Abstract
Pretreatment experiments were carried out to demonstrate high xylose yields at high solids loadings in two different batch pretreatment reactors under process-relevant conditions. Corn stover was pretreated with dilute sulfuric acid using a 4-l Steam Digester and a 4-l stirred ZipperClave® reactor. Solids were loaded at 45% dry matter (wt/wt) after sulfuric acid catalyst impregnation using nominal particle sizes of either 6 or 18 mm. Pretreatment was carried out at temperatures between 180 and 200 °C at residence times of either 90 or 105 s. Results demonstrate an ability to achieve high xylose yields (>80%) over a range of pretreatment conditions, with performance showing little dependence on particle size or pretreatment reactor type. The high xylose yields are attributed to effective catalyst impregnation and rapid rates of heat transfer during pretreatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of liquid transportation fuels from lignocellulosic biomass is seen as one of many paths forward to reducing industrial societies’ dependence on fossil fuels for its energy needs. As opposed to using edible feedstocks for biofuel production, which has many significant implications for world food supplies and land use change [1], the conversion of lignocellulosic materials for fuels such as ethanol has the potential to promote sustainable farming practices, improve rural economies, and reduce price volatility in the transportation fuel market. Over 1.2 billion tons of lignocellulosic biomass could be available annually on a sustainable basis for conversion to biofuels in the US to satisfy up to 30% of US national gasoline consumption by 2030 [2]. However, the conversion of lignocellulosic based feedstocks to fermentable sugars and ethanol presents significant challenges due to the intrinsic recalcitrance of plant matter [3]. Efficient, economical, and sustainable conversion technologies must be developed for cellulosic ethanol production to become a viable pathway to large-scale production of renewable transportation fuels.
Lignocellulosic biomass primarily refers to plant material which is not grown for human consumption and incorporates the majority of the tonnage yielded for a given field or forest. It consists of the stalks, leaves, wood, bark, cob, straw, and other materials which the plant creates to act as a structure for the plant during growth and seed production. Because this material is not produced by plants with the explicit end use of energy storage (as opposed to stored soluble sugars), structural plant matter exhibits significant recalcitrance upon attempts to liberate its structural sugars in a soluble form prior to fermentation. It is necessary to “treat” the biomass using biochemical and/or thermochemical processes in order to release fermentable sugars at high yields. This is currently done in two stages. The first stage, pretreatment, involves the chemical and/or thermal treatment of biomass to alter the structure of the biomass so that it is accessible for enzymatic attack [4]. The second stage, enzymatic hydrolysis, involves the use of enzymes to break down the remaining cellulose and hemicellulose not converted in pretreatment to its monomeric sugar units for use in subsequent hexose and pentose fermentation to bioethanol [5].
The purpose of this investigation was to demonstrate high hemicellulose hydrolysis yields from dilute acid pretreatment under process-relevant conditions and determine the robustness of the experimental space across different reactor configurations and biomass particle sizes. Many studies of pretreatment using dilute acid are carried out under process scenarios which might be economically disadvantageous at a commercial scale, although suitable for fundamental laboratory research and for determining comparative data across different feedstocks and pretreatment approaches [4, 6–8]. Examples of this include pretreatment at low solids concentrations, over longer time periods, and using indirect heating methods. In this study, we increased the initial solids loading in the pretreatment reactors to 45% solids and used direct steam injection into commercially available batch reactors while limiting reaction times to 90 to 105 s. While similar conditions have been investigated in continuous pilot-scale reactors, results obtained were not satisfactory in providing sufficient xylan yields from pretreatment [9, 10]. It is important to hydrolyze as much hemicellulose as possible during dilute acid pretreatment while maintaining high soluble hemicellulosic sugar yields, as increased xylan removal has been shown to make the cellulose fraction more digestible by cellulase enzymes and provides the possibility for separate fermentations of hexoses (glucose) and pentoses (xylose) [11]. Demonstrating high xylan hydrolysis yields under process-relevant conditions is crucial to developing cost-effective biomass conversion technologies [12].
Materials and Methods
Feedstock
Corn stover (Zea mays) was used as the model feedstock. The stover was obtained from Pioneer variety 33A14 plants, harvested in 2002 (from the Kramer farm, Wray, CO, USA), and was knife-milled to pass either a 6- or 18-mm round rejection screen (Reduction Technologies, Model 10 × 12, Leeds, AL, USA). The milling process resulted in a range of particle sizes smaller than the nominal rejection screen sizes. Table 1 presents the solids compositional analysis of the corn stover used in this study.
