Abstract
A heterotrophic nitrifying bacterium was isolated from the activated sludge and identified as Pseudomonas stutzeri GEP-01. Strain GEP-01 exhibited an efficient heterotrophic nitrification capability and a high nitrogen utilization rate, 48 mg/L NH4+-N was removed after culturing for 24 h without NO2−-N or NO3−-N accumulation, and 64.7% of the NH4+-N was removed by heterotrophic nitrification. Single-factor experiments indicated that factors such as the carbon source, temperature, NH4+-N load, and inoculum size had significant effects on the ammonium removal efficiency of strain GEP-01. The preferred conditions for heterotrophic nitrification were sodium citrate, 30 °C, 40 mg/L NH4+-N, and 5% inoculum size. When the initial NH4+-N amounts were 100, 200, 500 and 1000 mg/L, the removal rates were approximately 100%, 93%, 90.4%, and 78.9%, respectively, and higher ammonium concentrations require longer culture time. Nitrogen balance demonstrated that 40% of the initial nitrogen was lost, which was probably removed in the form of gas products under optimum culture conditions, and 36.3% of NH4+-N was converted to biomass. When incubated (adding a small amount of sodium citrate as carbon source and no carbon source) in swine wastewater containing 835 mg/L of ammonium, the removal ratio reached 56.3% and 24.8%. Strain GEP-01 has potential applications in the treatment of ammonium-rich wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the acceleration of urbanization and agricultural specialization in China and the impact of global warming [1], ammonia-nitrogen pollution in water is becoming more and more serious, especially from coking [2], food, aquaculture [3] and other industries, as well as high concentration ammonia-nitrogen wastewater from landfill leachate [4]. Untreated discharges of such wastewater can cause serious environmental hazards, such as eutrophication. In recent decades, eutrophication has occurred in a large number of lakes [5, 6] and coastal areas [7, 8] in China. With the increasingly serious eutrophication of water body and the increasingly stringent discharge standard of nitrogen in wastewater, how to economically and effectively remove ammonia-nitrogen in wastewater has become one of the urgent problems to be solved in the treatment of high concentration ammonia-nitrogen wastewater.
In recent years, bacteria with heterotrophic nitrification–aerobic denitrification have been discovered and have attracted extensive attention and research due to their potential value of biological removal of nitrogen. Heterotrophic nitrification and its genus research are riches and breakthroughs in traditional nitrification theory, such as Pseudomonas [9], Alcaligenes [10] and Halomonas [11]. Heterotrophic nitrifying bacteria have the advantages of fast growth rate, low temperature resistance [12], simultaneous nitrification, and denitrification, which makes the potential application value of heterotrophic nitrifying bacteria very significant. Pseudomonas stutzeri was proved to be a heterotrophic nitrification–aerobic denitrification bacterium [13]. The studies on Pseudomonas stutzeri have focused on their ability and pathway to remove nitrogen [14, 15], and studied the factors affecting C/N, temperature, dissolved oxygen, etc., [16,17,18]. However, the effect of NH4+-N load and inoculum size has rarely been reported. It has been reported that, compared to other bacteria, Pseudomonas stutzeris can more easily form biofilms in nutrient-poor environments [19]. Therefore, studying the effects of ammonia-nitrogen load and inoculum on the nitrogen removal capacity of Pseudomonas stutzeri has a good reference for engineering applications such as biological aerated filter and biological contact oxidation system. In this study, a heterotrophic nitrifying bacterium was isolated from activated sludge of sewage treatment plant and identified as Pseudomonas stutzeri and named GEP-01. Its heterotrophic nitrification capability and the factors affecting the nitrogen removal of strain GEP-01 and the optimal reaction conditions were studied. It is expected that the application of Pseudomonas stutzeri GEP-01 in water environment restoration can be expanded.
Materials and methods
Culture media
The enrichment medium (EM) was comprised of (per liter): 5.0 g sodium acetate, 5.0 g (NH4)2SO4, 0.25 g NaH2PO4, 0.01 g MnSO4·4H2O, 0.03 g MgSO4·7H2O, 0.75 g K2HPO4, 1.0 g CaCO3, and 1 mL trace elements solution consisting of (per liter) 50 g EDTA, 0.05 g H8MoN2O4, 5 g Fe2(SO4)3, 0.05 g H3BO3, 1.6 g CuSO4, 0.01 g KI, 2.2 g ZnSO4, and 0.05 g CoCl2.
The basal medium (BM) for strain cultivation and heterotrophic nitrification was comprised of (per liter): 5.0 g sodium acetate, 2.0 g (NH4)2SO4, 0.25 g NaH2PO4, 0.01 g MnSO4·4H2O, 0.03 g MgSO4·7H2O, 0.75 g K2HPO4, 1.0 g CaCO3, and 1 mL trace elements solution. Luria-Bertani (LB) agar plates were prepared by adding 15 g agar to the BM.
The initial pH of all the media mentioned above was adjusted to 7.2.
