Abstract
The nitrogen-removing characteristics of Pseudomonas stutzeri T13, a heterotrophic nitrifying-aerobic denitrifying bacterium, were investigated. The ammonium and nitrate removal of the bacterium was found to reach nearly 100 % at 15 h. However, the total nitrogen (TN) removal rate reached only about 23.47 % because of the dramatic accumulation of nitrite at a high dissolved oxygen (DO) level (160 rpm). The process of nitrite reduction was found to be the bottleneck for the efficiency of aerobic denitrification. Decreasing the shaking speed from 160 to 50 rpm to lower the DO concentration during cultivation was an effective method of improving nitrite utilization because nitrite removal increased from 62.37 to 100 %. The 99.21 % capability of simultaneous heterotrophic nitrification and aerobic denitrification with TN removal was achieved at a relatively low DO level (50 rpm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The efficiency of nitrogen removal is always based on biological processes, i.e., nitrification (NH4 + to NO3 −) and denitrification (NO3 − to N2). Traditionally, denitrification only reacts under anaerobic or anoxic conditions, thereby restricting the occurrence of denitrification to nitrification in different time zones (Zhu et al. 2008). Novel aerobic denitrifying bacteria have been reported in recent years, such as Thiosphaera pantotropha (Robertson et al. 1985), Thauera mechernichensis sp. nov.TL1T (Scholten et al. 1999), and Klebsiella pneumonia CF-S9 (Padhi et al. 2013). These bacteria can reduce nitrate to gaseous nitrogen under aerobic conditions, indicating the potential of simultaneous nitrification and denitrification.
Aerobic denitrifiers can conduct an aerobic respiratory process, in which nitrate is gradually reduced to N2 (Ji et al. 2013). A specific enzyme called periplasmic nitrate reductase (Nar), which is located on the periplasmic side of the cell membrane and is only slightly inhibited by oxygen, plays a key role in the conversion of nitrate to nitrite under aerobic conditions (Sparacino-Watkins et al. 2014). Moreover, nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos) are used to reduce oxynitrides (NO2 − → NO → N2O → N2) (Philippot et al. 2001). However, nitrite reductases in certain aerobic denitrification bacteria are still sensitive to oxygen the same as traditional denitrifiers. This sensitivity leads to low enzyme activities and impeded nitrite reduction during aerobic denitrification (Körner and Zumft 1989). The accumulation of nitrite during aerobic denitrification has been reported by Liang et al. (2011), yet research on the pertinent reason and regulatory method is very limited.
The aerobic denitrification bacterium Pseudomonas stutzeri strain T13 (hereafter denoted as T13) and its genome sequence information has been previously reported (Li et al. 2012). In the present study, the nitrogen removal ability of this strain was further investigated, and the characteristics of denitrification at different dissolved oxygen (DO) levels were compared. The results provided an efficient method of retarding the accumulation of nitrite during aerobic denitrification and improve total nitrogen (TN) removal.
Materials and methods
Strain source
P. stutzeri T13 (CGMCC 7.187) was provided by the State Key Laboratory of Urban Water Resource and Environment, Harbin Institute of Technology, China. The gene encoding the periplasmic nitrate reductase was determined from the genome sequence information of strain T13 downloaded from GenBank (Accession No. ALJB00000000). Strain T13 was ensured to be an aerobic denitrification bacterium (Li et al. 2012). The genes encoding nitrite reductase and ammonia monooxygenase were also present in the strain T13 genome (Li et al. 2012).
Medium
The basal medium used for bacteria cultivation contained (per liter of distilled water): 4.7 g of sodium succinate, 1.5 g of KNO3, 1.5 g of KH2PO4, 7.9 g of Na2HPO4, 0.5 g of MgSO4 · 7H2O, and 1 ml of a trace element solution. The components of the trace element solution were as follows (per liter of distilled water): 50 g of EDTA, 2.2 g of ZnSO4, 5.5 g of CaCl2, 5.06 g of MnCl2 · 4H2O, 5.0 g of FeSO4 · 7H2O, 1.1 g of (NH4)6Mo7O2 · 4H2O, 1.57 g of CuSO4 · 5H2O, and 1.61 g of CoCl2 · 6H2O.