Dilute Sulfuric Acid Impregnation Procedure
The corn stover biomass was impregnated with acid using an atmospheric pressure acid impregnation (APAI) recirculating acid bath system designed at the National Renewable Energy Laboratory (NREL) [13]. To ensure effective pretreatment, the impregnation was controlled to maximize acid penetration into the biomass. Six kilograms of corn stover was loaded into a Hastelloy® C-276 wire mesh (100 mesh) basket (approximately 92-L volume) and lowered into a 200-L polyethylene tank containing 120 L of 1.08% (wt/wt) sulfuric acid solution, which was recirculated through the basket. The temperature of the acid solution was controlled at 60 ± 5 °C. The biomass was soaked for 4 h, after which time the excess acid solution was allowed to drain off. The impregnation time and temperature conditions were selected based on previous research showing high xylose yields using this method of impregnation [13, 14]. The impregnated and drained feedstock was then further dewatered using a 15-L hydraulic press operated at a maximum pressure of 360 psig in the dewatering mold to reach the desired solids concentration range of 45–50% solids. The solids concentration range was also selected based on previous research at NREL [13]. The impregnated and dewatered feedstock was kept at 4 °C in sealed containers until pretreatment.
Pretreatment Reactors
Pretreatment was performed in two batch-type reactors, a 4-L steam digester (Autoclave Engineers) and a 4-L (2-L working volume) ZipperClave® reactor (Autoclave Engineers). The two reactors were selected to approximate reaction and reactor conditions in a commercial-scale continuous reactor. While the two reactors selected are not directly scalable to a commercial or pilot scale, the ability to pretreat biomass at high solids concentrations and using direct steam injection for rapid heating are important process parameters for an economical commercial reactor. The steam digester combines direct steam injection with explosive decompression through a shear die to provide rapid heating and cooling, as well as mechanical disruption of the biomass. The steam digester is similar to batch steam explosion pulping equipment used commercially in the pulp and paper industry and therefore may be scalable for biomass pretreatment [15]. The ZipperClave® reactor also uses direct steam injection for heating the biomass but depressurizes more gradually by slowly relieving head space pressure in approximately 15 to 20 s on the pretreatment chamber through a throttle valve. Pressure release in the ZipperClave® reactor is via the headspace, so there is no explosive shearing of the biomass in this system. Mixing is achieved in the ZipperClave® reactor using a modified anchor-type impeller with customized lifting wedges which sweep the reactor bottom and provide good mixing and lifting of the biomass under high solids loading conditions. Both reactor systems are capable of pretreatment at high solids concentrations (20 to 90 wt.%); however, we experienced difficulty with the ZipperClave® reactor mixing the 18-mm milled corn stover. Therefore, only 6-mm milled material was pretreated in the ZipperClave® reactor. Initial solids concentrations of the biomass upon addition to the reactor were between 45% and 49% dry matter (wt/wt). After steam injection and pretreatment, solids concentrations were 29 ± 4% dry matter (wt/wt), based on the amount of steam necessary to achieve the desired pretreatment temperature. These solids loadings are consistent with other high solids pretreatment reactors [9].
Experimental Design
Samples were pretreated over a range of reaction times and temperatures, with a severity [8] range of 2.5 to 3.2 (Eq. 1), where t is time in minutes and T is temperature in degrees Celsius [16].
Table 2 presents an outline of the experimental conditions investigated in the experiments. The experiment was designed to span a range of optimal conditions for high xylose yields during dilute acid pretreatment based on previous research conducted at NREL [9, 13]. Reaction temperatures ranged from 180 to 200 °C, and reaction times were either 90 or 105 s. Table 3 gives the reaction time and temperatures with the corresponding severities (R o). Pretreatment was carried out primarily in the steam digester with a limited number of pretreatments in the ZipperClave® reactor to demonstrate the effects of reactor type on pretreatment. The biomass was exposed to reaction temperatures (within 10° of target reaction temperature) for slightly longer periods of time (<5 s) in the ZipperClave® to compensate for the slower depressurization step at the end of the reaction in that pretreatment system. Pretreatment experiments were conducted in duplicate, with a limited number of experiments replicated four times to determine experimental error. Error bars were reported as ±one standard deviation of the pooled replicates.