Screening and isolation of strain GEP-01
First, 5 g of sludge sample (obtained from a sewage treatment plant in Zhengzhou, Henan, China) was placed in a conical flask containing 100 mL of 0.9% sterile saline and shaken at 150 rpm for 1 h. Then, 10 mL of the mixture was added to 90 mL of the EM in a conical flask and incubated on a rotary shaker at 30 °C and 150 rpm for 24 h to enrich the heterotrophic bacteria.
After 1 day of cultivation, 10 mL of the cell suspension was transferred to 90 mL fresh EM and incubated at 30 °C and 150 rpm for 2 days. Thereafter, 1 mL of the bacterial suspension was transferred to 100 mL fresh BM in a 250 mL flask for selective cultivation of the bacterial cultures the under conditions of 30 °C and 150 rpm for 2 days. The above process was repeated three times. The enriched bacterial culture was gradient diluted, and the suspensions with different concentrations were spread onto LB agar plates using the dilution plate method and incubated to grow single colonies that were clearly visible at 30 °C. Separate colonies exhibiting different morphological features were selected and further purified. Then, pure isolates were picked and individually tested to determine their capabilities for nitrogen removal. The bacterium with the highest nitrification capability was obtained and used for subsequent tests.
The obtained pure strain was streaked and stored at 4 °C as the source of the strain for further study. Every 2 weeks, the bacterium was inoculated onto a new medium to maintain the bacterial activity. The culture medium used for the preservation of the strain was a heterotrophic nitrification medium.
Identification of strain GEP-01
The morphological characteristics of the isolated microbial strains were observed using a microscope, and Gram staining and microscopic examination were performed. Then, the pure strain was subjected to genomic DNA extraction, PCR amplification of 16S rDNA, and purification of the PCR product, and the PCR product was placed on an agar gel for electrophoresis. Sequencing was performed using positive and negative universal primers. The sequence was edited using DNASTAR, and the gene sequence obtained by removing the vector sequence was analyzed using BLAST in the GenBank database.
Assessment of heterotrophic nitrification and growth curve of strain GEP-01
The bacterial solution was inoculated into 100 mL of BM (the initial NH4+-N concentrations was about 50 mg/L) at a 5% inoculum size and cultured at 30 °C, 150 rpm, and pH 7.0–8.0, during which the absorbance of the bacterial cells at 600 nm was sampled at regular intervals to prepare a growth curve.
The bacterial solution was inoculated into 100 mL of BM at a 5% inoculum size (OD600 ≈ 1.4) and cultured at 30 °C, 150 rpm, and pH 7.0–8.0. Samples were taken every 6 h, centrifuged (10,000 r/min) and then filtered through a 0.45 mm membrane filter, and the filtrate was used to determine the concentrations of NH4+-N, NO3−-N, NO2−-N and TN. Biomass nitrogen (bio-N) was calculated by subtracting TN of centrifuged sample (4 °C, 15 min, 3000 rpm) from that of non-centrifuged sample. All experiments were carried out in triplicate.
Assessment of optimum conditions and influencing factors for heterotrophic nitrification performance
To study the optimal heterotrophic nitrification performance of strain GEP-01 and its influencing factors, single-factor experiments and an orthogonal experiment of L9(34) were designed to study the effects of the carbon source, temperature, NH4+-N load, and inoculum size on the NH4+-N removal capability of strain GEP-01. Before each experiment, strain GEP-01 was inoculated onto LB by the spread-plate method and cultured at 30 °C. After the colonies grew, a single colony was picked and placed in BM for culturing at 150 rpm and 30 °C. The cultured bacterial solution (bacteria OD600 ≈ 1.4) was inoculated into BM and shaking flask experiments were carried out under the effects of different influencing factors. Three parallel experiments were conducted for each experimental group. Samples were taken at regular intervals, and the sample treatment and measurement were as described above.
Effect of carbon source
Seignette salt, sodium acetate, and sodium citrate were used as single carbon sources, and ammonium sulfate was used as the nitrogen source, with a 5% inoculum size (bacterial OD600 ≈ 1.4, wet cells after centrifugation). The initial NH4+-N concentration was 40 mg/L, and the bacteria were cultured at pH 7, 150 rpm, and 30 °C.
Effect of temperature
At 10, 20, and 30 °C, sodium acetate was used as the single carbon source, ammonium sulfate was used as the nitrogen source, and a 5% inoculum size (bacterial OD600 ≈ 1.4, wet cells after centrifugation) was used. The initial NH4+-N concentration was 40 mg/L, and the bacteria were cultured at pH 7 and 150 rpm.
Considering that the temperature will exceed 35 °C in summer, the culture condition of 40 °C was designed to evaluate the ammonium removal capability of GEP-01 at high temperature.
Effect of NH4+-N load
The initial NH4+-N concentrations were 40, 100, and 200 mg/L. Sodium acetate was used as the single carbon source, and ammonium sulfate was used as the nitrogen source, with a 5% inoculum size (bacterial OD600 ≈ 1.4, wet cells after centrifugation). The bacteria were cultured at pH 7, 150 rpm and 30 °C.