Ammonium removal performance
A preculture of T13 was inoculated in triplicate in 250 ml flasks each containing 100 ml of basal medium using ammonium as the sole nitrogen source instead of nitrate. A medium without inoculation was used as the control. The flasks were aerobically cultivated with constant shaking at 160 rpm and 30 °C for 24 h. The samples were taken from the flasks at 3 h intervals to determine the optical density at 660 nm (OD660), as well as the concentrations of ammonium nitrogen (NH4 +-N), nitrate nitrogen (NO3 −-N), and nitrite nitrogen (NO2 −-N). The effect of the initial ammonium concentration on ammonium removal was examined by adjusting the initial ammonium from 52.71 to 224.68 mg/L with the same initial chemical oxygen demand (COD) concentration of 1860.84 mg/L. The parameters were determined in all the samples after 18 h of inoculation. Each experiment was performed in triplicate.
Capability of aerobic denitrification
To evaluate the aerobic denitrification performance of T13, it was inoculated into the basal medium with nitrate or nitrite as the sole nitrogen source. The culture was aerobically cultivated with constant shaking at 160 rpm and 30 °C for 24 h. The samples were taken from the flasks at 3 h intervals to determine the optical density at 660 nm (OD660), as well as the concentrations of nitrate nitrogen (NO3 −-N), nitrite nitrogen (NO2 −-N), and TN. Each experiment was performed in triplicate.
Effect of DO on aerobic denitrification
T13 was inoculated in the basal medium with nitrate or nitrite. For aerobes, forced aeration of liquid cultures can be achieved by shaking the flask on shaker (Madigan et al. 2012). And the faster oxygen reaeration rate can be realized by increasing the shaking speed (McDaniel and Bailey 1969; Wittmann et al. 2003). Thus, the different DO concentrations were controlled by varying the shaking speed (0, 50, 100, and 160 rpm). The concentrations of nitrate nitrogen (NO3 −-N), nitrite nitrogen (NO2 −-N), and TN were determined. Both treatments were carried out in triplicate.
Simultaneous heterotrophic nitrification and aerobic denitrification capability of T13 under low shaking speed condition
T13 was cultivated in the basal medium with both ammonium and nitrate as nitrogen sources under high (160 rpm of shaking speed) and low (50 rpm of shaking speed) DO conditions. The comparison of TN removal during simultaneous heterotrophic nitrification and aerobic denitrification was examined.
Analytical methods
The concentrations of ammonium, nitrate, nitrite, and TN were determined according to standard methods, i.e., by Nessler’s reagent spectrophotometry, phenol disulfonic acid photometry, and N-(1-naphthyl)-ethylene diamine photometry, respectively (APHA 2012). The growth of T13 was measured using a spectrophotometer at a wavelength of 660 nm.
Results
Ammonium removal performance
The ammonium utilization of T13 was investigated under an aerobic culture condition, in which the ammonium chloride was used as the sole nitrogen source in the basal medium. The growth of T13 and its ammonium utilizing ability is shown in Fig. 1. The OD660 rapidly increased after inoculation. The log phase was observed between 3 and 12 h, and then the stationary phase was reached.
Ammonium was slowly utilized by the limited biomass in the first 3 h. Ammonium concentration dramatically decreased along with the log phase. Approximately 100 % NH4 +-N was removed in 18 h at an average ammonium removal rate of 4.50 mg L−1 h−1 (Table S1). A small amount of ammonium was generated again in the water after 18 h along with the slight decline in OD660 value. This finding was mainly due to microbial autolysis under famine condition when nutrient substance was exhausted (König et al. 2010).
