Abstract
Continuous, anaerobic fermentations of D-xylose were performed by Actinobacillus succinogenes 130Z in a custom, biofilm reactor at dilution rates of 0.05, 0.10 and 0.30 h−1. Succinic acid yields on xylose (0.55–0.68 g g−1), titres (10.9–29.4 g L−1) and productivities (1.5–3.4 g L−1 h−1) were lower than those of a previous study on glucose, but product ratios (succinic acid/acetic acid = 3.0–5.0 g g−1) and carbohydrate consumption rates were similar. Also, mass balance closures on xylose were up to 18.2 % lower than those on glucose. A modified HPLC method revealed pyruvic acid excretion at appreciable concentrations (1.2–1.9 g L−1) which improved the mass balance closure by up to 16.8 %. Furthermore, redox balances based on the accounted xylose consumed and the excreted metabolites, indicated an overproduction of reducing power. The oxidative pentose phosphate pathway was shown to be a plausible source of the additional reducing power.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dicarboxylic acids are expected to play a crucial role in the feasibility of future biorefineries due to their potential as platform molecules for deriving both commodity and specialty chemicals [1], and their value-addition as co-products in complementing biofuels production [2]. Succinic acid (SA), a four carbon dicarboxylic acid produced as an intermediate in the tricarboxylic acid (TCA) cycle, is of particular interest in this regard. SA is considered a prospective high value, bio-based chemical due to its potential as a building block for a number of renewable products (e.g., 1,4-butanediol and tetrahydrofuran) [3], and due to the attractive and growing market for both SA and its derivatives. The current market size for SA is around 30–50 kton/annum [4] with an estimated increase to 700 kton/annum by 2020 [5]. Although commercial scale bio-production of SA has been demonstrated to some degree of success [6], the expected growth in the SA market hinges on efficient, bulk bio-production that is cost-competitive with the conventional petrochemicals route [7, 8]. The conventional route, through which the majority of commercial SA is currently produced, follows an unsustainable and comparatively expensive petroleum-based process where butane is converted into SA via maleic anhydride [9]. As such, bio-based SA can serve as an attractive, sustainable replacement for petroleum-derived SA as well as related products such as, for example, maleic anhydride and adipic acid [7]. Furthermore, bio-based SA can be used in the synthesis of bio-polymers such as polybutylene succinate and polyurethane [10], thereby further driving an increase in the attractiveness and market size of SA.
Various microbial platforms have been explored for bio-production of SA with the best bench-scale performance achieved by Actinobacillus succinogenes [11–14], Anaerobiospirillum succiniciproducens [15, 16], Mannheimia succiniciproducens [17–19], and engineered strains of Escherichia coli [20–22]. Current commercial bio-production of SA is based primarily on industrial proprietary yeasts (Saccharomyces cerevisiae and Candida krusei) and bacteria (Escherichia coli and Basfia succiniciproducens) that utilise starch-derived, refined sugars (e.g., sucrose, glucose and fructose) as feedstocks [4]. Wild type Actinobacillus succinogenes is a top-performing succinate producer and has been shown to produce SA at high productivities, yields and titres in mixed-acid, bench-scale fermentations [11–14, 23, 24]. Furthermore, A. succinogenes is tolerant to high acid titres [25] and can consume a variety of carbohydrates including glucose, xylose, arabinose and galactose [26], making it an attractive contender for industrial bio-production of SA from diverse feedstocks.
In addition to the biocatalyst, the choice of feedstock is an important consideration as it drives the overall economics of the process and directs process development [2]. An ideal feedstock for commercial bio-production should tie in with larger biorefinery strategies to leverage the advantages of economies of scale thereby further reducing production costs. The focus, therefore, is to produce SA at productivities, yields and titres that are industrially competitive and to utilise a renewable feedstock that is aligned with the long-term biorefinery strategy. Lignocellulosic plant biomass meets the criteria of such a feedstock as it is renewable, relatively low in cost, abundant and readily available [27]. In addition, plant biomass is non-edible and therefore does not compete with starch-based food crops. Achieving cost-competitive SA production on lignocellulosic biomass would be an ideal bio-based SA process. The feasibility of such a process depends on efficient utilisation and conversion of all the carbohydrates present in lignocellulose.
Xylose is a major carbohydrate in hemicellulose, and the xylan polymer constitutes between 20 and 50 % of grassy biomass, and 8–25 % of woody biomass [28]. Furthermore, current promising pretreatment technologies are able to generate xylose-rich hydrolysates from corn stover, a model lignocellulosic feedstock [29–31]. Therefore, it is necessary to determine the feasibility and limits of SA production on a clean xylose feed to establish a baseline for lignocellulosic fermentations, and to gain insight into the extent to which C5 carbohydrates can be utilised by A. succinogenes. Furthermore, insight into the metabolism of the organism when grown on xylose is crucial for integrated process development and fermentation optimisation, and is instructive in determining potential targets for metabolic engineering. In this regard, continuous operation is advantageous as it allows for analysis of the system at steady-state. Steady-state conditions are crucial for performing accurate mass balances and metabolic flux analysis which provide insight into the physiology of the organism. In addition, continuous operation, especially with immobilised cells, allows for higher productivities making it a potential process option for bulk scale production.
This paper presents the first continuous fermentations of xylose to succinic acid using immobilised, wild type Actinobacillus succinogenes 130Z. Fermentations were performed in a custom biofilm reactor since continuous operation of A. succinogenes is necessarily biofilm based. Moreover, continuous operation allowed for proper mass and redox balances through steady-state conditions, which provided insights into the xylose metabolism of A. succinogenes. Finally, the paper explores the fermentation performance on xylose relative to a previous study on glucose in terms of yield, titre and productivity.