To evaluate the high-strength ammonium removal efficiency of GEP-01, the BM with an initial ammonium concentration of 500 and 1000 mg/L was prepared. The bacteria were cultured at pH 7, 150 rpm, and 30 ℃.
Effect of inoculum size
Inoculum sizes of 1%, 5%, and 10% (bacteria OD600 ≈ 1.4, wet cells after centrifugation) were used, with sodium acetate as the single carbon source and ammonium sulfate as the nitrogen source. The initial NH4+-N concentration was 40 mg/L, and the bacteria were cultured at pH 7, 150 rpm and 30 °C.
Orthogonal experiment
Four factors (the carbon source, temperature, NH4+-N load, and inoculum size) were selected, and each factor was set at three levels. The orthogonal experiment was designed using SPSS software according to the factors and levels selected above, and the results were analyzed.
Ammonium removal under optimum culture conditions
Ammonium removal was carried out under the optimum culture conditions resulting from the single-factor experiments and nitrogen balance was analyzed by measuring NH4+-N, TN, NO3−-N, NO2−-N, NH2OH, and bio-N.
Assessment of ammonium removal in swine wastewater
The strain GEP-01 was inoculated into 100 mL of swine wastewater (adding sodium citrate as carbon source and no carbon source) at a 5% inoculum size (OD600 ≈ 1.4) and cultured at 30 °C, 150 rpm, and pH 7.0–8.0. Samples were taken every 12 h. Swine wastewater was from a pig farm in Zhengzhou, Henan Province. The swine wastewater used for the experiment was the effluent treated by biogas digester, hydrolytic acidification tank and sedimentation tank, and its water quality index is shown in Table 1.
Analytical methods
The pH was measured with a pH meter (PHS-3C, Leici, Shanghai, China). Bacterial growth was measured using spectrophotometry at 600 nm and represented as OD600. Bio-N was calculated by subtracting TN of centrifuged sample (4 °C, 15 min, 3000 rpm) from that of non-centrifuged sample [20]. COD was determined by a COD detector (DR 1010, HACH, USA). The NH4+-N concentration was determined by Nessler’s reagent spectrophotometry. The NO2−-N concentration was determined by the N-(1-naphthalene)-diaminoethane photometry method. The TN was determined by spectrophotometry. The NO3−-N concentration was determined by the phenol disulfonic acid photometry method. The NH2OH was analyzed by indirect spectrophotometry at a wavelength of 705 nm [21].
Result and discussion
Isolation and identification of strain GEP-01
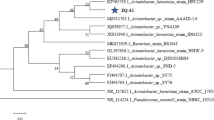
Using sludge samples as the source of the strain, after enrichment and separation, nine strains of microorganisms were screened. Strain GEP-01 was selected based on the NH4+-N and TN removal efficiencies. The colony of strain GEP-01 on the agar plate was round, beige, smooth, opaque, and shiny, and it was slightly protruding in the middle with neat edges. Strain GEP-01 was Gram negative, nonspore forming, and flagellated and had the shape of short rods. The sequence was submitted to the NCBI for BLAST sequence alignment. The 16S rDNA sequence similarity with multiple strains of Pseudomonas stutzeri was 98%. Based on the morphological, physiological, and biochemical characteristics of strain GEP-01, it was identified as Pseudomonas stutzeri. A phylogenetic tree was established on the basis of the neighbor-joining method (Fig. 1). The result further confirmed the identification of strain GEP-01 as Pseudomonas stutzeri. It has been submitted to the China General Microbiological Culture Collection Center (CGMCC) for preservation (CGMCC NO. 16359). It has been reported that the heterotrophic nitrification aerobic denitrification ability of Pseudomonas has been confirmed [22,23,24].
Heterotrophic nitrification characteristic and growth curve of strain GEP-01
The NH4+-N removal characteristics and the growth curve of strain GEP-01 are shown in Fig. 2. The growth of the isolate resulted in sigmoid curves. The lag period of strain GEP-01 was observed at 3–4 h, but it was not obvious. During this time, the NH4+-N concentration was reduced from 48.65 to 36.12 mg/L, and the average nitrification ratio was 3.13 mg NH4+-N/L/h. Then, the strain entered the exponential phase, which lasted for approximately 10 h, and the OD value reached the maximum of 1.39. At this stage, ammonium was oxidized for cell propagation. Meanwhile, the NH4+-N concentration descended significantly, with the removal efficiency reaching 92.0% within 12 h, and the average nitrification ratio was 3.73 mg NH4+-N/L/h. Then, the strain began to enter the declining period. The removal rate of NH4+-N at 24 h was 98.3%. Similar to Pseudomonas sp. ASM-2–3 [22] and Pseudomonas putida strain NP5 [24], strain GEP-01 showed the characteristics of rapid growth, a short start-up time, and good potential for heterotrophic nitrification.