Analytical Methods
Sugar analyses of pretreated liquors were performed using an Agilent 1100 HPLC with a Biorad Aminex HPX-87P column. Both pretreated sample liquors and solids were analyzed for their composition and sugar content. Solids were analyzed for the fraction of insoluble solids (FIS) remaining following pretreatment. The FIS was used to close the mass balance and determine the fractions of the major sugars hydrolyzed in pretreatment. Effective acid concentration was determined in both the liquor remaining in the APAI system after impregnation and the impregnated solids by titration with the National Institute of Standards and Technology traceable 0.1 N NaOH standards (J.T. Baker, Phillipsburg, NJ, USA). All analytical methods used followed standard NREL Laboratory Analytical Procedures [17, 18].
Results
Figure 1 shows the amount of xylan remaining in the solid fraction as a function of pretreatment severity [8]. Xylan removal was high under all pretreatment conditions and increased with severity in both reactors and both particle sizes. The highest level of xylan removal, approximately 97%, occurred at the highest severity conditions [8]. Pretreatment in the ZipperClave® reactor resulted in hydrolyzing slightly less xylan (2–4%) at the less severe conditions than the steam digester, but, over the entire severity range explored, xylan removal was not significantly different between the two reactors. We observed little difference in xylan removal when comparing the two different corn stover particle sizes in the Steam Digester pretreatment experiments.
Residual xylan remaining in the pretreated solids as a function of severity [8]. This was calculated from the difference of the xylan content of native and pretreated corn stover
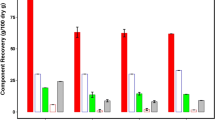
Figures 2 and 3 show total xylan solubilization in the pretreatment hydrolyzate liquors based on the initial xylan present in the biomass. Total xylose yields (defined as the sum of the oligomeric and monomeric xylose yields) were found to be higher at lower pretreatment severities, with the highest yield of 87% occurring at a severity of 2.5 in the Steam Digester (180 °C, 90 s). Total xylose yields remained in the 75% to 85% range up to a severity of 2.9 and only dipped below 75% at the higher severities. The overall trend is a decreasing xylose yield with increasing pretreatment severity, suggesting that xylose was degrading more rapidly at the higher severities. Xylan was primarily hydrolyzed to monomeric xylose, as shown in Fig. 3. The ZipperClave® reactor was shown to produce slightly less monomeric sugars than pretreatments from the Steam Digester.
Figure 4 shows furfural production as a function of severity of pretreatment. As expected, degradation of xylan to furfural increased with increasing pretreatment severity. This is consistent with previous research findings [19]. Xylan conversion to furfural increased from 2 wt.% at 180 °C to 10 wt.% at 200 °C. When compared to the mass balance of xylan after pretreatment (Fig. 3), these values directly correlate to the decrease in total xylose measured in the hydrolyzate liquors. At the higher severity conditions tested, the reduction in total xylose yield cannot be fully attributed to the increase in furfural yield, suggesting that additional sugar degradation products besides furfural are being generated.
Cellulose hydrolysis during dilute acid pretreatment in the temperature range investigated in this study has been shown to yield only a fraction of the total glucose in the hydrolyzate liquors [6, 14, 20–22]. Figure 5 shows the amount of glucose solubilized during pretreatment as a fraction of total initial glucan in the native biomass. Glucan hydrolysis yields ranged from 10% to 20% and increased with increasing severity. The majority of the glucose released was present as monomers. There was relatively little degradation to 5-hydroxymethylfurfural during these pretreatment conditions, even at the most severe conditions (data not shown). Increased glucose yields correlated directly with increased severity in all three pretreatment process variations. Somewhat more glucose was hydrolyzed in the Steam Digester experiments as compared to the ZipperClave® runs at equivalent pretreatment severities.
Discussion
This study demonstrated that total xylose hydrolysis yields above 80% are readily attainable under high solids loading (45% wt/wt) dilute acid pretreatments. In some cases, monomeric xylose yields above 80% were achieved. The reaction conditions were sufficiently robust to yield similar high xylose yields in both reactors and at two different particle sizes. There was a significant impact of temperature on the reaction yields, suggesting that temperature control and uniform temperature profiles are important in biomass pretreatment reactor design. While it is relatively easy to achieve uniform mixing and even steam penetration of the biomass in bench-scale systems, it will be much more difficult to achieve effective heat and mass transfer in large-scale reactors when operating at high solids loadings. Pretreatment residence time was a less important factor in this study; however, this was primarily due to a small window of variation. Overall xylan hydrolysis was found to decrease as the severity of pretreatment was increased from 2.5 to 3.2. This suggests that the optimum xylose yields (where degradation is minimized) may occur at reaction conditions below those included in the study. Previous dilute acid pretreatment data [23] have shown similarly high xylose yields at lower temperatures than were investigated. While high xylose yields were achieved in this study, there is still significant room for optimization of the pretreatment process.
