Abstract
Sulfide from anaerobic treatment of high-sulfate wastewater would always have some adverse effects on downstream processes. In this study, a coupling anaerobic/aerobic system was developed and operated under haloalkaliphilic condition to realize deep and high-efficiency removal of sulfate without production of sulfide. A haloalkaliphilic sulfur-oxidizing strain, Thioalkalivibrio versutus SOB306, was responsible for oxidation of sulfide. The anaerobic part was first operated at optimum condition based on a previous study. Then, its effluent with an average sulfide concentration of 674 ± 33 mg·l−1 was further directly treated by a set of 1 l biofilter with SOB306 strain under aerobic condition. Finally, 100% removal rate of sulfide was achieved at aeration rate of 0.75 l·l−1·min−1, ORP of − 392 mV and HRT of 4 h. The average yield of elemental sulfur reached 79.1 ± 1.3% in the filter, and the CROS achieved a conversion rate of sulfate to sulfur beyond 54%. This study for the first time revealed the characteristics and performance of the haloalkaliphilic CROS in deep treatment of high-sulfate wastewater, which paved the way for the development and application of this method in the real world.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of modern industrialization, a large amount of highly concentrated sulfate organic wastewater is discharged from some processes, such as chemical industry, monosodium glutamate production, pharmacy, leather, paper and so on. Even though sulfate does not result in distinct hazards in our surrounding environment, sulfate pollution can lead to several indirect environmental effects [1]. Wastewater with sulfate is normally treated with physicochemical and biological methods. It is well-known that, however, the physicochemical methods possess some underlying disadvantages which limit their applications, such as separation and appropriate disposal of solid phase, high cost and energy consumption [2].
As regards biological methods, organic sulfate-rich wastewater was generally treated in anaerobic processes in which sulfate-reducing bacteria (SRBs) could be responsible for sulfate reduction into sulfide [3]. As a matter of fact, a high concentration of sulfide could poison methane-producing archaea (MPA) which was another important member in the processes and cause a decline in methane production. In some conventional biological systems with about pH 6.0, sulfide existed in the form of H2S which could penetrate into cells easily and generate a direct toxicity to MPAs, SRBs and other kinds of microorganisms, and inhibited the treatment effect of anaerobic processes [4, 5]. Moreover, treating wastewater using biological methods is economic, effective, and thorough, but the capacity of biosystems was easily subject to limitation under conditions of high salinity and pH [4, 6].
However, some haloalkaliphilic microorganisms displayed remarkable advantages in the treatment of such sulfate-rich wastewaters [7]. In this kind of haloalkaliphilic system, distinctly, the presence of hydrogen sulfide in solution was in the form of HS− that cannot penetrate into cells easily [8, 9]. In our group, some interesting studies on performance of haloalkaliphilic bioreactors and bacterial communities were performed in recent years [4, 10, 11]. Generally, if there existed a high concentration of sulfide in effluent of an anaerobic reactor, the activity of some key microorganisms in some downstream processes was also suppressed. Sulfide is a kind of known inhibitor of nitrification and affects microbial communities in nitrifying treatment process, for instance, it can differentially inhibit ammonium-oxidizing and nitrite-oxidizing bacteria [12,13,14]. Therefore, removal of sulfide in solution using some methods would help minimize adverse effects on downstream processes as much as possible.
Biodesulfurization based on sulfur-oxidizing bacteria (SOBs) was a useful technology for removal of sulfide, and some relevant technologies have been presently developed. A specific group of haloalkaliphilic SOBs belonging to the genus Thioalkalivibrio isolated from soda lake sediments was successfully applied in some fed reactor systems for sulfide removal [15,16,17]. The members of Thioalkalivibrio were Gram-negative, halophilic, alkaliphilic, sulfur-oxidizing and chemolithotrophic bacteria. They could tolerate Na+ concentrations up to 5 M and their optimum pH for growth was between 9 and 10 [18]. They mainly gained energy by oxidizing reduced or partially reduced sulfur compounds and also fixed CO2 from the atmosphere [19]. Under oxygen-limited conditions, dissolved sulfide was mainly oxidized into elemental sulfur (S0) by these SOBs, whilst a part (typically less than 10%) was oxidized into sulfate (SO42−) [20]. The use of these SOBs could circumvent many of the obstacles associated with conventional assays. As mentioned above, the effluent of anaerobic bioreactor using haloalkaliphilic microorganisms was also characteristic of high pH, high concentration of sodium and a certain concentration of sulfide, so it might be directly treated by haloalkaliphilic SOBs for sulfide removal.
