Abstract
In this work, an integrated and optimized production process for 99 % pure 1,3-propanediol (PDO) from raw glycerol is presented. Glycerol fermentation is carried out applying a newly isolated strain Clostridium pasteurianum K1 under non-sterile conditions without any complex ingredients in the fermentation media. In this environment over 55 g/L PDO, yields of 0.52 g/g and space time yields over 2.3 g/(Lh) were achieved in up to 1 m3 pilot scale. The downstream process for PDO purification consists of an ultrafiltration for biomass and protein separation, an evaporation step for concentration of PDO and a two-step rectification for final purification. For a proof of concept, process optimization and especially investigation of interactions of individual steps, the downstream process was performed in miniplant scale. A minimum salt input into the downstream process was shown to be important to overcome precipitation in evaporation as well as rectification. Thus, raw glycerol is desalinated before fermentation and the fermentation medium was minimized and complex nutrients, such as yeast extract, were avoided totally to prevent furthermore dark color formation. Furthermore, by titration of fermentation with ammonia instead of sodium hydroxide, the later separation of the major by-products, organic acids, in the evaporation step was significantly enhanced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of white biotechnology for the industrial production of chemicals is growing continuously. Beside the traditional production of ethanol for beverages, which is carried out for several thousand years [1], industrial application of biotechnology became interesting in the early 20th century. Around that time the acetone–butanol–ethanol (ABE) fermentation was discovered [2]. It replaced production processes based on crude oil during 2nd world war and was applied even later in some countries such as South Africa, Soviet Union or China, where it is still in use today [3].
Around 1980, advanced biotechnological tools such as genetic modification were developed and offered new horizons for the development of highly productive production strains [4]. This method opened the way in biochemical production to create new products such as enzymes, vitamins and amino acids, for which chemical production is either difficult or even impossible [4]. Enzymes or vitamins are usually high value products whereby amino acids are produced in amounts of million tons per year, making them to become bulk commodities. These products demand highly efficient production processes to fit the market prices around 1 €/kg [5].
Looking at the recent development of rising oil prices and climatic changes, industrial biotechnology is more and more seen as an alternative sustainable concept. The world’s largest chemical companies launch fermentation processes for lactic acid [6, 7], succinic acid [8, 9], acrylic acid [10] or 1,3-propanediol (PDO) [11] to generate, eg., monomers for polymer production.
The industrially established bioproduction process for PDO is based on glucose or starch, respectively, as raw material and uses genetically modified Escherichia coli strains for the bioconversion [11]. Besides this route, also the utilization of glycerol with wild-type strains is feasible and of economical interest [12, 13].
This alternative route is in focus of research since more than 20 years [14] with corresponding success. Plenty of publications were made, not only about fermentation, but also about the downstream processing of fermentation broth [15]. The fermentation of glycerol can be done with different wild-type strains like Clostridium butyricum, Klebsiella pneumonia or C. pasteurianum in submersed culture in batch, fed-batch, continuous or continuous fermentation with cell retention [13, 16–18]. In fed-batch culture best titers of up to 100 g L−1 PDO can be reached with wild-type strains. Continuous fermentation with cell retention leads to the best space time yields [13, 19, 20].
Beyond the bioconversion, downstream process steps such as extracting PDO in different ways or its adsorption on different packed beds have been discussed [15, 21–25]. Efforts were also made to have an evaluation of the whole process from glycerol to pure PDO including apparatus design and cost calculations [26]. Grothe [26] discussed a process based on a publication of Deckwer [18], in which glycerol is fermented in fed-batch culture. Biomass is afterwards separated in a decanter with help of flocculation agents. PDO is then concentrated in an evaporation step and finally purified and separated from residual water, glycerol and salts in a 2-step rectification. Experimental work of Grothe [26] covered fermentation in fed-batch, biomass separation and concentration in pilot scale experiments.