The overall solubilization of xylan and furfural production was found to be very similar between pretreatments using the two reactor systems studied. The decrease in the total xylan solubilized at higher pretreatment severities in the ZipperClave® reactor (Fig. 2) suggests that degradation of xylose may be slower in the ZipperClave® reactor. This may be caused by the differences in the geometries of the two reactor systems, differences in mixing and steam addition systems, and/or feedstock heating variations between the two reactor systems, although no definitive explanation is apparent from these data. Figure 3 shows that a larger fraction of xylose is hydrolyzed as oligomers from the ZipperClave® pretreatments, which may also be attributable to the reactor system differences that result in slightly different overall reaction kinetics and may account for the differences in the monomeric xylose and furfural found in the steam digester under identical pretreatment conditions.
We believe that one of the key factors in achieving high process yields is the effective impregnation of the biomass feedstock with sulfuric acid prior to steam injection and pretreatment. This was done by soaking the biomass feedstock in excess acid for an extended period of time. Previous diffusion studies on the penetration of sulfuric acid into finely milled corn stover [24] suggest that the diffusion time (4 h) and method of acid impregnation (soaking) used for this study were sufficient to provide effective acid impregnation of the biomass. Studies comparing soaking and spraying impregnation methods have demonstrated higher yields of fermentable sugars and reduced amounts of degradation products when the biomass is soaked in excess acid as opposed to spraying on of the acid [25]. Limited studies using indicator dyes to assess catalyst penetration into biomass particles have shown that varying the method of impregnation can significantly affect the extent of catalyst penetration into the biomass structure [26]. While the current scenario of an extended soaking time with a high solid to liquid ratio, followed by a mechanical dewatering step, may not be scalable in an economically feasible manner, it is possible to envision a scenario where biomass is soaked in a limited amount of excess acid solution and dewatered using a belt press or a compression screw. Equipment for practical and effective catalyst impregnation should be factored into any process design for larger-scale continuous pretreatment processes.
In this study, we did not observe a marked difference in pretreatment between the different feedstock particle sizes in the steam digester. This suggests that the dilute sulfuric acid impregnation methodology was equally effective at distributing the acid catalyst throughout both the small and large particle sizes and that mass transfer of the acid catalyst was not significantly different between the smaller and larger particle sizes. The data show that the 18-mm particles pretreated better at lower severities, suggesting a peak in xylose yield outside of the experimental space. However, this may have been caused by the fact that there was a slight difference in the acid concentrations between the two particle sizes due to normal variation in the impregnation method. We also recognize that practical commercial-scale processes may be economically constrained in their ability to carry out size reduction to an 18-mm nominal particle size and that larger particle sizes may more strongly impact the process yields as well as the equipment used for catalyst impregnation.
Conclusions
Xylose yields (both on a total sugar and monomeric sugar basis) above 80% were demonstrated in two batch reactor systems over a limited range of pretreatment severities. With initial solids concentrations of 45%, these data are consistent with solids concentrations that would be expected for actual pretreatment processes on a commercial scale. The short residence times used are also important in allowing for relatively smaller pretreatment reactors in a commercial plant, which will reduce capital equipment costs. We believe that one of the key components to achieving high hydrolysis yields of hemicellulose during pretreatment at short residence times is the effective impregnation of the biomass with sulfuric acid in a method which allows total penetration of the acid into the biomass structure. Further research needs to be carried out to understand how these results can be improved upon and translated to a continuous pretreatment system, as well as better understanding what processes are necessary for effective catalyst impregnation, especially with the larger feedstock particles that may be used in a commercial setting. The data suggest that process optimization may depend on scalable catalyst impregnation and the ability to control temperature profiles within suitable pretreatment reactor systems.
References
Searchinger, T., Heimlich, R., Houghton, R. A., et al. (2008). Science, 319, 1238–1240. doi:10.1126/science.1151861.
Perlack, R. D., et al. (2005). Biomass as feedstock for a bionergy and bioproducts industry: the technical feasibility of a billion-ton annual supply. US Department of Energy and US Department of Agriculture Report.
Himmel, M. E., Ding, S. Y., Johnson, D. K., et al. (2007). Science, 315, 804–807. doi:10.1126/science.1137016.
Mosier, N., Wyman, C., Dale, B., et al. (2005). Bioresource Technology, 96, 673–686. doi:10.1016/j.biortech.2004.06.025.
Mcmillan, J. D. (1994). In M. E. Himmel, et al. (Ed.), Enzymatic conversion of biomass for fuels production pp. 411–437. Washington DC: American Chemical Society.
