditions in comparison to the initial standard conditions without pH control. The black arrow indicates the start of the pH control and the grey arrows the addition of glucose to the cultivation under optimized conditions (1.5 L bioreactor, 470 rpm)
The final itaconic acid concentration of 129 g/L was reached after 4.7 days of cultivation. Therefore, the process had very good productivity of 1.15 g/L/h and maximum productivity of 2.64 g/L/h. This is an increase of 48 % in the final itaconic acid concentration to the standard cultivation without pH control, where 87 g/L itaconic acid was formed. The yield was 0.58 (w/w) under optimized conditions. The main results are summarized in Table 1.
Discussion
In this work, we investigated the influence of the pH on the itaconic acid production with A. terreus. Previous studies concentrated on variations of the initial pH and the pH control throughout the cultivation. Until now, the highest achieved concentration in the literature was 90 g/L (Kuenz et al. 2012) without any pH control.
In this study, the initial pH showed no influence on the product formation at pH values equal to or higher than 3.1. The pH in the unbuffered medium dropped from the germination of the spores, because of the uptake of ammonium ions (Soccol et al. 2006). Low initial pH values delayed the germination of the spores (Batti and Schweiger 1963), but showed no influence on the product formation. This insensitivity towards the initial pH would explain the wide range of initial pH values in the literature. It ranges from pH 2 (Park et al. 1993; Yahiro et al. 1995) (when mycelium was used as inoculum) up to pH 5.9 (Gyamerah 1995).
When the pH regulation was studied, the majority of the authors concentrated on the continuous pH regulation throughout the cultivation. Riscaldati et al. (2000) found an increase in the final itaconic acid concentration with increasing pH control from pH 1.95 to 2.4. But the final concentration decreased drastically at a higher pH control at pH 2.8. Other studies found the best product concentrations during pH control at pH 2.1 to 2.2 (Lockwood and Reeves 1945; Rychtera and Wase 1981). But the final itaconic acid concentrations were well below the cultivation without pH control.
The increase in the final itaconic acid concentration first occurred after a single pH shift after 2.1 days of cultivation. At this time, the cultivation had entered the production phase and itaconic acid was already formed. Here, the maximum productivity dropped after the pH shift when NaOH and KOH were used as lye and conidiophores were formed. The final itaconic acid concentration was raised from 100 g/L without pH control to 131 and 133 g/L, respectively. The addition of an equivalent quantity of sodium ions in the form of NaCl lacked the previously mentioned effects of the pH shift, including the increase of the final itaconic acid concentration. This indicates that the rise of the final itaconic acid concentration was caused by the change in the pH value. The use of ammonia solution as lye resulted in an increase of the product concentration and a steady maximum productivity. This could be caused by the lack of conidiophore formation, which might be caused by the increase in the ammonium concentration.
The pH shift also had a positive effect on the by-product formation. The concentration of the by-products was reduced from 3.3 % to a maximum of 2.1 %. Interestingly, when ammonia solution was used as lye, the malic acid concentration increased after the pH shift to a maximum of 3.0 g/L and then decreased again. The addition of high amounts of ammonium during a pH shift could increase the production of malate from the pyruvate over oxaloacetate (Jaklitsch et al. 1991). The decrease in the by-product formation after a pH shift was also reported by Batti and Schweiger (1963). Here, the influence on the final itaconic acid concentration was not mentioned. The effects of a pH shift in the itaconic acid production were quite different from a pH shift in the citric acid production using Aspergillus niger. Here, the increase of the pH during the cultivation results in a change in the product formation from citric acid (at low pH) to oxalic acid (high pH) (Cleland and Johnson 1956; Mischak et al. 1985).
The scale-up of the process to the 1.5 L bioreactor showed a similar behavior to the 400 mL bioreactor. Only the final itaconic acid concentration was lower in the cultivation without pH control. This difference was also visible after the single pH shift, which increased the final itaconic acid concentration, but the achieved concentrations were below the ones from the 400 mL bioreactor. After the height of the pH shift was varied from pH 3 to pH 6, only slight differences were visible between the pH shifts from pH 4 to 6. Here, the final itaconic acid concentration was raised from 87 to 102–110 g/L. In contrast to the pH shift to pH 3, where no increase in the product concentration occurred, the pH values of the cultivations did not decrease to their previous value of pH 2. They decreased to pH values 2.4–2.7.
Instead of a pH shift, the pH was then controlled to a pH of 3 after 2.1 days of cultivation. This resulted in a large increase in the itaconic acid concentration to 146 g/L, which was considerably higher than after a single pH shift, where 110 g/L itaconic acid was formed. When performing the pH control instead of the pH shift, the cells were not exposed to the stress caused by the pH shift. This lack of stress probably allowed them to reach higher product concentrations. To reach this high product concentration, it was necessary to start the pH control in the production phase of the cultivation. When the pH was controlled from the beginning, only very low productivities could be reached. The cells have to grow at a low pH value to gain the ability to produce itaconic acid (Larsen and Eimhjellen 1955).