The effect of initial ammonium concentration on the heterotrophic nitrification capability of T13 is shown in Table 1. A high ammonium removal rate of 100 % was observed from the two setups at initial NH4 +-N concentrations of 52.71 and 77.47 mg/L. By contrast, increased initial ammonium concentration (E2 to E6) decreased the NH4 +-N removal rate from 100 to 39.56 %. Moreover, during the heterotrophic nitrification process, the organics are necessary for the microbes to utilize the ammonium. Thus, the more available organics exist, the more ammonium can be utilized. The calculated maximum ammonium utilization of the T13 was approximately 96.93 mg/L under the specific initial COD concentration (1860.84 mg/L) condition.
Capability of aerobic denitrification
The aerobic denitrification performance of T13 was investigated with nitrate as the sole nitrogen source at 30 °C and 160 rpm, as depicted in Fig. 2a. The nitrate concentration continuously decreased and eventually reached 2.47 mg/L at 15 h. The maximum removal rate of 17.44 mg L−1 h−1 was calculated between 3 and 12 h during the log phase. Notably, nitrite dramatically accumulated to 147.44 mg/L at 15 h, which led to the low TN removal of only 18.63 %.
The capability of T13 to utilize nitrite was examined by making nitrite as the sole nitrogen source at 30 °C and 160 rpm, as shown in Fig. 2b. Nitrite concentration decreased from 161.71 to 76.1 mg/L in the first 15 h after inoculation. As T13 bred into the stead phase, a nearly constant nitrite concentration and TN removal rate were observed. The unsatisfactory reduction rate of nitrite (52.94 %) by strain T13 was much lower than the nitrate reduction rate (98.69 %) when nitrate was used as the sole nitrogen. The same performance by a psychrophilic aerobic denitrifier S1-1 has been reported (Zheng et al. 2011). Meanwhile, no nitrate was detected throughout the entire process, which may be due to the preference of strain T13 on utilizing nitrate over utilizing nitrite.
Effect of DO on aerobic denitrification
The effect of dissolved oxygen (DO) on T13 nitrate reduction is shown in Fig. 3a. The significant impacts of oxygen were exhibited more on nitrate depletion rate than on removal efficiency. Nearly 100 % nitrate was exhausted under each condition. The slower reaction rate was observed during decreasing the shaking speed. The entire amount of nitrate was expended at only 15 h at 160 rpm, with the depletion rate of 12.40 mg NO3 −-N L−1 h−1. At 18, 21, and 30 h, the cultures were examined under 100, 50, and stationary cultivation conditions. The utilization rates are 10.40, 8.77, and 6.27 mg NO3 −-N L−1 h−1, respectively (Table S2). The low shaking speed could retard the connection between microorganisms and substrates.
Simultaneous heterotrophic nitrification-aerobic denitrification under low shaking speed condition
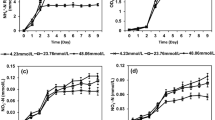
Simultaneous utilization of ammonium and nitrate was rapidly realized in the first 15 h under 160 rpm cultivation condition, which provided sufficient DO to T13 (Fig. 4a). Nearly 100 % of ammonium and nitrate removal were both observed. However, the severe accumulation of nitrite, which reached 194.8 mg/L at 15 h, led to inefficiency in TN removal of only 23.47 %.
By comparison, the aerobic denitrification capability of T13 in low DO conditions controlled by reducing the cultivation shaking speed to 50 rpm was shown in Fig. 4b. Likewise, ammonium and nitrate could be consumed simultaneously by T13 at 50 rpm with the average removal rate of 4.52 mg NH4 +-N L−1 h−1 (0–18 h) and 8.48 mg NO3 −-N L−1 h−1 (0–21 h). Throughout the entire process, only a small quantity of nitrite was produced. The peak value of 39.18 mg/L was reached at 9 h, then reduced to zero at 18 h. A TN removal of 99.21 % was observed, which was due to the high nitrite reductase activity unlimited by the low dissolved oxygen.