Lloyd, T. A., & Wyman, C. E. (2005). Bioresource Technology, 96, 1967–1977. doi:10.1016/j.biortech.2005.01.011.
Saha, B. C., Iten, L. B., Cotta, M. A., et al. (2005). Biotechnology Progress, 21, 816–822. doi:10.1021/bp049564n.
Zimbardi, F., Viola, E., Nanna, F., et al. (2007). Industrial Crops and Products, 26, 195–206. doi:10.1016/j.indcrop.2007.03.005.
Schell, D. J., Farmer, J., Newman, M., et al. (2003). Applied Biochemistry and Biotechnology, 105, 69–85. doi:10.1385/ABAB:105:1-3:69.
Schell, D. J., Walter, P. J., & Johnson, D. K. (1992). Applied Biochemistry and Biotechnology, 34–35, 659–665. doi:10.1007/BF02920586.
Jeoh, T., Ishizawa, C. I., Davis, M. F., et al. (2007). Biotechnology and Bioengineering, 98, 112–122. doi:10.1002/bit.21408.
Aden, A. M. R., Ibsen, K., Jechura, J., Neeves, K., Sheehan, J., Wallace, B., & National Renewable Energy Laboratory (2002). Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis for corn stover. Golden: National Renewable Energy Laboratory.
Tucker, M. P., Kim, K. H., Newman, M. M., et al. (2003). Applied Biochemistry and Biotechnology, 105, 165–177. doi:10.1385/ABAB:105:1-3:165.
Nguyen, Q. A., Tucker, M. P., Keller, F. A., et al. (2000). Applied Biochemistry and Biotechnology, 84–86, 561–576. doi:10.1385/ABAB:84-86:1-9:561.
Ahmed, A., & Kokta, B. (1998). In R. A. Young, & M. Akhtar (Eds.), Environmentally friendly technologies for the pulp and paper industry pp. 191–212. Hoboken: Wiley.
Overend, R. P., & Chornet, E. (1987). Philosophical Transactions of the Royal Society of London Series a-Mathematical Physical and Engineering Sciences, 321, 523–536.
Sluiter, A. B. H. (2006). Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Golden: National Renewable Energy Laboratory NREL/TP-510-42623 ed.
Sluiter, A. B. H. (2008). Determination of structural carbohydrates and lignin in biomass. Golden: National Renewable Energy Laboratory NREL/TP-510-42681 ed.
Mcmillan, J. D. (1994). In M. E. Himmel, et al. (Ed.), Enzymatic conversion of biomass for fuels production pp. 292–324. Washington DC: American Chemical Society.
Varga, E., Reczey, K., & Zacchi, G. (2004). Applied Biochemistry and Biotechnology, 113–116, 509–523. doi:10.1385/ABAB:114:1-3:509.
Um, B. H., Karim, M. N., & Henk, L. L. (2003). Applied Biochemistry and Biotechnology, 105, 115–125. doi:10.1385/ABAB:105:1-3:115.
Wyman, C. E., Dale, B. E., Elander, R. T., et al. (2005). Bioresource Technology, 96, 2026–2032. doi:10.1016/j.biortech.2005.01.018.
Bhandari, N., Macdonald, D. G., & Bakhshi, N. N. (1984). Biotechnology and Bioengineering, 26, 320–327. doi:10.1002/bit.260260405.
Kim, S. B., & Lee, Y. Y. (2002). Bioresource Technology, 83, 165–171. doi:10.1016/S0960-8524(01)00197-3.
Marie Linde, M. G., & Guido, Z. (2006). Applied Biochemistry and Biotechnology, 129–132, 546–572.
Viamajala, S., Selig, M. J., Vinzant, T. B., Tucker, M. P., Himmel, M. E., McMillan, J. D., & Decker, S. R. (2006). Applied Biochemistry and Biotechnology, 130, 509–527.
Acknowledgements
The authors of this paper would like to thank Jeff Wolfe, Justin Sluiter, and David Templeton from the National Renewable Energy Laboratory for their technical and laboratory help. We also gratefully acknowledge the funding for this project provided by the US Department of Energy’s Office of Energy Efficiency and Renewable Energy, Office of the Biomass Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weiss, N.D., Nagle, N.J., Tucker, M.P. et al. High Xylose Yields from Dilute Acid Pretreatment of Corn Stover Under Process-Relevant Conditions. Appl Biochem Biotechnol 155, 115–125 (2009). https://doi.org/10.1007/s12010-008-8490-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8490-y