Materials and methods
Microorganism
Actinobacillus succinogenes 130Z (DSM 22257; ATCC 55618) was acquired from the German Collection of Microorganisms and Cell Cultures (DSMZ). Cultures were maintained in 66 % w/w glycerol solutions at −40 °C for medium-terms and in tryptone soy broth (TSB) suspensions at 4 °C for short-terms. Inoculum was incubated at 37 °C and 150 rpm for 16–24 h in 30 mL sealed vials containing 15 mL sterilised TSB at 30 g L−1. Prior to inoculation, the inoculum was analysed using HPLC to ensure purity and consistent metabolite distributions.
Fermentation medium
The fermentation medium comprised three parts: (1) a nutrient and salts mixture, (2) a xylose solution and (3) a phosphate buffer. The nutrient and salts mixture consisted of 6.0 g L−1 yeast extract, 10.0 g L−1 clarified corn steep liquor (Sigma-Aldrich), 1.0 g L−1 NaCl, 0.2 g L−1 MgCl2·6H2O, 0.2 g L−1 CaCl2·2H2O, 1.36 g L−1 sodium acetate, 0.5 mL L−1 Antifoam SE-15 (Sigma-Aldrich) and 0.16 g L−1 Na2S·9H2O (to ensure anoxic feed). Note, all chemicals were obtained from Merck KgaA (Darmstadt, Germany) unless stated otherwise. To clarify the corn steep liquor, a 200 g L−1 solution was boiled at 105 °C for 15 min. The solution was then cooled over night at 4 °C to facilitate solids precipitation, and the supernatant was removed and used for the nutrient mixture. The phosphate buffer comprised 3.2 g L−1 KH2PO4 and 1.6 g L−1 K2HPO4. The xylose solution was prepared at approximately 50–85 g L−1 with the effective operational concentration of the feed (accounting for dilution due to base and antifoam addition) varying between 43 and 84 g L−1 during fermentation. To maintain CO2 saturation in the broth and to avoid CO2 limitations, CO2 gas (Afrox, Johannesburg, South Africa) was fed at 0.10 vvm.
Fermentations
Continuous fermentations (runs) were performed using a custom bioreactor (fermenter) similar to that presented in [11] except with a working volume of approximately 360 mL. Temperature was controlled at 37 °C by means of a hotplate connected to a thermocouple for control feedback. pH was controlled at 6.80 using a Liquiline CM442 (Endress+Hauser, Gerlingen, Germany) coupled to a Ceragel CPS71D glass electrode (Endress+Hauser, Gerlingen, Germany). Control was achieved by dosing unsterilised 10 N NaOH in an on–off fashion actuated by an internal relay of the Liquiline. The average flow rate of NaOH was calculated in real-time and used to calculate effective inlet xylose and inlet organic acid concentrations, as small amounts of succinic- (~0.4 g L−1), lactic- (~0.7 g L−1) and acetic acid (~1.0 g L−1) were present in the feed due to corn steep liquor and sodium acetate, respectively.
The reactor system (reactor, fittings, liquids and bottles) was autoclaved at 121 °C for 60 min with the three feed components contained in separate bottles to prevent unwanted reactions, and to avoid the formation of precipitates. After inoculation (~10 mL), the fermenter was operated in batch mode to increase cell density and then switched to continuous mode at a low dilution rate (0.05 h−1) to avoid cell washout. Once a stable biomass population was established, the dilution rate was increased (0.1–0.3 h−1) to accelerate the formation of biofilm. Antifoam was added as needed and its dilution of the feed medium was accounted for similarly to that of base addition. The total biomass content in the fermenter was increased by using support structures and packing to facilitate biofilm attachment, growth and stability. The supports included tightly bound wooden sticks (run 1), silicone-tubing segments (run 2) and loosely spaced wooden sticks (run 3). Different supports were used in each fermentation in an attempt to overcome blockages of the system due to excessive biofilm growth. All supports were cleaned with distilled water and dried prior to insertion.
Analytical methods
Sampling of the reactor was performed on a bed of ice to suppress metabolic activity outside of the reactor. The concentrations of organic acids, ethanol and xylose present in the feed medium and fermentation broth were determined by means of high-performance liquid chromatography (HPLC). An Agilent 1260 Infinity HPLC (Agilent Technologies, USA), equipped with a 300 × 7.8 mm Aminex HPX-87H ion-exchange column (Bio-Rad Laboratories, USA), and an RI detector was used. For the first two fermentations, a 5 mM solution of H2SO4 at a flowrate of 0.6 mL min−1 served as the mobile phase with column and RID temperatures of 60.0 and 55.0 °C, respectively. To avoid co-elution of xylose and pyruvate, a 20 mM solution of H2SO4 (all else equal) was used for the mobile phase in the third fermentation. In the given system, acid retention times decrease with increasing mobile phase acidity, whereas carbohydrates are minimally influenced. It was found that suitable xylose and pyruvic acid separation occurred with a 20 mM H2SO4 mobile phase without leading to co-elution of other compounds.
Data analysis and collection
A total of three independent fermentations were performed at dilution rates of 0.05, 0.10 and 0.30 h−1 with a total fermentation time of approximately 1500 h. Steady-states were achieved at each dilution rate in each fermentation. Averages for each variable are based on the steady-state results at each dilution rate for each fermentation. In the case where multiple steady-states were obtained at a single dilution rate within a fermentation, the average of these was used in determining the average for the variable. Using a single set of data from each fermentation ensured that the overall average was not biased towards any one fermentation and that an equal contribution was made from each fermentation. Standard deviation, calculated using the averages of each fermentation run (three values in total), was used to reflect the variation in each variable. The redox analyses performed on the third fermentation data include averages of all the steady-state data within that fermentation.