As shown in Fig. 2, the changes in the TN were consistent with those of the NH4+-N. The TN in the medium decreased rapidly within 12 h. At 12 h, the TN decreased to 23.46 mg/L, and the removal rate reached 54.55%. In the later period, the TN decreased slowly. After 24 h, the TN decreased to 18.42 mg/L, and the removal rate finally reached 64.3%. Nitrification products, including NO3−-N and NO2−-N, were detected when ammonium was consumed in large quantities, and the concentrations of NO3−-N and NO2−-N reached the maximum after 18 h, at 0.52 mg/L and 0.14 mg/L, respectively. Then, the concentrations were reduced to 0.43 mg/L and 0.098 mg/L. The changes in the NO3−-N concentration in the medium were consistent with those of the NO2−-N concentration, which showed a trend of increasing first and then decreasing due to the removal of NH4+-N and the enhancement of denitrification. During the whole reaction process, NO2−-N did not accumulate in a large amount, and some of the ammonium nitrogen may have been converted to gaseous nitrogen products during nitrification by strain GEP-01. Some papers have reported that heterotrophic nitrification and aerobic denitrification bacteria were able to oxidize ammonium to nitrite or nitrate and simultaneously denitrify these products to N2O and/or N2 [25, 26].
To verify the presence of ammonium assimilation and the proportion of nitrification during NH4+-N removal, bio-N was determined. As shown in Fig. 2, in the first 12 h, along with reproduction of strain GEP-01, concentration of NH4+-N dropped rapidly, bio-N increased from 0.13 to 14.65 mg/L. Most initial ammonium was converted into biomass. In the stationary phase, OD660 remained constant, bio-N increased slowly from 15.02 to 16.32 mg/L. Results demonstrated that 64.7% of the NH4+-N was removed by heterotrophic nitrification during this process.
In this experiment, the NH4+-N was almost completely degraded, while the TN was not completely degraded, and NO3−-N and NO2−-N accumulated little. It is possible ammonium assimilation [20] and heterotrophic nitrification occurred at the same time when ammonia nitrogen was the main nitrogen source. At this time, part of the NH4+-N provided a nitrogen source for biological proliferation, thus reducing the NH4+-N. Part of the ammonium was oxidized to nitrite and nitrate due to heterotrophic nitrification and then converted to nitrogen-containing gas by denitrification, or ammonium was oxidized to hydroxylamine and directly converted to nitrogen-containing gas [27].
Optimum conditions and influencing factors of ammonium removal
Carbon sources
The effects of the carbon source on ammonium removal are plotted in Table 2. When sodium citrate and sodium acetate were used as carbon sources, the NH4+-N was completely removed after 30 h of cultivation. The rate of heterotrophic nitrification was the fastest when using sodium citrate. The initial NH4+-N concentration (39.89 mg/L) decreased to 0.97 mg/L (97.5% removal efficiency) at 6 h, with an average removal rate of 6.48 mg NH4+-N/L/h. The heterotrophic nitrification rate was slightly slower when using sodium acetate, and the NH4+-N removal rate was 98.7% after 30 h. The NH4+-N removal rate of strain GEP-01 was only 44.5% at 30 h when the carbon source was seignette salt. It can be seen that ammonium removal was significantly affected by the type of carbon source.
The maximum removal rate of TN appeared at 18–30 h, which was slightly delayed compared to that of NH4+-N. The maximum TN removal rates of sodium citrate, sodium acetate, and seignette salt tartrate were 61.7%, 44%, and 21.3%, respectively (Table 2). As shown in Table 2, during the cultivation, with the progress of heterotrophic nitrification and aerobic denitrification, the concentrations of NO3−-N and NO2−-N did not exceed 3 mg/L. Moreover, the concentration of nitrate nitrogen was the highest when sodium citrate was used as the carbon source, also proving that strain GEP-01 can more easily perform heterotrophic nitrification with sodium citrate.
It can be seen that strain GEP-01 utilizes sodium citrate better than sodium acetate and seignette salt, this utilization is more conducive to the direct utilization and absorption of heterotrophic nitrifying bacteria. This result was consistent with previous findings [10, 11]. The plausible reason is that sodium citrate can be directly inserted into the metabolic process without modification, and it makes the medium more alkaline, which is better for nitrification [28]. Among the three carbon sources, sodium citrate and sodium acetate are oxidized compounds, while seignette salt is a reduced compound. Another reason for this finding is that the differences in the nitrogen removal efficiency relative to the carbon sources might be related to their oxidoreduction potentials [23].