The increase of the pH during the production phase had an immense influence on the final itaconic acid concentrations. By raising the pH from about pH 2 to pH 3, the dissociation grade of itaconic acid changes. The share of single dissociated acid increased about tenfold to 12 %, which also increased the solubility of itaconic acid from 122 to 166 g/L. Whether the rise in the final product concentration was only caused by the increase of the solubility is yet unknown. It is also possible that the changed dissociation grade enabled an easier transport of the acid through the cell membrane or had an influence on the characteristics of the cell membrane or that the rise in the pH caused a change in the gene expression (Caddick et al. 1986).
The combination of the developed pH control with previously found optimizations resulted in a slightly lower final concentration of 129 g/L itaconic acid than in the pH control to pH 3 after 2.1 days of cultivation. But the productivity increased immensely under the optimized conditions. It increased slightly from the reference cultivation to the pH control after 2 days of cultivation from 0.41 to 0.48 g/L/h, but increased 2.8-fold to 1.15 g/L/h when using the combined optimized conditions. The increase was caused by the earlier germination of the submerged conidia and the high maximum productivity caused by the increased phosphate concentration (Hevekerl et al. 2014). The increased phosphate concentration only had an impact on the maximum productivity, but not on the final itaconic acid concentration (Hevekerl et al. 2014).
In summary, we were able to raise the final itaconic acid concentration to 146 g/L, which is an increase of 62 % to the previously published concentration by Kuenz et al. (2012). By combining different optimization steps, the productivity of the itaconic acid production could be raised to 1.15 g/L/h with a final concentration of 129 g/L itaconic acid.
References
Batti M, Schweiger LB (1963) Process for the production of itaconic acid. US patent 3,078,217 (to Miles Laboratories)
Caddick M, Brownlee A, Arst H Jr (1986) Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol Gen Genet 203(2):346–353
Cleland W, Johnson MJ (1956) Studies on the formation of oxalic acid by Aspergillus niger. J Biol Chem 220(2):595–606
Gyamerah MH (1995) Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL 1960. Appl Microbiol Biotechnol 44(1–2):20–26
Hevekerl A, Kuenz A, Vorlop KD (2014) Filamentous fungi in microtiter plates—an easy way to optimize itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol 98(16):6983–6989
Jaklitsch WM, Kubicek CP, Scrutton MC (1991) The subcellular organisation of itaconate biosynthesis in Aspergillus terreus. J Gen Microbiol 137(3):533–539
Kautola H (1990) Itaconic acid production from xylose in repeated-batch and continuous bioreactors. Appl Microbiol Biotechnol 33(1):7–11
Kinoshita K (1932) Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz. Aspergillus itaconicus. Acta Phytochimica 5:271–287
Kuenz A, Gallenmüller Y, Willke T, Vorlop K-D (2012) Microbial production of itaconic acid: developing a stable platform for high product concentrations. Appl Microbiol Biotechnol 96(5):1209–1216
Larsen H, Eimhjellen K (1955) The mechanism of itaconic acid formation by Aspergillus terreus. 1. The effect of acidity. Biochem J 60(1):135
Lockwood LB, Reeves MD (1945) Some factors affecting the production of itaconic acid by Aspergillus terreus. Arch Biochem 6(3):455–469
Mischak H, Kubicek CP, Röhr M (1985) Formation and location of glucose oxidase in citric acid producing mycelia of Aspergillus niger. Appl Microbiol Biotechnol 21(1–2):27–31
Okabe M, Lies D, Kanamasa S, Park EY (2009) Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol 84(4):597–606
Park YS, Ohta N, Okabe M (1993) Effect of dissolved oxygen concentration and impeller tip speed on itaconic acid production by Aspergillus terreus. Biotechnol Lett 15(6):583–586
Riscaldati E, Moresi M, Federici F, Petruccioli M (2000) Effect of pH and stirring rate on itaconate production by Aspergillus terreus. J Biotechnol 83(3):219–230
Rychtera M, Wase DAJ (1981) The growth of Aspergillus terreus and the production of itaconic acid in batch and continuous cultures. The influence of pH. J Chem Technol Biotechnol 31(8):509–521
Soccol CR, Vandenberghe LP, Rodrigues C, Pandey A (2006) New perspectives for citric acid production and application. Food Technol Biotechnol 44(2):141–149
Tate BE (1981) Itaconic acid and derivatives. Grayson M Eckroth E (eds) Kirk-Othmer Encycl Chem Technol 3:865–873
Willke T, Vorlop K-D (2001) Biotechnological production of itaconic acid. Appl Microbiol Biotechnol 56(3–4):289–295
Yahiro K, Takahama T, Park YS, Okabe M (1995) Breeding of Aspergillus terreus mutant TN-484 for itaconic acid production with high yield. J Ferment Bioeng 79(5):506–508
Acknowledgments
Many thanks for the financial support by the Fachagentur Nachwachsende Rohstoffe e.V. (grant no. 22020908).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hevekerl, A., Kuenz, A. & Vorlop, KD. Influence of the pH on the itaconic acid production with Aspergillus terreus . Appl Microbiol Biotechnol 98, 10005–10012 (2014). https://doi.org/10.1007/s00253-014-6047-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6047-2