Samples (data) are only meaningful or only considered within the definition of a contunuous reactor once the system is at steady-state. Steady-state (or pseudo steady-state) was assumed when (1) the time-averaged NaOH flowrate remained within 5 % of a 4-h average and (2) metabolite and residual xylose concentrations showed fluctuations within 3 % over a period of at least two volume turnovers.
Mass balance calculations
Mass balances were performed by calculating the stoichiometric amount of substrate required to produce the measured metabolite concentrations based on elemental balances, and comparing this amount to the actual amount of substrate consumed. The elemental balances were performed on C, H and O between the carbohydrate substrate and the products, since for each element appearing in the substrate, an equal amount must appear in the products. The products comprise all the measured metabolites of the fermentation (i.e., succinic-, acetic-, formic- and pyruvic acid), and H2O. Since CO2 can serve as both a substrate and a metabolite, it remains an unknown in the system and its net production or consumption will be reflected in the mass balance solution. The elemental balances in this case provide three equations (C, H and O), and given a system of M products (including CO2 and H2O), M-3 specifications (measurements) are required to solve for the unknown metabolite production rates and the corresponding element-balanced substrate consumption rate. The system can be solved using linear algebra techniques. Once the substrate consumption rate is determined, it is divided by the actual (measured) substrate consumption rate and expressed as a percentage that represents the mass balance closure. Note that the accuracy of the mass balance is dependent upon including all the metabolites in the calculation; if metabolites are neglected, the closures will necessarily be lower. A generalised approach to elemental balancing is presented in [32].
Results and discussion
Continuous xylose fermentations
Succinic acid (SA) was successfully produced continuously from xylose by A. succinogenes in a custom biofilm reactor in three separate fermentations each including dilution rates (D) of 0.05, 0.10 and 0.30 h−1. The fermentations spanned a total of approximately 1500 h cumulatively and showed generally good stability. The only major operational challenge was caused by accumulation of biofilm which led to clogging of the support packing resulting in regions of preferential flow; hence different supports were used in each fermentation in an attempt to overcome this issue. Erosion or sloughing of the biofilm, known features of biofilm behaviour [33], resulted in disturbances of the steady-states. However, biofilm was able to regrow and once the fermenter had stabilised, steady-states were re-established.
The SA productivity and rate of xylose consumption were similar in all three fermentations (Fig. 1). Therefore, the performance of the three fermentations can be directly compared despite different types of biofilm supports being employed in each fermentation. Similarly, the rate of glucose consumption in a previous SA study with A. succinogenes [11] compared remarkably well with the average rate of xylose consumption in this study (Fig. 2a), and therefore it is reasonable to make direct comparisons between the two studies. As such, it was found that while the rates of xylose and glucose consumption were similar, the SA productivity was noticeably lower for the xylose fermentations (Fig. 2b) and varied between 1.5 and 3.4 g L−1 h−1. A maximum SA productivity of 3.6 g L−1 h−1 (single steady-state) was obtained at D = 0.3 h−1 which is 11.4 % lower than the maximum achieved on glucose (4.4 g L−1 h−1) across the same range of dilution rates. The only other prominent pure xylose fermentation study with A. succinogenes reports a productivity of 0.54 g L−1 h−1 [34]. The nearest approximations to pure xylose fermentations are those on lignocellulosic biomass containing high xylose concentrations where the best SA productivities reported are: 0.99 g L−1 h−1 on acid-pretreated sugarcane bagasse [35], 1.01 g L−1 h−1 on sugar cane bagasse [36], and 1.21 g L−1 h−1 on straw hydrolysate [37]. Liang et al. [38] achieved productivities of 2.31 and 1.69 g L−1 h−1 on sugar cane bagasse and corn stover hydrolysate respectively using engineered E. coli. Although fermentation inhibitors may have negatively impacted productivities in these studies, the sugar streams also contained glucose which may have increased SA productivities above that of a pure xylose stream due to the higher yields attainable on glucose. Importantly, all these studies were conducted in batch or repeat-batch mode and the high productivities achieved in this study highlight the benefits of operating continuously. Also, the use of biofilm to increase cell densities and enhance productivities [39] is clearly demonstrated in the current study.
The average volumetric rate of xylose consumption (Xyl cons.) and succinic acid production (SA prod.) for all three runs (R). Packing types: R1 tightly bound wooden sticks, R2 silicone segments, R3 loosely spaced wooden sticks. Open diamond xylose consumption R1, filled diamond SA productivity R1, open circle xylose consumption R2, filled circle SA productivity R2, open triangle xylose consumption R3, filled triangle SA productivity R3
Summary of the xylose fermentations relative to a previous study on glucose [11]. Each data point represents the average of the steady-states at each dilution rate for all three fermentations. The error bars reflect the standard deviation (σ) for each data set. For the cases where the error bars are not visible, the markers are covering the bars and σ is, therefore, small. Xylose (Xyl) and glucose (Glc) runs were performed at the same dilution rates, but the glucose data sets are shifted to the left on the x axis in each graph to compare the error bars more clearly with those of xylose. SA succinic acid, AA acetic acid, FA formic acid. Filled circle glucose study, filled triangle xylose study, In d and f: open circle glucose study, open triangle xylose study
The lower SA productivities on xylose correspond to lower SA concentrations (C SA) (Fig. 2c), where C SA was between 10.9 and 29.4 g L−1 on average. The acetic acid concentration (C AA) ranged from 3.6 to 6.0 g L−1 (Fig. 2d) increasing at lower dilution rates but to a lesser extent than in the glucose study, whereas the formic acid concentration (C FA) remained between 2.5 and 2.8 g L−1. Overall, the metabolite concentrations in the xylose fermentations followed similar trends to those of the glucose fermentations and no ethanol formation was observed.