Different temperature
The ammonium removal by strain GEP-01 occurred over a wide temperature range (Table 2). Higher temperatures promoted the heterotrophic nitrifying ability of strain GEP-01 significantly. The variation of NH4+-N was marginal at 10 °C, at only 48.8%, but as the temperature increased from 20 to 30 °C, the NH4+-N was completely removed within 24 h and 18 h, respectively. During this period, the average nitrification ratios were 1.65 mg and 2.22 mg NH4+-N/L/h. The removal of NH4+-N accelerated with the increase of the temperature, and the remove curve of NH4+-N within 12 h at 10 °C was relatively flat, which showed that growth at lower temperatures required a longer lag period. The TN removal rates at 20 °C and 30 °C were not significantly different, at 47.0% and 58.7%, respectively, while the TN removal rate at 10 °C was only 24.6% but was better than that of Pseudomonas putida strain NP5 [24]. At 30 °C, the concentration of NO3−-N peaked at 12 h, at 2.31 mg/L. The possible reason for this result was that the rate of nitrification was stronger than that of denitrification at this time, and the NO3−-N was not converted into N2 or N2O in time after the conversion of NO2−-N to NO3−-N, causing the peak to appear earlier. At 10 °C, the accumulation of NO3−-N and NO2−-N was small, at only 0.82 and 0.079 mg/L, and heterotrophic nitrification and aerobic denitrification were weak.
The results in Table 2 indicated that Pseudomonas stutzeri GEP-01 was capable of growing at 40 °C and has a good removal of NH4+-N. At 40℃, the removal rates of NH4+-N and TN were 97% and 53.7%, respectively. There was no accumulation of nitrate and nitrite in this process. The strain GEP-01 has better removal capability of NH4+-N and TN at 20–40 °C, which will benefit its engineering application. The optimal temperatures for most reported heterotrophic nitrifying bacteria range from 25 to 37 °C [29,30,31]. The effect of the temperature on the nitrification efficiency of heterotrophic nitrifying bacteria can be explained by the effects of the temperature on the organism. For bacteria, for every 10 °C increase in temperature over a suitable temperature range, the enzymatic reaction rate will increase by a factor of 1–2, and thus, the metabolic rate will increase accordingly [32]. The results showed that the removal rate of NH4+-N by strain GEP-01 increased with the increase of the temperature in the range of 30 °C, and was inhibited over 40℃.
NH4+-N load
The NH4+-N removal curve of strain GEP-01 under different NH4+-N loads is shown in Table 2. At the initial concentration of 40 mg/L, NH4+-N was degraded rapidly and was almost completely removed within 6 h. At the initial concentration of 100 mg/L, NH4+-N was completely degraded within 18 h. When the initial concentration was increased to 200 mg/L, the NH4+-N removal rate was approximately 94.3% within 30 h after an adaptation period, and the average nitrification ratio was 6.25 mg NH4+-N/L/h (Table 2). In the removal of high-strength ammonium wastewater, Pseudomonas Schneider needs a longer adaptation period and a longer reaction time. As shown in Table 2, when the initial NH4+-N concentration was 500 mg/L, only 17.4% of NH4+-N was removed within 24 h. Within the next 48 h, the concentration of NH4+-N gradually decreased until the final concentration was 47.3 mg/L. At this time, the removal rate of NH4+-N was 90.4%. When the initial NH4+-N concentration was 1000 mg/L, the removal rate at 72 h was only 79.2%. This may be due to the exhaustion of carbon in the medium, and the heterotrophic nitrification process is hindered, thereby affecting the removal of NH4+-N. When the initial NH4+-N concentration was 500 and 1000 mg/L, the C/N ratio was 2.92 and 1.46, respectively, and most optimum C/N ratio on nitrogen removal by heterotrophic nitrifying bacteria were 8–20 [17, 33, 34]. Compared with some of the previously reported Pseudomonas stutzeri, such as Pseudomonas stutzeri T13 (at 160 rpm and 30 ℃, when the initial concentration was 224.68 mg/L, the NH4+-N removal rate after 18 h was 39.56%) [18] and Pseudomonas stutzeri XL-2 (at 120 rpm and 30 ℃, when the initial concentration was 100 mg/L, the NH4+-N removal amount was 69.86 mg/L in 48 h) [35], strain GEP-01 has better nitrogen removal efficiency.
The removal rates of the TN were 63.8%, 54.6%, 48.2%, 47.4%, and 43.2%, respectively (Table 2). At the NH4+-N concentrations of 40 mg/L, 100 mg/L, 200 mg/L and 500 mg/L, the process of degrading NH4+-N by strain GEP-01 produced no accumulation of NO3−-N or NO2−-N. The accumulation of NO3−-N was only detected under the condition of high NH4+-N. This is similar to the case for the bacterium Aeromonas sp. HN-02 [36]. Ammonium is the only nitrogen source used in the growth process of ammonia-oxidizing bacteria. The concentration of ammonium in the matrix is essential for the growth of heterotrophic nitrifying bacteria. In the absence of ammonium, the matrix cannot meet the growth requirements of the cells. Excessive ammonium will have a toxic effect on cell growth [37], and at the same time, it will inhibit the activity of enzymes in the nitrification reaction through substrate inhibition, thus affecting the nitrification activity of nitrifying bacteria [38]. The high NH4+-N removal rate and low contents of intermediate products make strain GEP-01 have engineering application value in treating high-NH4+-N concentration water, such as eutrophic water.