In the present work, we offered a coupling bioprocess for treatment of wastewater with a high concentration of sulfate and sodium based on haloalkaliphilic and biological sulfate-reducing system and sulfur-oxidizing system. The performance of this process was investigated by treating modeling wastewater under optimum sulfate-reducing condition. Here we also come up with a significant demonstration that haloalkaliphilic sulfur-oxidizing biosystem could be directly applied in deep treatment of sulfate wastewater and high-efficiency removal of sulfide from some kind of wastewater.
Materials and methods
System description
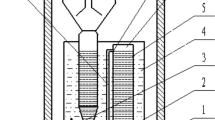
A coupling sulfate-reducing and sulfur-oxidizing system (CROS) consisted of one bioreactor for removal of sulfate under anaerobic condition and the other for removal of sulfide under aerobic condition. The profile of CROS is displayed in Fig. 1. The anaerobic part with a working volume of 6 l has been described detailedly and reported by our group [11]. Here, we attempted to construct a continuous and synchronized system for deep removal of sulfate by adding an aerobic biofilter as shown in Fig. 1 and Fig. S1A. The effluent of anaerobic process with highly concentrated sulfide was directly pumped into the biofilter at some constant flow rate, and sulfide was further oxidized into sulfur and a small amount of sulfate through keeping suitable aeration rate and redox potential (ORP). The biofilter was filled with hollow ceramic rings with the size of Φ12 mm × 5 mm, and its working volume was 1 l. Two kind of sensors, pH (InPro3250i, Mettler-Toledo) and ORP (Pt4805-DPA, Mettler-Toledo, Switzerland), were installed on the lid of biofilter for real-time monitoring of the running state.
Inoculum and nutrient media
The liquid culture of biofilter was directly taken from an 80 l haloalkaliphilic sulfur-oxidizing bioreactor (HSOB) which was used for biodesulfurizing experiments in the long term in our group (Fig. S1B). Thioalkalivibrio versutus SOB306 was solely the functional strain responsible for oxidization of sulfide [21]. Medium FTD was used in contrast tests. The medium contained: Na2CO3 46.0 g·l−1, NaHCO3 23.0 g·l−1, K2HPO4·3H2O 2 g·l−1, KNO3 g·l−1, NH4Cl 0.3 g·l−1, MgCl2·6H2O 0.1 g·l−1, Na2S·9H2O 5.3 g·l−1. A trace elements solution was added (1 ml·l−1) as described elsewhere [22]. The preparation of medium for anaerobic bioreactor was totally based on our previous study [11].
Operation of CROS
The anaerobic bioreactor was first operated according to a previous study [11]. After the performance of bioreactor reached the optimum condition stably, effluent was collected into the tank as shown in Fig. 1. Then, the effluent was pumped into biofilter with HSOB culture at a certain feed rate and aeration was regulated timely based on the value of ORP to guarantee CROS running continuously and stably. Through adjusting HRT and aeration rate, performance of bioreactor reached an optimal state with the greatest removal rate of sulfide. Samples were taken from A, C and D of CROS every 4 h.