The average yield of succinic acid on xylose was between 0.55 and 0.68 g g−1 and increased with decreasing dilution rate (Fig. 2e). The previous study on xylose reported a yield of 0.76 g g−1 in batch mode [34], and similar to the productivity comparison, studies with A. succinogenes using xylose-rich lignocellulosic feed streams report higher overall yields (e.g., 0.79 g g−1 on sugarcane bagasse hydrolysate [35] and 0.83 g g−1 on straw hydrolysate [37]). However, as mentioned before, the lignocellulosic feed streams all contained glucose which likely increased the net yield above that of pure xylose as seen in the comparison made in this study. More importantly, the SA yield on xylose was substantially lower than that on glucose across all dilution rates which dovetails with the correspondingly lower C SA values in Fig. 2c. Given that the rate of xylose consumption was similar to that of glucose, a lower SA yield at similar overall by-product concentrations implies that there is mass unaccounted for in the system. In other words, in the xylose runs, C SA together with the by-product concentrations is lower than the equivalent amount in the glucose fermentations and can, therefore, not account for the total amount of xylose consumed.
The average SA/AA mass ratio (Y AASA), or selectivity to SA, was higher at lower dilution rates implying that carbon flux to SA increased as the dilution rate decreased (i.e., as acid titres increased) which relates to the higher SA yields at lower dilution rates. Also, Y AASA (3.0–5.0 g g−1) was similar for both xylose and glucose fermentations (Fig. 2f) which indicates that a similar flux shift towards SA occurred with both substrates. Similar to the glucose study, the FA/AA mass ratio (Y AAFA) on xylose showed a decreasing trend with decreasing dilution rate, but to a lesser extent, which corresponds to the trend in C FA. A decrease in Y AAFA implies that pyruvate dehydrogenase and/or formate dehydrogenase activity is greater at lower dilution rates as detailed in previous continuous fermentations on glucose by A. succinogenes [11, 12]. However, the dehydrogenase activity appears to be lower in the case of xylose since Y AAFA does not decrease to the same extent as in the glucose study.
Regarding the stability and time-course of the fermentations, it can be seen that once stable biofilm was achieved, steady-state conditions remained fairly stable in terms of productivity (Fig. 3a) and yield (Fig. 3b), especially at the lower dilution rates. At D = 0.3 h−1, there appears to be more scatter in the variables (last two points at 0.3 h−1), most likely due to biofilm development under lower acid titres, where sections of biofilm detached along with regions of gradual regrowth. It is interesting to note that the steady-state at D = 0.05 h−1 was repeatable at three distinct time intervals during the fermentation.
Time-course of the third fermentation (R3) for a succinic acid productivity and b succinic acid yield on xylose. In each figure, region 1 represents the start-up and biofilm growth phase, region 2 shows a disturbance causing loss of productivity and a decrease in yield, and region 3 is where stable operation and steady-states were achieved. Not all data points are under steady-state conditions. Filled triangle D = 0.05 h−1, filled circle D = 0.1 h−1, filled diamond D = 0.3 h−1
Mass and redox balances
While the above results are valuable in themselves and suggest that A. succinogenes can effectively convert a xylose-rich feedstock to SA, it is instructive to further understand the differences between the xylose and glucose fermentations. To investigate the yield differences between the two fermentations, and the possibility of unaccounted mass presented above, the mass balances of each system were compared. Mass balances were performed by calculating the stoichiometric amount of substrate required to produce the measured metabolite concentrations based on elemental balances, and comparing this amount to the actual amount of substrate consumed (see “Materials and methods”, “Mass balance calculations”). The mass balance closures were found to be lower for the xylose fermentations (Table 1). The previously mentioned studies on xylose-containing fermentations by A. succinogenes do not interpret the results using mass balances and are performed exclusively in batch reactors where mass balances cannot be accurately performed; therefore, no mass balance comparisons are available.
Incomplete mass balance closures can be attributed to undetected metabolites or biomass growth when no dry cell weight (DCW) measurements are included in the mass balance. In biofilm fermentations, cell mass accumulates in the fermenter [39], and erosion and sloughing of biofilm [33] cause segments to exit the fermenter unpredictably. As such, DCW measurements either do not account for biomass incorporated into the biofilm or contain biofilm fragments of accumulated biomass that overestimate mass measurements; therefore, DCW in a biofilm reactor is not a true reflection of biomass growth or suspended cell concentrations. Consequently, it is difficult to determine the amount of substrate used for biomass growth at steady-state during a biofilm fermentation. However, the growth rate of A. succinogenes has been shown to decrease significantly with increasing C SA due to product inhibition and becomes negligible beyond a C SA of 15 g L−1 in continuous fermentations [40], and cell growth terminates at a C SA of around 13 g L−1 in batch fermentations [41]. In addition, it has been shown that over extended periods of steady-state operation (96 h) in a biofilm fermentation with A. succinogenes, DCWs were low on average (0.19 g L−1) compared to the average amount of glucose consumed (42 g L−1) [11] and so at most 0.45 % of the glucose consumed was used for biomass. In light of these two observations, and considering that C SA was between 10.9 and 29.4 g L−1 throughout this study, it was assumed that substrate consumption through biomass growth was negligible. Any contribution of biomass growth to the overall mass balance would be more apparent at high dilution rates (e.g., 0.3 h−1) where C SA is lower (10.9 g L−1); however, the mass balance showed similar closures across all dilution rates. As such, it was assumed that biomass growth at steady-state had a negligible contribution to the mass balance across all dilution rates and it was suspected that an undetected metabolite was produced. Lin et al. [25] reported appreciable pyruvic acid concentrations (~4.8 g L−1) together with succinic-, acetic- and formic acid in batch fermentations of glucose with A. succinogenes. Furthermore, Guettler et al. [26] reported small amounts of pyruvic acid when A. succinogenes was grown on an enriched medium. Despite good mass balance closures without pyruvic acid production in the comparative glucose fermentations, pyruvic acid was considered a likely candidate for the missing metabolite.