Since Grothe’s [26] work, in which raw material was identified as main cost driver, significant market chances for glycerol occurred due to growing biodiesel production [27], where glycerol is a by-product [28]. Thus, huge amounts of so-called raw glycerol became available, being an impure but cost attractive raw material for PDO production. Raw glycerol has a purity of 60–80 % [28] and contains large amounts of salts such as K2SO4 or NaCl beside other substances such as fatty acids. The use of raw glycerol for fermentation was successfully tackled by several authors [29–33].
Beyond the use of raw glycerol, also the possibility of non-sterile fermentation conditions is a promising strategy for cost reduction and was shown for K. oxytoca [34], C. freundii [35] and C. butyricum [31, 36].
For process development miniplants are a very useful tool to achieve a “prove of concept”. The miniplant scale describes a scale of apparatus and plants, which process around kilograms product per hour [37]. Miniplant apparatus reflect a scale-down of production scale apparatus and can even work continuously. They are supposed to reflect production environment at a scale as small and cost efficient as possible but still relevant for scale-up. In this way, for standard apparatus, such as stirred tank reactors or rectification columns, a direct scale-up from miniplant to production scale and the validation of flow sheet simulations are possible [38, 39]. The miniplant technology has so far seldom been applied in the research of bioproduction processes.
This work aims at a complete and overall experimental validation and optimization of a process concept for raw glycerol conversion to highly pure PDO. It includes handling of impurities in raw glycerol and application of the concept of non-sterile glycerol fermentation and downstream processing. For a technically relevant proof of concept, all process steps were performed at least in a miniplant scale.
Materials and methods
Microbial cultivation
For fermentation of glycerol and PDO production a microbial strain was used, which was isolated from an environmental sample and identified as identical to C. pasteurianum ATCC 6013 via 16 s rRNA analysis (99 % identical) by the German Collection of Microorganisms and Cell Cultures (DSMZ), Braunschweig, Germany.
The strain was stored at −80 °C in 2 mL cryo vials containing 80 % active pre-culture and 20 % of glycerol by volume. A 2-step pre-culture was prepared for 1 L fermentation inoculation in anaerobic bottles containing 100 mL of pre-culture medium for 8–12 h at 37 °C. For 10 L and 1,000 L fermentations, additional pre-culture steps were carried in 1, 10 and 3 × 33 L vessels to achieve inoculation volumes of 10 % for the main culture correspondingly. The pre-culture medium was prepared with ingredients in demineralized water as follows: 2 g/L NH4Cl, 0.75 g/L KCl, 2.45 g/L NaH2PO4, 4.58 g/L Na2HPO4, 0.28 g/L Na2SO4, 0.42 g/L Citric acid, 0.2 g/L L-Cystein, 0.26 g/L MgCl2·6 H2O, 2.9 mg/L CaCl2·2 H2O, 1 mL/L vitamin solution, 1.0 mL/L FeCl3·6 H2O solution, 2.0 mL/L trace element solution and 20 g/L glycerol. It was degassed with N2 for 10 min while it was kept boiling at 100 °C.
The fermentation medium consisted of 1 g/L NaH2PO4, 0.1 g/L K2SO4, 0.3 g/L MgSO4·7 H2O, 0.2 g/L L-Cystein, 3 mg/L CaCl2·2H2O, 5 mL trace element solution, 1 mL Fe-solution; 1 mL vitamin solution and adapted amounts of raw glycerol, 80 %purity, purchased from ADM, Hamburg, Germany. The vitamin solution contained: 80 mg of biotin and 100 mg of pantothenate. The trace element solution contained 0.684 g/L ZnCl2, 2 g/L MnCl2·4 H2, 0.476 g/L CoCl2·6 H2, 0.17 g/L CuCl2·2 H2O; 4.35 mg/L Na2MoO4·2H2O, 0.025 g/L NiCl2·6 H2O, 0.062 g/L H3BO3, 10 mL/L HCl 37 %. The iron solution contained 5 g/L FeCl3. All solutions were sterilized by filtration and added to the media after thermal sterilization and pH adjustment in the reactor. In case NaOH was used for pH control 2 g/L of NH4Cl was added to the fermentation media.