Inoculum size
As can be seen from Table 2, the culture time was the same, and increasing the inoculum size within a certain range could increase the NH4+-N removal rate. When the inoculum size was 1% and 5%, the NH4+-N was completely removed within 24 h and 12 h, respectively, and the average nitrification ratio was increased from 1.67 to 3.35 mg NH4+-N/L/h. The NH4+-N removal rate was 80% within 30 h when the inoculum size was 10%. When the inoculum amount was 5%, the TN concentration reached the lowest level, which was 21.5 mg/L, after 24 h of culture. Although the high inoculum amount increased the TN removal efficiency in the early stage, the final removal rate was only 39.07%. As shown in Table 2, the maximum accumulation amounts were 2.91 and 0.09 mg/L at 10% inoculum. That is, as the inoculum size increased, the maximum concentrations of NO3−-N and NO2−-N increased gradually, but within the range of 1% to 10%, the accumulation amount was low.
At present, the inoculum size has rarely been studied in the research of heterotrophic nitrifying bacteria. The amount of inoculum determines the number of bacteria in the culture medium. Theoretically, the larger the amount of inoculum, the greater is the number of bacteria, and the better will be the nitrification effect. However, bacteria are subject to the restrictions of nutritional conditions, and excessive bacteria will consume a high amount of nutrients; this level of consumption is unfavorable for nitrification [39, 40]. Excessive inoculum size will lead to the rapid consumption of nutrients, thus restricting the continued growth of bacteria and leading to the autolysis of existing bacteria [41]; this outcome results in the decrease of microbial activity. This may be the reason why the concentration of NH4+-N increased when the inoculum was large. Therefore, it can be seen that the effect of changes in the inoculum size on the remove of NH4+-N is that the removal rate increases first and then decreases as the inoculum size increases. For strain GEP-01, the inoculum size of 5% may be a critical point, but perhaps, the critical point is a value between 5 and 10%, which is a postulation that requires further experimental verification.
Orthogonal experiment of analysis
The results of the orthogonal experiment were analyzed using SPSS software, as shown in Table 3. The best experimental conditions were obtained by comparing the K value: A2B3C1D2. That is, when the carbon source was sodium citrate, the temperature, NH4+-N load, and inoculum size were 30 °C, 40 mg/L and 5%, and the TN removal rate was the highest at 58.3%. The range of the temperature was 29.7, the range of the NH4+-N load was the second largest, and the range of the inoculum size was the smallest. The order of influence on the TN removal rate was: temperature > NH4+-N load > carbon source > inoculum size. The preferred conditions for nitrogen removal were sodium citrate, 30 °C, 40 mg/L NH4+-N, and 5% inoculum size.
Nitrogen balance under optimum culture conditions
The results of the nitrogen balance are shown in Table 4. 100% of NH4+-N was removed under the optimum conditions in 24 h, and the removal rate of TN was 76.5%. The results of the nitrogen balance analysis indicated that 23.5% of the initial nitrogen was converted to NO3−-N, NO2−-N, and other forms of nitrogen (hydroxylamine and organic nitrogen). On the other hand, the bio-N increased to 14.51 mg/L and that 36.2% of the initial nitrogen was converted to biomass. Comparing the initial and final N amount, 40% of the initial nitrogen was lost, which was probably removed in the form of gas products through heterotrophic nitrification and aerobic denitrification. The results disclose that part of the NH4+-N was used for biological proliferation, and others was oxidized to nitrite and nitrate and then converted to nitrogen-containing gas by denitrification, or ammonium was oxidized to hydroxylamine and directly converted to nitrogen-containing gas.
Ammonium removal in swine wastewater
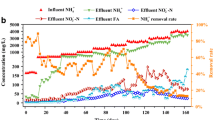
The ammonium removal in the swine wastewater inoculated with strain GEP-01 are shown in Fig. 3. The concentrations of COD, NH4+-N, and TN gradually decreased with increasing time (Fig. 3), but whether carbon source is added or not has a great influence on the removal efficiency of TN and NH4+-N. When the initial COD concentration was 1032 mg/L, after 108 h of culture, the removal rates of NH4+-N and TN were only 24.8% and 16.3% (Fig. 3a). When the initial COD concentration increased to 2295 mg/L (sodium citrate as carbon source), the final removal rates of NH4+-N and TN increased to 56.3% and 37.7%, respectively (Fig. 3 b). In the last 24 h, the concentrations of NH4+-N and TN tended to be stable. The possible reason is that with the consumption of carbon that can be utilized by microorganisms, bacterial growth and heterotrophic nitrification processes were hindered. Some studies have reported that adequately C/N ratio was necessary for good nitrogen removal by heterotrophic nitrification bacteria [17, 42]. The nitrogen removal capability of strain GEP-01 has been confirmed in real wastewater. The effect of C/N on nitrogen removal will be studied in the following research.