Due to the incomplete mass balance closures described above, a modified HPLC method was used for analysis of metabolites and xylose in the third fermentation. Analysing a known solution of xylose and pyruvic acid by HPLC showed complete co-elution of the two species as seen by a single, enlarged peak on the chromatogram. By applying the same HPLC method as in the first two fermentations, but with a more acidic mobile phase (20 mM versus 5 mM H2SO4), it was possible to separate the peaks distinctly. Given the new analysis method, a third fermentation of xylose was performed. The third fermentation revealed that pyruvic acid was indeed produced at appreciable concentrations at all three dilution rates with an associated improvement in the mass balances compared to the first two fermentations (Fig. 4). Note, Fig. 2 is based on all three fermentations since none of the fermentation variables, except yield and xylose consumption rate, were influenced by the detection of pyruvate. Yield and xylose consumption rate were marginally affected because residual xylose forms the basis of each calculation and the quantification of residual xylose was impacted by co-elution of pyruvic acid. Fortunately, since high xylose feed concentrations resulted in residual xylose concentrations substantially greater than the detected pyruvic acid concentrations, the presence of pyruvate was shown to negligibly influence the xylose peak area, as seen by similar yields and xylose consumption rates when including and excluding the third fermentation data in the calculation of averages.
Despite the improvement in mass balance closures with pyruvic acid inclusion, the balances were still lower than those of the glucose fermentations and complete closures were not achieved. However, the improvement in mass balance closures, together with a more accurate picture of the metabolite distribution during xylose fermentations, underscores the necessity and utility of performing mass balances on fermentation data. In addition, with an improved view on the active metabolic network of A. succinogenes, it becomes possible to perform redox balances with better accuracy which are instructive in understanding metabolic flux distributions. These benefits further highlight the advantages of steady-state analysis which is only possible during continuous operation.
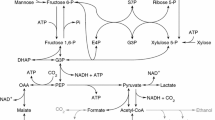
Redox balances were performed by considering the NADH contributions from the overall, balanced metabolic pathways that convert xylose to metabolites. The overall pathways can be viewed as either reductive or oxidative, where a net production of NADH in a pathway is viewed as oxidative while a net consumption of NADH is reductive. In a redox balanced system, the net NADH of the oxidative and reductive pathways should be zero. Equations 1–4 give overall pathways for the molar conversion of xylose (Xyl) to excreted metabolites with the associated amounts of NADH, and can be further visualised with reference to the proposed central metabolic network of A. succinogenes (Fig. 5), which includes pyruvic acid excretion. An equation for cell growth has been excluded as detailed in the above discussion.
Proposed central metabolic network of A. succinogenes showing the uptake of xylose and subsequent conversion to succinic acid and by-products. The genome of A. succinogenes has been published, therefore insight into the metabolism can be gained from the genome [42–44]. This insight has been incorporated into Fig. 5. Relevant enzymes with EC numbers: 1 High affinity ATP-dependent xylose transporter (3.6.3.17); 2 xylose isomerase (5.3.1.5); 3 xylulose kinase (2.7.1.17); 4 ribulose-phosphate 3-epimerase (5.1.3.1); 5 pyruvate formate-lyase (2.3.1.54); 6 pyruvate dehydrogenase (1.2.4.1; 1.8.1.4; 2.3.1.12); 7 formate dehydrogenase (1.2.1.2); 8 transhydrogenase (1.6.1.2). Xyl int intracellular xylose, Xu5P xylulose-5-phosphate, Ru5P ribulose-5-phosphate, R5P ribose-5-phosphate, G3P glyceraldehyde-3-phosphate, S7P sedoheptulose-7-phosphate, E4P erythrose-4-phosphate, F6P fructose-6-phosphate, PEP phosphoenolpyruvate, Pyr pyruvate, AA acetic acid, FA formic acid, EtOH ethanol
Equation 1 represents the formation of acetic acid (AA) via the pyruvate dehydrogenase (PDH) route, and Eq. 2 represents the formation of acetic acid and formic acid (FA) via the pyruvate formate-lyase (PFL) route. If formic acid is further converted to CO2 and NADH by formate dehydrogenase, the overall pathway is equivalent to Eq. 1. Pyruvic acid (Pyr) production followed by excretion is given by Eq. 3, and succinic acid (SA) formation is represented by Eq. 4 and involves net consumption of NADH. Thus, Eqs. 1–3 can be seen as the oxidative pathways while Eq. 4 is the reductive pathway. Note, in balancing xylose uptake via the non-oxidative pentose phosphate pathway (Fig. 5), three xylose molecules are converted into two fructose-6-phosphate (F6P) molecules and one glyceraldehyde-3-phosphate molecule, yielding five phosphoenolpyruvate molecules which in turn produce five NADH molecules. Therefore, for each xylose molecule consumed, 5/3 NADH molecules are generated. Performing an NADH balance using the measured metabolites as described here can be seen as a product-based redox balance since the closure is relative to the measured metabolites (products) only.