Bioreactors used were either a 2 L (1 L working volume) fermenter (Bioengineering, Wald, Switzerland) a 300 L (200 L working volume) fermenter (Bioengineering, Wald, Switzerland) or a 8,000 L (1,000 L working volume) pilot fermenter of the company Biokraftwerke Fürstenwalde, Fürstenwalde, Germany. For pH indication a Mettler Toledo pH probe was used and the pH was kept at 6.5 with 25 % NH3 solution or 20 % NaOH. If performed, sterilization procedure for the fermenter was steam sterilization for 20 min at 121 °C.
Feeding of the cultivations was performed with diluted raw glycerol (50 % w/w) or desalinated raw glycerol (50 % w/w).
Electrodialysis
Downstream processing of the fermentation broth was performed in Miniplant apparatus (See Fig. 1).
Flow chart of the miniplant for glycerol fermentation and PDO purification. Fermentation broth was concentrated in several steps in the identical evaporator. Rectification 1 and 2 were carried out in the same apparatus. The unit operations were only conducted separately and not connected for continuous processing
For cross-flow filtration either hollow fiber modules of Pall, Dreieich, Germany, type UMP-1147R with 0.1 m2 active membrane area made of polypropylene or hollow fiber modules made by Microdyn-Nadir, Wiesbaden, Germany, type MOLSEP FB02-CC-FUS0181 with 0.19 m2 active membrane area made of polyethersulfone was used. To circulate the fermentation broth through the membrane module a membrane pump, Quattroflow, Kamp-Linfort, Germany, type 1000-S was charged. The permeate flow was controlled by a MiniCori mass flow meter, Bronckhorst, Kamen, Germany, controlling a peristaltic pump.
Thermal concentration of fermentation broth was conducted in an up-flow evaporator of the manufacturer Normag, Ilmenau, Germany, equipped with a 1.5 kW electric heating and power input control.
Rectification experiments for PDO purification were carried out in a 50 mm vacuum-isolated glass column equipped with 2 × 800 mm of structured packing type A500, Montz, Hilden, Germany. The reflux ratio was controlled by a magnetic swing funnel. The evaporator vessel contained four submersed electric heating coils each at 0.6 kW maximum power. Feed addition was controlled by a MiniCori mass flow meter, Bronckhorst, Kamen, Germany and a gear pump preheated in the middle of the column. Bottom and distillate product were collected in weighted vessels.
Analysis
Analysis of metabolites was done using a Knauer Smartline HPLC equipped with a Biorad Aminex HPX-87 H column and a Knauer Eurokat H pre-column, both running at 75 °C with 5 mM H2SO4 as eluent at isocratic flow of 0.6 mL/min. The injected sample volume was 50 µL and calibration range from 0.1 to 5 g/L for glycerol, acetic acid, PDO, butyric acid and ethanol. All samples were centrifuged, filtered (0.2 µm) and diluted to calibration range.
Ammonia concentration was detected with a Spectroquant ammonia kit of Merck for the range of 2.6–193 mg/L.
Bacterial growth was followed by OD measurement at 650 nm with a Genesys 10 UV Scanning photometer manufactured by Thermo Scientific using 1-mm cuvettes.
Flowchart simulations
Flowchart simulations were carried out with Apsen Plus 2006®. The thermophysical properties of the included components water, PDO, acetic acid, acetate, butyric acid, butyrate, NH3, NH4 +, NaOH and Na+ were taken from the Aspen Plus® database or predicted by Aspen Plus® internal methods. The dissociation reaction equilibria were taken from the Aspen database as well or internally calculated from Gibbs free energy.
Results and discussion
Desalination of raw glycerol
To avoid precipitation of salts in the downstream process of fermentation broth, desalination of raw glycerol was investigated. Diluted raw glycerol with 50 % w/w (3.2 % w/w NaCl) glycerol was desalinated by electrodialysis to salt concentrations beyond 0.05 % w/w. From these experiments an energy demand of 174 kJ/kg glycerol, a membrane-specific desalination capacity of 7.65 kg/(m2h) and glycerol yields of 90 % were determined. Compared to this, the specific energy demand for a thermal desalination by distillation would be 850 kJ/kg based on the enthalpy of evaporation [40].