Conclusions
A newly isolated bacterium Pseudomonas stutzeri GEP-01 has great nitrification ability and high nitrogen utilization rate for NH4+-N, 48 mg/L NH4+-N was removed after cultured 12 h without NO3−-N and NO2−-N accumulation, and 63.3% of the NH4+-N was removed by heterotrophic nitrification. The factors such as carbon source, temperature, NH4+-N load, and inoculum size had significant effects on the NH4+-N removal efficiency, the preferred conditions for heterotrophic nitrification were sodium citrate as the carbon source, 40 mg/L NH4+-N load, 30 °C and 5% inoculum size. In addition, higher ammonium concentrations require longer culture time. Initial NH4+-N was 1000 mg/L, the NH4+-N and TN removal rate was about 78.9% and 43.2% within 72 h. Nitrogen balance demonstrated that 40% of the initial nitrogen was lost under optimum culture conditions, which was probably removed in the form of gas products. When the initial NH4+-N concentration of the swine wastewater was 835 mg/L and the culture conditions were not changed, adding a small amount of carbon source increased the NH4+-N removal rate from 24.8 to 56.3%.
References
Gao G, Clare AS, Rose C, Caldwell GS (2017) Eutrophication and warming-driven green tides (Ulva rigida) are predicted to increase under future climate change scenarios. Mar Pollut Bull 114(1):439–447
Xiang Y, Xiang Y, Wang L, Jiao Y (2018) Effects of coking wastewater on the growth of five wetland plant species. Bull Environ Contam Toxicol 100(2):265–270
Cao L, Wang J, Xiang S, Huang Z, Ruan R, Liu Y (2019) Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. Environ Sci Pollut Res 26(7):6725–6734
Wang S, Wu X, Wang Y, Li Q, Tao M (2008) Removal of organic matter and ammonia nitrogen from landfill leachate by ultrasound. Ultrason Sonochem 15(6):933–937
Le C, Zha Y, Li Y, Sun D, Lu H, Yin B (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manag 45(4):662–668
Wang J, Fu Z, Qiao H, Liu F (2019) Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci Total Environ 650:1392–1402
Hu J, Zhang G, Li K, Pa P, Chivas AR (2008) Increased eutrophication offshore Hong Kong, China during the past 75 years: evidence from high-resolution sedimentary records. Mar Chem 110(1–2):7–17
Wang B, Xin M, Wei Q, Xie L (2018) A historical overview of coastal eutrophication in the China Seas. Mar Pollut Bull 136:394–400
Gao F, Liao S, Liu S, Bai H, Wang A, Ye J (2019) The combination use of Candida tropicalis HH8 and Pseudomonas stutzeri LZX301 on nitrogen removal, biofloc formation and microbial communities in aquaculture. Aquaculture 500:50–56
Shoda M, Ishikawa Y (2014) Heterotrophic nitrification and aerobic denitrification of high-strength ammonium in anaerobically digested sludge by Alcaligenes faecalis strain no. 4. J Biosci Bioeng 117(6):737–741
Guo L, Chen Q, Fang F, Hu Z, Wu J, Miao A, Xiao L, Chen X, Yang L (2013) Application potential of a newly isolated indigenous aerobic denitrifier for nitrate and ammonium removal of eutrophic lake water. Biores Technol 142:45–51
Yao S, Ni J, Chen Q, Borthwick AGL (2013) Enrichment and characterization of a bacteria consortium capable of heterotrophic nitrification and aerobic denitrification at low temperature. Biores Technol 127:151–157
Zhang J, Wu P, Hao B, Yu Z (2011) Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Biores Technol 102(21):9866–9869
Zhao B, Ran XC, Tian M, An Q, Guo JS (2018) Assessing the performance of a sequencing batch biofilm reactor bioaugmented with P. stutzeri strain XL-2 treating ammonium-rich wastewater. Biores Technol 270:70–79
Zhou M, Ye H, Zhao X (2015) Ammonium removal by a novel heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas stutzeri KTB from wastewater. Water Qual Res J Can 50(3):219–227
Ji B, Wang H, Yang K (2014) Tolerance of an aerobic denitrifier (Pseudomonas stutzeri) to high O-2 concentrations. Biotechnol Lett 36(4):719–722
Qing H, Donde OO, Tian C, Wang C, Wu X, Feng S, Liu Y, Xiao B (2018) Novel heterotrophic nitrogen removal and assimilation characteristic of the newly isolated bacterium Pseudomonas stutzeri AD-1. J Biosci Bioeng 126(3):339–345
Sun Y, Li A, Zhang X, Ma F (2015) Regulation of dissolved oxygen from accumulated nitrite during the heterotrophic nitrification and aerobic denitrification of Pseudomonas stutzeri T13. Appl Microbiol Biotechnol 99(7):3243–3248
Ueda A, Saneoka H (2015) Characterization of the ability to form biofilms by plant-associated Pseudomonas species. Curr Microbiol 70(4):506–513
Sun Y, Feng L, Li A, Zhang X, Yang J, Ma F (2017) Ammonium assimilation: an important accessory during aerobic denitrification of Pseudomonas stutzeri T13. Biores Technol 234:264–272