Using known metabolite concentrations from each steady-state in the third fermentation, the NADH in each pathway was calculated using Eqs. 1–4, and the produced and consumed NADH amounts were compared. From Fig. 6a it can be seen that more NADH was consumed than produced and the deviation is more pronounced at increasing amounts of NADH consumption. Furthermore, the deviation increased with decreasing dilution rate which dovetails with increased Y AASA at lower dilution rates since more carbon is directed towards the reductive, C 4 pathway. The implication of excessive NADH consumption is that the oxidative pathways of the metabolic network (Fig. 5; excluding the oxidative pentose phosphate pathway) are unable to generate the NADH required for the observed amount of SA production. This notion can be visualised in Fig. 6b where the total amount of xylose consumed in order to satisfy the product-based redox balance (ΔX r ) is compared to the xylose consumed in each pathway based on Eqs. 1–4 and the corresponding metabolite measurements. Across all dilution rates, the sum of the xylose consumed in each pathway does not account for the total ΔX r and another pathway (“other pathway”) is required in each case to make up the xylose deficit. Moreover, it is precisely this “other pathway” (necessarily oxidative) that supplies the NADH required to close the product-based redox balance.
Redox balances of the third fermentation, a Parity plot of the NADH produced in the oxidative pathways versus the NADH consumed in the reductive pathway for each steady-state, using the excreted metabolite concentrations. The three data groupings correspond to the three dilution rates (0.05, 0.1 and 0.3 h−1). In a redox balanced system, the points would lie on the solid diagonal. Open circle NADH balance of third fermentation, solid line redox balanced system, dashed line 10 % above redox closure, dotted line 10 % below redox closure; b a comparison of the actual (measured) xylose consumed (ΔX m ) to the xylose required to satisfy the product-based redox balance (ΔX r ), and the individual contribution of each detected metabolic pathway to xylose. For each dilution rate, the averages of the metabolites and xylose consumed of all the steady-states are used in calculating individual pathway contributions and xylose consumed
A possible metabolic pathway that can supply reducing power and that is within the central metabolic network of A. succinogenes is the oxidative pentose phosphate pathway (OPPP). The OPPP provides a route from glycolysis to the non-oxidative pentose phosphate pathway (NPPP) with an associated release of reduction power as NADPH (Fig. 5). If a fraction of the F6P produced in the NPPP is cycled through the OPPP instead of proceeding through glycolysis, an overall pathway can be defined where xylose is converted to CO2 and NADPH exclusively (Eq. 5). Also, since transhydrogenase activity has been detected in A. succinogenes [42], the NADPH produced in the OPPP can be converted to NADH which can serve as the unaccounted NADH needed for the observed SA production.
If one assumes that the xylose consumed in the “other pathway” segment occurs via the OPPP, it is possible to re-check the overall redox distribution similar to the calculation in Fig. 6a, but with the inclusion of the designated OPPP xylose using Eq. 5. This results in perfect closure of the redox balance and supports the notion that reduction power is generated through the OPPP. Therefore, the OPPP is a likely candidate pathway due to not only its presence in the central metabolism of A. succinogenes, but also because it can oxidise xylose to the exact extent needed to satisfy the product-based redox balance. Furthermore, it has been previously shown that carbon flux through the pentose phosphate pathway (PPP) in a mutant strain of E. coli, grown on glucose, increased by 325 % with increasing succinic acid production resulting from increased CO2 flow rates [45]. Increased flux through the PPP can be attributed to an increased demand for reduced cofactors at greater CO2 concentrations. Also, given that the biochemical route of xylose uptake is via the PPP, increased expression of the PPP is expected in xylose fermentations as has been reported with Saccharomyces cerevisiae [46] and Aspergillus niger, where glucose-6-phosphate dehydrogenase activity was shown to be 92.4 % greater in cells grown on xylose compared to those grown on glucose [47].
A further consequence of the stoichiometry of Eq. 5 is the distinct difference in proportion between the NADH discrepancy (Fig. 6a) and the “other pathway” segment (Fig. 6b). Since a single mole of xylose can provide 10 mol of NADH (as NADPH), only a minor flux through the OPPP would be sufficient to account for missing reduction power. For example, at the highest C SA in this study when D = 0.05 h−1 (third fermentation), the OPPP would consumed only 2.4 % of the total carbon (ΔX r ) to close the redox balance. Therefore, the product-based redox balance can be closed by consuming only a small fraction of xylose in the OPPP. However, this does not close the overall mass balance and the gap between ΔX r and the actual amount of xylose consumed (ΔX m ) remains (Fig. 6b).
Given that the HPLC configuration used for metabolite analysis was capable of detecting all the organic acids in the central metabolism of A. succinogenes and no unidentified peaks were observed with no further traces of co-elution, it is unlikely that the unaccounted mass is an organic acid. To this end, it is possible that gaseous CO2 is the missing metabolite. CO2 can only be generated by simultaneously forming reduction power via the OPPP—the likely route whereby this is achieved. This implies that excess reduction power will be produced if CO2 is used to close the mass balance. It is clear from the product distribution that this additional reduction power cannot be consumed by the know pathways and accordingly it must be consumed by an external oxidising agent. Since the fermentations occurred under anaerobic conditions, the oxidising agent would likely be a component within the fermentation medium. One possible candidate is yeast extract since it is known to behave as an external electron acceptor in glycerol fermentations with A. succinogenes [48] and could therefore react with reduced cofactors from the cell. If this is the case, then the fraction of carbon (as xylose) consumed in the OPPP would increase from 2.4 to 16.5 % in the example above, where the increased consumption translates directly to CO2 production. Since this increase is considerably high and would necessitate a substantial amount of oxidant in the broth, it is more likely that a smaller fraction of additional carbon is lost as CO2 via the OPPP, thereby improving but not fully closing the mass balance. The incomplete mass balance is informative and does not detract from the process relevance of the results; rather it invites further investigation on xylose metabolism of A. succinogenes.