Analytical methods
Before making measurement, all samples were centrifuged at 13,000 rpm and 10 min, and then the supernatant was taken and diluted based on the requirement. The concentration of dissolved sulfide was measured by colorimetry with a spectrophotometer (U-2910; Hitachi, Tokyo, Japan) [23]. Sulfate and thiosulfate were analyzed by ion chromatography (Dionex model ICS-900, Dionex, Sunnyvale, CA), which was equipped with an electrical conductivity detector (Dionex Sunnyvale, CA). A Dionex IonPacTM AS14A analytical column (4 × 250 mm) was operated at 25 °C, the mobile phase was 8.0 mM Na2CO3/1.0 mM NaHCO3 at a flow rate of 1.0 ml·min−1 [24]. The injection volume was 10 μl. The concentration of elemental sulfur of effluent was calculated by the mass balance between total concentration of all dissolved sulfur products in the inlet and outlet. All experimental data were processed based on sulfur balance as the following equations:
Herein, R and O represented anaerobic bioreactor and aerobic biofilter, respectively.
Results and discussion
Start-up of CROS
In this study, the anaerobic part of CROS was first operated under optimum condition totally based on the method reported by a previous study, with sulfate concentration of 3000 mg·l−1 in influent, COD/SO42− ratio of 4.0 and HRT of 24 h [11]. After stable operation, the average removal rate of sulfate reached 68.5%, and the average concentration of sulfide in effluent achieved 674 ± 33 mg·l−1, as shown in Fig. 2a. Its performance was line with that in the previous study, which demonstrated that the capacity of this anaerobic bioreactor was very stable [11]. 1 l culture was collected from previous HSOB. The values of pH and salinity of this kind culture were 9.5 and 1.0 M Na+, respectively. Figure 2b displayed the dose of some key sulfur compounds in the culture. Owing to long-term running, accumulation concentrations of sulfate and thiosulfate reached 9235 ± 182 mg·l−1 and 5166 ± 71 mg·l−1, respectively. However, the concentration of sulfide was only 32 ± 1 mg·l−1. Then, the CROS was started up by coupling anaerobic bioreactor with biofilter through the action of peristaltic pump. The anaerobic bioreactor could produce around 6 l effluent per day, so the pump rate was set to 4.15 ml·min−1 to synchronously run biofilter. HRT of biofilter was about 4 h. The performance of CROS was regulated by adjusting aeration rate based on ORP.
Aeration rate and ORP
The change laws of ORP with aeration rate are demonstrated in Fig. 3. The value of ORP gradually increased from − 400 mV to − 340 mV as aeration rate was improved under constant concentration of sulfide in the influent. ORP was one of the most important operating parameters for sulfide oxidizing selectively to elemental sulfur [25]. Under optimal ORP for sulfur formation, a maximum amount of sulfide would be converted into elemental sulfur [26]. When ORP was kept at − 400 mV, the removal rate of sulfide was just 93.1 ± 1.5%, but when the aeration rate was adjusted to 0.75 l·l−1·min−1 and even higher, sulfide was totally removed from liquid and transferred into other kinds of sulfur compounds without toxicity. And the value of ORP was − 392 mV at this time. In the whole process, oxidizing reactions were dependent on some key enzyme systems in cells, like Fcc, Sox, Hdr, and so on [27, 28].
Moreover, selectivity of oxidization of sulfide to sulfur and sulfate was also obviously different at different aeration rates. As shown in Fig. 4, the change of sulfate was positively correlated with aeration rate. Even though sulfide had been oxidized completely, the selections for production of sulfate and elemental sulfur were 40% and 50% at the highest aeration rate, which was not an ideal state owing to lower production rate of sulfur and higher energy expenditure. When aeration rate was kept at 0.75 l·l−1·min−1, the production rate of elemental sulfur reached the highest level, namely 78%, and the rate of sulfate was decreased to 20%. As a matter of fact, the production rate of sulfate could be still reduced at a lower aeration rate, but the selection for production of elemental sulfur was dropped below 60% instead. In addition, when the value of ORP dropped, a lower level of oxygen led to a low oxidization level of sulfide and accumulation of thiosulfate [20]. Thiosulfate was formed during sulfide oxidation, which was likely related with some abiotic processes [29, 30]. As the aeration rate decreased, the oxidation capacity of the system also decreased, and sulfide could not be abundantly removed. That was mainly because the activity of SOB306 cells was repressed at a certain extent under lower oxygen input. Yet, the more elemental sulfur was generated, the better sulfur pollutants were removed thoroughly [31]. Taken together, the capacities of biofilter and CROS were optimum at aeration rate of 0.75 l·l−1·min−1 and ORP of − 392 mV under continuous feeding.