Discussion
Ammonium removal performance
Generally, nitrite and nitrate should be produced during heterotrophic nitrification and denitrified to gaseous nitrogen to say that heterotrophic nitrification and aerobic denitrification are simultaneously occurring (Chen and Ni 2011). However, neither obvious accumulation of nitrate nor nitrite occurred throughout the entire process. This phenomenon may be due to the assimilation of ammonium (Zhao et al. 2010a, b) and the rapid denitrification of trace nitrite and nitrate produced during heterotrophic nitrification using ammonia monooxygenase (Zhao et al. 2010a, b) to nitrogenous gas. Similar results are observed during heterotrophic nitrification by Acinetobacter sp. Y16 (Huang et al. 2013) and P. stutzeri YZN-001 (Zhang et al. 2011). On the contrary, trace nitrite was formed as an intermediate during the oxidation of ammonium in Acinetobacter sp. HA2 (Zhang et al. 2011), and both nitrate and nitrite were obviously accumulated as terminal products for Rhodococcus sp. CPZ24 (Chen et al. 2012). All these reports indicate that the gap of nitrogen removal capability between different heterotrophic nitrification bacteria is based not only on ammonium removal rate but also on the by-product and its utilization.
A higher OD660 was observed along with more ammonium utilized by T13 (Table 1), indicating that assimilation played an important role in ammonium removal. Furthermore, the accumulation of both nitrate and nitrite were not examined at the end of each experiment because of the rapid aerobic denitrification.
Capability of aerobic denitrification
The nitrite accumulation characteristics of T13 during nitrate reduction were similar to those found for Paracoccus denitrificans DL-23 under high nitrate load condition (Liang et al. 2011). Generally, the aerobic denitrificans can catalyze the transformation of nitrate to nitrite under aerobic condition by periplasmic nitrate reductase (Nap), which locates the periplasmic side of the cell membrane, and be less inhibited by oxygen (Sparacino-Watkins et al. 2014). However, the Nir, as another key role in the whole aerobic denitification process, is more sensitive to oxygen. The activity of Nir will decline when DO is increased (Körner and Zumft 1989). Therefore, research on the effect of oxygen on aerobic denitrification is significant.
Effect of DO on aerobic denitrification
The nitrite reduction process is sensitive to DO. High DO concentration could inhibit the activity of nitrite reductase (Ka et al. 1997). The DO had an obvious conspicuous influence on T13 nitrite removal, as described in Fig. 3b. A remarkable increase in nitrite removal was found when the shaking speed was decreased from 160 to 0 rpm. Relatively lower nitrite utilization rates of 62.37 % at 160 rpm and 71.38 % at 100 rpm were presented compared with the slower shaking speed cultivation, which reached up to approximately 100 % under both 50 rpm and stationary conditions. However, increasing nitrite removal by reducing the shaking speed from 100 to 50 rpm would be at the cost of extending the reaction time from 18 to 24 h (Table S3).
Simultaneous heterotrophic nitrification-aerobic denitrification under low shaking speed condition
Nitrite reductase is most sensitive to dissolved oxygen. However, thresholds for all denitrification enzymes shifted to lower oxygen levels (Körner and Zumft 1989). Thus, T13 would express better denitrification performance under low dissolved oxygen conditions. It is effective to promote the nitrite reduction by minimizing the inhibition of dissolved oxygen of Nir, and achieve the high-efficiency TN removal of 99.21 %. In summary, this is the first research on improving the heterotrophic nitrification-aerobic denitrification efficiency by DO regulation. Nitrite reduction is the bottleneck of the aerobic denitrification process. For practical application, integrating with immobilization may be a good way to promote the efficiency of TN removal, because it can provide a micro-aerobic/anoxic environment for nitrite reduction inside the carriers.