Frear D, Burrell R (1955) Spectrophotometric method for determining hydroxylamine reductase activity in higher plants. Anal Chem 27:1664–1665
Kariminiaae-Hamedaani HR, Kanda K, Kato F (2004) Denitrification activity of the bacterium Pseudomonas sp ASM-2-3 isolated from the Ariake Sea tideland. J Biosci Bioeng 97(1):39–44
Li C, Yang J, Wang X, Wang E, Li B, He R, Yuan H (2015) Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a phosphate accumulating bacterium Pseudomonas stutzeri YG-24. Biores Technol 182:18–25
Yang L, Wang X-H, Cui S, Ren Y-X, Yu J, Chen N, Xiao Q, Guo L-K, Wang R-H (2019) Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Bioresour Technol 285:121360
Chen S, He S, Wu C, Du D (2019) Characteristics of heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter sp. T1 and its application for pig farm wastewater treatment. J Biosci Bioeng 127(2):201–205
Zhao B, An Q, He YL, Guo JS (2012) N2O and N-2 production during heterotrophic nitrification by Alcaligenes faecalis strain NR. Biores Technol 116:379–385
Zhao B, He YL, Zhang XF (2010) Nitrogen removal capability through simultaneous heterotrophic nitrification and aerobic denitrification by Bacillus sp LY. Environ Technol 31(4):409–416
Sun Z, Lv Y, Liu Y, Ren R (2016) Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a novel metal resistant bacterium Cupriavidus sp S1. Biores Technol 220:142–150
Liu Y, Ai G-M, Miao L-L, Liu Z-P (2016) Marinobacter strain NNA5, a newly isolated and highly efficient aerobic denitrifier with zero N2O emission. Biores Technol 206:9–15
Ren Y-X, Yang L, Liang X (2014) The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Biores Technol 171:1–9
Rout PR, Dash RR, Bhunia P, Rao S (2018) Role of Bacillus cereus GS-5 strain on simultaneous nitrogen and phosphorous removal from domestic wastewater in an inventive single unit multi-layer packed bed bioreactor. Biores Technol 262:251–260
Krishna C, Van Loosdrecht MCM (1999) Effect of temperature on storage polymers and settleability of activated sludge. Water Res 33(10):2374–2382
Chen Q, Ni JR (2012) Ammonium removal by Agrobacterium sp LAD9 capable of heterotrophic nitrification-aerobic denitrification. J Biosci Bioeng 113(5):619–623. https://doi.org/10.1016/j.jbiosc.2011.12.012
Lei Y, Wang Y, Liu H, Xi C, Song L (2016) A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Zobellella taiwanensis DN-7, can remove high-strength ammonium. Appl Microbiol Biotechnol 100(9):4219–4229. https://doi.org/10.1007/s00253-016-7290-5
Yu Y, An Q, Zhou Y, Deng S, Miao Y, Zhao B, Yang L (2019) Highly synergistic effects on ammonium removal by the co-system of Pseudomonas stutzeri XL-2 and modified walnut shell biochar. Biores Technol 280:239–246
Chen M, Wang W, Feng Y, Zhu X, Zhou H, Tan Z, Li X (2014) Impact resistance of different factors on ammonia removal by heterotrophic nitrification-aerobic denitrification bacterium Aeromonas sp HN-02. Biores Technol 167:456–461
Vadivelu VM, Keller J, Yuan Z (2007) Effect of free ammonia on the respiration and growth processes of an enriched Nitrobacter culture. Water Res 41(4):826–834
Anthonisen AC (1976) Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Control Fed 48:835–852
Batchelor SE, Cooper M, Chhabra SR, Glover LA, Stewart GS, Williams P, Prosser JI (1997) Cell density-regulated recovery of starved biofilm populations of ammonia-oxidizing bacteria. Appl Environ Microbiol 63(6):2281–2286
Ramadan MA, El-Tayeb OM, Alexander M (1990) Inoculum size as a factor limiting success of inoculum for biodegradation. Appl Environ Microbiol 56(5):1392–1396
Jolliffe LK, Doyle RJ, Streips UN (1981) The energized membrane and cellular autolysis in Bacillus subtilis. Cell 25(3):753–763
Zhang D, Li W, Huang X, Qin W, Liu M (2013) Removal of ammonium in surface water at low temperature by a newly isolated Microbacterium sp strain SFA13. Biores Technol 137:147–152
Acknowledgements
This work was supported by the Key R&D and Promotion Project of Henan Province (No. 192102310498); the Major Special Science and Technology Project of Henan Province (No. 181100310300); the National Science and Technology Major Project (No. 2017ZX07602-003-002); and the Key Scientific Research Project Plan of Colleges and Universities in Henan Province (No. 19B180012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, J., Zhu, T., Liu, C. et al. Ammonium removal characteristics of heterotrophic nitrifying bacterium Pseudomonas stutzeri GEP-01 with potential for treatment of ammonium-rich wastewater. Bioprocess Biosyst Eng 43, 959–969 (2020). https://doi.org/10.1007/s00449-020-02292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02292-x