Performance of CROS
Under aeration rate of 0.75 l·l−1·min−1 and ORP of − 392 mV, the conversion of sulfide was further explored in this study. As shown in Fig. 5, before the first 20 h, the sulfur compounds in original culture from HSOB were oxidized by SOB306 strain and continuously discharged outside. Therefore, the concentrations of sulfate and thiosulfate were gradually reduced until it reached stable conditions after 20 h. The concentration of sulfate was kept below 1,400 mg·l−1, the accumulation of thiosulfate was relatively low and the average yield of elemental sulfur reached 79.1 ± 1.3%. Eventually, the conversion rate of sulfate to sulfur achieved beyond 54% by the CROS.
Lastly, some contrast tests were performed by substituting effluent of anaerobic bioreactor with FTD medium. The concentration of sulfide was around 700 mg·l−1, which was similar to that in effluent of anaerobic part. Then, the performance of biofilter was tested at different flow rates, and aeration rate was still stayed at 0.75 l·l−1·min−1. From Fig. 6, the performance of biofilter achieved optimum condition at HRT of 3 h, with 80.2 ± 2.3% of productive rate of sulfur and 19.6 ± 0.5% of accumulation rate of sulfate. It was speculated that the activity of SOB306 cells was suppressed partly by some kind of organic matters, metal ions and other microorganisms from effluent of anaerobic bioreactor. Certainly, S0 formation was accompanied by the growth of strain SOB306 which obtained energy by oxidizing sulfide into sulfur and sulfate under aerobic conditions. Compared with neutrophilic SOBs which just grew under some conditions with lower pH and salinity, SOB306 strain could be more adaptable to this kind of complex environment and the performance of biofilter and CROS could be further improved once cells covered onto packings by formation of biofilm over time [32]. However, it was still demonstrated that the effluent of anaerobic bioreactor could be directly treated by biofilter with SOB306 strain owing to its haloalkaliphilic and sulfur-oxidizing properties. Most of previously reported coupled anaerobic/aerobic treatments of high-sulfate systems were operated around neutral pH [32,33,34]. Therefore, this study revealed, for the first time, the characteristics and performance of haloalkaliphilic coupled anaerobic/aerobic system in deep removal of sulfate.
Conclusion
This work demonstrated successful operation of an integrated anaerobic/aerobic biosystem CROS for deep and high-efficiency treatment of high-sulfate model wastewater under high pH and salinity. The anaerobic part of CROS was operated at optimum condition with sulfate concentration of 3000 mg·l−1 in influent, COD/SO42− ratio of 4.0 and HRT of 24 h. The effluent with 674 ± 33 mg·l−1 of sulfide was further treated by a set of 1 l biofilter under aerobic condition. To keep synchronous operation of two bioreactors, the flow rate of biofilter was set to 4.15 ml·min−1. The capacity of biofilter got optimum condition with 100% removal of sulfide at aeration rate of 0.75 l·l−1·min−1, ORP of − 392 mV and HRT of 4 h. The average yield of elemental sulfur in biofilter reached 79.1 ± 1.3%, and the conversion rate of sulfate to sulfur achieved beyond 54% by the CROS. The main sulfur-oxidizing bacterium involved in this process was a haloalkaliphilic Thioalkalivibrio versutus SOB306, which could tolerate high pH and salinity. It turned out that the effluent of anaerobic bioreactor could be directly treated by biofilter with SOB306 strain owing to its haloalkaliphilic and sulfur-oxidizing properties. This study first revealed the characteristics and performance of haloalkaliphilic coupled with anaerobic/aerobic system in the advanced treatment of high-sulfate wastewater.