Conclusions
This study demonstrates that xylose can be successfully and efficiently converted to succinic acid by A. succinogenes in a continuous, biofilm reactor. Furthermore, succinic acid production on xylose compares well with that on glucose, but lower SA yields, titres and productivities were observed. By-product concentrations on xylose were overall similar to those on glucose, but with lower acetic acid and higher formic acid levels. Akin to glucose fermentations, xylose fermentations displayed incomplete mass balances but to a larger extent. Pyruvic acid excretion, as detected by a modified HPLC method, improved but did not fully close the mass balances. Furthermore, redox balances of the observed metabolites showed an overproduction of reduction power, and carbon flux through the oxidative pentose phosphate pathway (OPPP) was shown to be a plausible source of this additional reduction power which affords elevated C4 pathway flux. However, inclusion of OPPP flux was unable to close the overall mass balance. In this regard, it is possible that a fraction of carbon is lost as CO2 via an overactive OPPP where oxidation of the additional reduction power (beyond that needed to close the redox balance) occurs external to the metabolism, possibly by an oxidant in the fermentation medium. Overall, the results are useful to integrated biorefinery development as they suggest that xylose-rich, lignocellulosic feedstocks, such as corn stover hydrolysate, may be suitable for SA production by A. succinogenes. In addition, the results provide a comparative baseline for future work involving more complex, biomass feedstocks. Going forward, process optimisation is essential and a cheaper fermentation medium should be explored (e.g., replacement of yeast extract by corn steep liquor or using spent yeast from ethanol production) in addition to the organism’s tolerance to inhibitors present in actual hydrolysates.
References
Werpy T, Petersen G (2004) Top value added chemicals from biomass, volume 1. Results of screening for potential candidates from sugars and synthesis gas
Lynd L, Wyman C, Gerngross T (1999) Biocommodity Engineering. Biotechnol Prog 15:777–793. doi:10.1021/bp990109e
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “top 10” revisited. Green Chem 12:539. doi:10.1039/b922014c
Jansen MLA, van Gulik WM (2014) Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol 30C:190–197. doi:10.1016/j.copbio.2014.07.003
Choi S, Woo C, Ho J, Yup S (2015) Biorefineries for the production of top building block chemicals and their derivatives. Metab Eng 1–17. doi: 10.1016/j.ymben.2014.12.007
Cok B, Tsiropoulos I, Roes AL, Patel MK (2014) Succinic acid production derived from carbohydrates: an energy and greenhouse gas assessment of a platform chemical toward a bio-based economy. Biofuels Bioprod Biorefining 8:16–29. doi:10.1002/bbb.1427
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740. doi:10.1007/s00253-007-1057-y
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552. doi:10.1007/s002530051431
Howe-Grant M, Kroschwitz J (1991) Kirk-Othmer encyclopedia of chemical technology, 4th edn. Wiley, New York
Bechthold I, Bretz K, Kabasci S et al (2008) Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol 31:647–654. doi:10.1002/ceat.200800063
Bradfield MFA, Nicol W (2014) Continuous succinic acid production by Actinobacillus succinogenes in a biofilm reactor: steady-state metabolic flux variation. Biochem Eng J 85:1–7. doi:10.1016/j.bej.2014.01.009
Maharaj K, Bradfield MFA, Nicol W (2014) Succinic acid-producing biofilms of Actinobacillus succinogenes: reproducibility, stability and productivity. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-5779-3
Yan Q, Zheng P, Tao S-T, Dong J-J (2014) Fermentation process for continuous production of succinic acid in a fibrous bed bioreactor. Biochem Eng J 91:92–98. doi:10.1016/j.bej.2014.08.002
Van Heerden C, Nicol W (2013) Continuous succinic acid fermentation by Actinobacillus succinogenes. Biochem Eng J 73:5–11. doi:10.1016/j.bej.2013.01.015
Lee PC, Lee SY, Chang HN (2010) Kinetic study on succinic acid and acetic acid formation during continuous cultures of Anaerobiospirillum succiniciproducens grown on glycerol. Bioprocess Biosyst Eng 33:465–471. doi:10.1007/s00449-009-0355-4
Nghiem NP, Davison BH, Suttle BE, Richardson GR (1997) Production of succinic acid by Anaerobiospirillum succiniciproducens. Appl Biochem Biotechnol 63–65:565–576. doi:10.1007/BF02920454
Kim DY, Yim SC, Lee PC et al (2004) Batch and continuous fermentation of succinic acid from wood hydrolysate by Mannheimia succiniciproducens MBEL55E. Enzyme Microb Technol 35:648–653. doi:10.1016/j.enzmictec.2004.08.018
Lee PC, Lee SY, Hong SH, Chang HN (2003) Batch and continuous cultures of Mannheimia succiniciproducens MBEL55E for the production of succinic acid from whey and corn steep liquor. Bioprocess Biosyst Eng 26:63–67. doi:10.1007/s00449-003-0341-1
Oh I, Lee H, Park C et al (2008) Succinic acid production by continuous fermentation process using Mannheimia succiniciproducens LPK7. J Microbiol Biotechnol 18:908–912
Beauprez JJ, De Mey M, Soetaert WK (2010) Microbial succinic acid production: natural versus metabolic engineered producers. Process Biochem 45:1103–1114. doi:10.1016/j.procbio.2010.03.035
Vemuri GN, Eiteman MA, Altman E (2002) Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 68:1715–1727. doi:10.1128/AEM.68.4.1715-1727.2002