References
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, D.C
Chen Q, Ni JR (2011) Heterotrophic nitrification–aerobic denitrification by novel isolated bacteria. J Ind Microbiol Biotechnol 38:1305–1310
Chen P, Li J, Li QX, Wang Y, Li S, Ren T, Wang L (2012) Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcussp. CPZ24. Bioresour Technol 116:266–270
Huang XF, Li WG, Zhang DY, Qin W (2013) Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification–aerobic denitrification at low temperature. Bioresour Technol 146:44–50
Ji B, Wang H, Yang K (2013) Tolerance of an aerobic denitrifier (Pseudomonas stutzeri) to high O2 concentrations. Biotechnol Lett 36:719–722
Ka JO, Urbance J, Ye RW, Ahn TY, Tiedje JM (1997) Diversity of oxygen and N-oxide regulation of nitrite reductases in denitrifying bacteria. FEMS Microbiol Lett 156:55–60
König H, Claus H, Varma A (2010) Prokaryotic cell wall compounds. Springer, Berlin, pp 383–406
Körner H, Zumft WG (1989) Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol 55:1670–1676
Li A, Gai ZH, Cui D, MaF YJX, Zhang XX, Sun YL, Ren NQ (2012) Genome sequence of a highly efficient aerobic denitrifying bacterium, Pseudomonas stutzeri T13. J Bacteriol 194:5720
Liang SC, Zhao M, Lu L, Wang CL, Zhao LY, Liu WJ (2011) Isolation and characteristic of an aerobic denitrifier with high nitrogen removal efficiency. Afr J Biotechnol 10:10648–10656
Madigan M, Mrtinko JM, Stahl D, Clark DP (2012) Brock biology of microorganisms, 13th edn. Pearson, San Francisco, pp 171–175
McDaniel LE, Bailey EG (1969) Effect of shaking speed and type of closure on shake flask cultures. Appl Microbiol 117(2):286–290
Padhi SK, Tripathy S, Sen R, Mahapatra AS, Mohanty S, Maiti NK (2013) Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. Int Biodeterior Biodegrad 78:67–73
Philippot L, Mirleau P, Mazurier S, Siblot S, Hartmann A, Lemanceau P, Germon JC (2001) Characterization and transcriptional analysis of Pseudomonas fluorescens denitrifying clusters containing the nar, nir, nor and nos genes. Biochim Biophys Acta 1517:436–440
Robertson LA, Kuenen JG, Kleijntjens R (1985) Aerobic denitrification and heterotrophic nitrification by Thiosphaera pantotropha. Antonie Van Leeuwenhoek 51:445
Scholten E, Lukow T, Auling G, Kroppenstedt RM, Rainey FA, Diekmann H (1999) Thauera mechernichensis sp. nov., an aerobic denitrifier from a leachate treatment plant. Int J Syst Bacteriol 49:1045–1051
Sparacino-Watkins C, Stolz JF, Basu P (2014) Nitrate and periplasmic nitrate reductases. Chem Soc Rev 43:676–706
Wittmann C, Kim HM, John G, Heinzle E (2003) Characterization and application of an optical sensor for quantification of dissolved O2 in shake-flasks. Biotechnol Lett 25(5):377–380
Zhang J, Wu P, Hao B, Yu Z (2011) Heterotrophic nitrification and aerobic denitrification by the bacterium Pseudomonas stutzeri YZN-001. Bioresour Technol 102:9866–9869
Zhao B, He YL, Hughes J, Zhang XF (2010a) Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour Technol 101:5194–5200
Zhao B, He Y-L, Zhang XF (2010b) Nitrogen removal capability through simultaneous heterotrophic nitrification and aerobic denitrification by Bacillus sp. LY. Environ Technol 31:409–416
Zheng H, Liu Y, Sun G, Gao X, Zhang Q, Liu Z (2011) Denitrification characteristics of a marine origin psychrophilic aerobic denitrifying bacterium. J Environ Sci 23:1888–1893
Zhu G, Peng Y, Li B, Guo J, Yang Q, Wang S (2008) Biological removal of nitrogen from wastewater. Rev Environ Contam Toxicol 192:159–195
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (51108120 and 51178139), the National Creative Research Group of the National Natural Science Foundation of China (51121062), the 4th Special Financial Grant from the China Postdoctoral Science Foundation (201104430), and the 46th China Postdoctoral Science Foundation (20090460901).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 184 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Li, A., Zhang, X. et al. Regulation of dissolved oxygen from accumulated nitrite during the heterotrophic nitrification and aerobic denitrification of Pseudomonas stutzeri T13. Appl Microbiol Biotechnol 99, 3243–3248 (2015). https://doi.org/10.1007/s00253-014-6221-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6221-6