References
Liamleam W, Annachhatre AP (2007) Electron donors for biological sulfate reduction. Biotechnol Adv 25:452–463
Silva AJd, Varesche M, Foresti E, Zaiat M (2002) Sulphate removal from industrial wastewater using a packed-bed anaerobic reactor. Process Biochem 37:927–935
Sinbuathong N, Khaodhiar S, Liengcharernsit W, Sirirote P, Watts D (2007) Effect of sulfate on the methanogenic activity of a bacterial culture from a brewery wastewater during glucose degradation. J Environ Sci 19:1025–1027
Zhou JM, Song ZY, Yan DJ, Liu YL, Yang MH, Cao HB, Xing JM (2014) Performance of a haloalkaliphilic bioreactor and bacterial community shifts under different COD/SO42- ratios and hydraulic retention times. J Hazard Mater 274:53–62
Reis M, Almeida J, Lemos P, Carrondo M (1992) Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol Bioeng 40:593–600
Philip L, Deshusses MA (2003) Sulfur dioxide treatment from flue gases using a biotrickling filter-bioreactor system. Environ Sci Technol 37:1978–1982
Sorokin DY, Rusanov II, Pimenov NV, Tourova TP, Abbas B, Muyzer G (2010) Sulfidogenesis under extremely haloalkaline conditions in soda lakes of Kulunda Steppe (Altai, Russia). FEMS Microbiol Ecol 73:278–290
Yongsiri C, Vollertsen J, Hvitved-Jacobsen T (2005) Influence of wastewater constituents on hydrogen sulfide emission in sewer networks. J Environ Eng 131:1676–1683
Mora-Naranjo N, Alamar-Provecho C, Meima J, Haarstrick A, Hempel D (2003) Experimental investigation and modelling of the effect of sulfate on anaerobic biodegradation processes in municipal solid waste. Water Sci Technol 48:221–227
Zhou J, Zhou X, Li Y, Xing J (2015) Bacterial communities in haloalkaliphilic sulfate-reducing bioreactors under different electron donors revealed by 16S rRNA MiSeq sequencing. J Hazard Mater 295:176–184
Mu T, Xing J, Yang M (2019) Sulfate reduction by a haloalkaliphilic bench-scale sulfate-reducing bioreactor and its bacterial communities at different depths. Biochem Eng J 147:100–109
Bejarano Ortiz DI, Thalasso F, Cuervo López FdM, Texier AC (2013) Inhibitory effect of sulfide on the nitrifying respiratory process. J Chem Technol Biotechnol 88:1344–1349
Erguder TH, Boon N, Vlaeminck SE, Verstraete W (2008) Partial nitrification achieved by pulse sulfide doses in a sequential batch reactor. Environ Sci Technol 42:8715–8720
Vela JD, Dick GJ, Love NG (2018) Sulfide inhibition of nitrite oxidation in activated sludge depends on microbial community composition. Water Res 138:241–249
De Graaff M, Bijmans MF, Abbas B, Euverink GJ, Muyzer G, Janssen AJ (2011) Biological treatment of refinery spent caustics under halo-alkaline conditions. Bioresour Technol 102:7257–7264
Klok JB, van den Bosch PL, Buisman CJ, Stams AJ, Keesman KJ, Janssen AJ (2012) Pathways of sulfide oxidation by haloalkaliphilic bacteria in limited-oxygen gas lift bioreactors. Environ Sci Technol 46:7581–7586
Roman P, Klok JB, Sousa JA, Broman E, Dopson M, Van Zessen E, Bijmans MF, Sorokin DY, Janssen AJ (2016) Selection and application of sulfide oxidizing microorganisms able to withstand thiols in gas biodesulfurization systems. Environ Sci Technol 50:12808–12815
Sorokin DY, Lysenko AM, Mityushina LL, Tourova TP, Jones BE, Rainey FA, Robertson LA, Kuenen GJ (2001) Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp. nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Inter J Syst Evol Microbiol 51:565–580