Jantama K, Zhang X, Moore JC et al (2008) Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101:881–893. doi:10.1002/bit.22005
Yan Q, Zheng P, Dong J-J, Sun Z-H (2014) A fibrous bed bioreactor to improve the productivity of succinic acid by Actinobacillus succinogenes. J Chem Technol Biotechnol 89:1760–1766. doi:10.1002/jctb.4257
Urbance SE, Pometto AL, Dispirito AA, Denli Y (2004) Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl Microbiol Biotechnol 65:664–670. doi:10.1007/s00253-004-1634-2
Lin SKC, Du C, Koutinas A et al (2008) Substrate and product inhibition kinetics in succinic acid production by Actinobacillus succinogenes. Biochem Eng J 41:128–135. doi:10.1016/j.bej.2008.03.013
Guettler M, Rumler D, Jain M (1999) Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int J Syst Bacteriol 49:207–216
FitzPatrick M, Champagne P, Cunningham MF, Whitney RA (2010) A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol 101:8915–8922. doi:10.1016/j.biortech.2010.06.125
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels, Bioprod Biorefining 6:465–482. doi:10.1002/bbb.1331
Chen X, Shekiro J, Franden MA et al (2012) The impacts of deacetylation prior to dilute acid pretreatment on the bioethanol process. Biotechnol Biofuels 5:8. doi:10.1186/1754-6834-5-8
Weiss ND, Nagle NJ, Tucker MP, Elander RT (2009) High xylose yields from dilute acid pretreatment of corn stover under process-relevant conditions. Appl Biochem Biotechnol 155:418–428. doi:10.1007/s12010-008-8490-y
Schell D, Farmer J (2003) Dilute-sulfuric acid pretreatment of corn stover in pilot-scale reactor. Appl Biochem Biotechnol 105:69–85
Villadsen J, Nielsen J, Lidén G (2011) Bioreaction engineering principles, Third. Engineering. doi: 10.1007/978-1-4419-9688-6
Cheng K-C, Demirci A, Catchmark JM (2010) Advances in biofilm reactors for production of value-added products. Appl Microbiol Biotechnol 87:445–456. doi:10.1007/s00253-010-2622-3
Liu Y-P, Zheng P, Sun Z-H et al (2008) Economical succinic acid production from cane molasses by Actinobacillus succinogenes. Bioresour Technol 99:1736–1742. doi:10.1016/j.biortech.2007.03.044
Xi Y, Dai W, Xu R et al (2013) Ultrasonic pretreatment and acid hydrolysis of sugarcane bagasse for succinic acid production using Actinobacillus succinogenes. Bioprocess Biosyst Eng 36:1779–1785. doi:10.1007/s00449-013-0953-z
Borges ER, Pereira N (2011) Succinic acid production from sugarcane bagasse hemicellulose hydrolysate by Actinobacillus succinogenes. J Ind Microbiol Biotechnol 38:1001–1011. doi:10.1007/s10295-010-0874-7
Zheng P, Dong J-J, Sun Z-H et al (2009) Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Bioresour Technol 100:2425–2429. doi:10.1016/j.biortech.2008.11.043
Liang L, Liu R, Li F et al (2013) Repetitive succinic acid production from lignocellulose hydrolysates by enhancement of ATP supply in metabolically engineered Escherichia coli. Bioresour Technol 143:405–412. doi:10.1016/j.biortech.2013.06.031
Qureshi N, Annous BA, Ezeji TC et al (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact 4:24. doi:10.1186/1475-2859-4-24
Brink HG, Nicol W (2014) Succinic acid production with Actinobacillus succinogenes: rate and yield analysis of chemostat and biofilm cultures. Microb Cell Fact 13:111. doi:10.1186/s12934-014-0111-6
Corona-González RI, Bories A, González-Álvarez V, Pelayo-Ortiz C (2008) Kinetic study of succinic acid production by Actinobacillus succinogenes ZT-130. Process Biochem 43:1047–1053. doi:10.1016/j.procbio.2008.05.011
McKinlay JB, Shachar-Hill Y, Zeikus JG, Vieille C (2007) Determining Actinobacillus succinogenes metabolic pathways and fluxes by NMR and GC-MS analyses of 13C-labeled metabolic product isotopomers. Metab Eng 9:177–192. doi:10.1016/j.ymben.2006.10.006
McKinlay JB, Vieille C (2008) 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab Eng 10:55–68. doi:10.1016/j.ymben.2007.08.004
McKinlay JB, Laivenieks M, Schindler BD et al (2010) A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genom 11:680. doi:10.1186/1471-2164-11-680
Lu S, Eiteman MA, Altman E (2009) Effect of CO2 on succinate production in dual-phase Escherichia coli fermentations. J Biotechnol 143:213–223. doi:10.1016/j.jbiotec.2009.07.012
Runquist D, Hahn-Hägerdal B, Bettiga M (2009) Increased expression of the oxidative pentose phosphate pathway and gluconeogenesis in anaerobically growing xylose-utilizing Saccharomyces cerevisiae. Microb Cell Fact 8:49. doi:10.1186/1475-2859-8-49
Witteveen CFB, Busink R, van de Vondervoort P et al (1989) L-Arabinose and D-Xylose catabolism in Aspergillus niger. J Gen Microbiol 135:2163–2171
Schindler BD, Joshi RV, Vieille C (2014) Respiratory glycerol metabolism of Actinobacillus succinogenes 130Z for succinate production. J Ind Microbiol Biotechnol 41:1339–1352. doi:10.1007/s10295-014-1480-x
Acknowledgments
The financial assistance of the National Research Foundation (NRF) towards this research is hereby acknowledged. Opinions expressed and conclusions arrived at, are those of the author and are not necessarily to be attributed to the NRF. We thank Andre Naude for his help in developing the HPLC methods.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Bradfield, M.F.A., Nicol, W. Continuous succinic acid production from xylose by Actinobacillus succinogenes . Bioprocess Biosyst Eng 39, 233–244 (2016). https://doi.org/10.1007/s00449-015-1507-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1507-3