Sorokin DY, Banciu H, Robertson LA, Kuenen JG (2006) Haloalkaliphilic Sulfur-Oxidizing Bacteria. Prokaryotes 2:969–984
van den Bosch PL, van Beusekom OC, Buisman CJ, Janssen AJ (2007) Sulfide oxidation at halo-alkaline conditions in a fed-batch bioreactor. Biotechnol Bioeng 97:1053–1063
Mu T, Yang M, Zhao J, Sharshar MM, Tian J, Xing J (2016) Improvement of desulfurizing activity of haloalkaliphilic Thialkalivibrio versutus SOB306 with the expression of Vitreoscilla hemoglobin gene. Biotechnol Lett 39:1–6
Pfennig N, Lippert KD (1966) Über das vitamin B 12-bedürfnis phototropher Schwefelbakterien. Arch Microbiol 55:245–256
Cord-Ruwisch R (1985) A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiol Meth 4:33–36
Zhou JM, Song ZY, Yan DJ, Liu YL, Yang MH, Cao HB, Xing JM (2014) Performance of a haloalkaliphilic bioreactor under different NO3-/SO42- ratios. Bioresour Technol 153:216–222
Song Z, Li Q, Wang D, Zhang J, Xing J (2012) A novel up-flow inner-cycle anoxic bioreactor (UIAB) system for the treatment of sulfide wastewater from purification of biogas. Water Sci Technol 65:1033–1040
Janssen A, Meijer S, Bontsema J, Lettinga G (1998) Application of the redox potential for controling a sulfide oxidizing bioreactor. Biotechnol Bioeng 60:147–155
Mu T, Zhou J, Yang M, Xing J (2016) Complete genome sequence of Thialkalivibrio versutus D301 isolated from Soda Lake in northern China, a typical strain with great ability to oxidize sulfide. J Biotechnol 227:21–22
Berben T, Overmars L, Sorokin DY, Muyzer G (2017) Comparative genome analysis of three thiocyanate oxidizing Thioalkalivibrio species isolated from soda lakes. Front Microbiol 8:254
Kleinjan WE, de Keizer A, Janssen AJ (2005) Kinetics of the chemical oxidation of polysulfide anions in aqueous solution. Water Res 39:4093–4100
Chen KY, Morris JC (1972) Kinetics of oxidation of aqueous sulfide by oxygen. Environ Sci Technol 6:529–537
Cai J, Zheng P, Qaisar M, Zhang J (2017) Elemental sulfur recovery of biological sulfide removal process from wastewater: a review. Crit Rev Environ Sci Technol 47:2079–2099
Okabe S, Ito T, Sugita K, Satoh H (2005) Succession of internal sulfur cycles and sulfur-oxidizing bacterial communities in microaerophilic wastewater biofilms. Appl Environ Microbiol 71:2520–2529
Fox P, Venkatasubbiah V (1996) Coupled anaerobic/aerobic treatment of high-sulfate wastewater with sulfate reduction and biological sulfide oxidation. Water Sci Technol 34:359–366
Xu X, Chen C, Wang A, Fang N, Yuan Y, Ren N, Lee D (2012) Enhanced elementary sulfur recovery in integrated sulfate-reducing, sulfur-producing rector under micro-aerobic condition. Bioresource Technol 116:517–521
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31800030, 31872633 and 21878307), the Major Science and Technology Program for Water Pollution Control and Treatment in China (No. 2017ZX07402003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mu, T., Yang, M. & Xing, J. Deep and high-efficiency removal of sulfate through a coupling system with sulfate-reducing and sulfur-oxidizing capacity under haloalkaliphilic condition. Bioprocess Biosyst Eng 43, 1009–1015 (2020). https://doi.org/10.1007/s00449-020-02298-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02298-5