Raw glycerol batches from different biodiesel production plants were tested for applicability in cultivation (pre-culture conditions) at initial glycerol concentrations between 10 and 30 g/L. The different kinds of raw glycerol were tested without pretreatment as well as with desalination by electrodialysis. Several batches showed a total inhibition of the fermentation independent of the pretreatment. According to Chatzifragkou et al. [41] inhibition of raw glycerol may be due to impurities like unsaturated fatty acids. These molecules cannot be separated by electrodialysis. This means that it was impossible to completely overcome inhibitory effects by electrodialysis. Thus, electrodialysis is an effective method to desalinate glycerol but it is not efficient to reduce growth inhibiting components.
Alternatively, the desalination of glycerol by distillation turned out to be a feasible route to overcome inhibition by raw glycerol impurities. The inhibitor containing glycerol was totally evaporated and taken over head in a 1-stage vacuum distillation at 30 mbar in 0.2 L scale. The resulting distillate was afterwards successfully used for fermentation with the drawback of high energy demand.
As a conclusion, electrodialysis offers an energy saving alternative for desalination of raw glycerol but applicability is dependent on the raw glycerol quality. Furthermore the desalination by distillation not only reduces the salt input into the PDO process but also removes inhibitors and makes contaminated glycerol accessible for fermentation.
Fed-batch fermentation
Glycerol fermentation was adapted to a concept of minimum impurity input by three key parameters to facilitate the downstream process: (1) utilization of a chemically defined, minimum salt fermentation medium without yeast extract, (2) use of desalted raw glycerol and (3) use of ammonia for pH control. Beyond this, the application of the new strain C. pasteurianum K1 under non-sterile fermentation conditions was established.
An examination of the chemically defined medium used by Reimann for C. butyricum [20] showed that MgCl2 and NaSO4 could be merged to MgSO4. NH4Cl was replaced by ammonia titration during the fermentation to supply sufficient nitrogen. Citric acid and KCl were assumed not to fulfill any essential metabolic need and were left out. These actions reduced the total salt input into the process but kept relevant ion concentrations constant. The titration with ammonia results in reduced raw material costs and significantly simplifies the downstream processing, beside the reduction of salt input (see also evaporation and integrate process analysis).
The second key improvement of the process presented here is the robustness of the fermentation with the strain K1. It allows non-sterile conditions. Average space time yields of 2.3 g/(Lh) and final concentrations of C PDO > 55 g/L can be achieved using raw glycerol or desalted raw glycerol in combination with the minimal medium (See Fig. 2). Under these conditions, the substrate-specific yield was determined to be 0.63 molPDO/molGly. The maximum productivity with C. pasteurianum K1 was observed during the early feed phase and reached 5.2 g/(Lh) (See Fig. 2: t = 8–15 h). Feeding of the culture was started at total consumption of glycerol in the batch phase. This time point was indicated by stagnating base addition (5-10 min) of the pH controller, which is related stagnation of acid (by-product) production due to starvation. To keep the process control simple, the feeding rate was chosen to be constant at a rate of 10 gglycerol/(h·lstarting vol.) which achieved a maximum PDO productivity of 5 g/(Lh) at an expected yield of 0.5 g/g. The total amount of feed was limited in 200 and 1,000 L scale to achieve total conversion and avoid residual glycerol in the downstream process.
The fermentation under sterile conditions with sodium hydroxide as titration agent showed comparable results regarding space time yields and final PDO concentration (See Fig. 2). The molar amounts of sodium hydroxide and ammonia added to the fermentation runs are in comparable ranges (see Table 1). As sodium hydroxide is known to be a stronger base than ammonia, it would have been expected, that base addition with sodium hydroxide is lower. This was not observed as the results summarized in Table 1. Potentially different kinds and amounts of organic acids may not explain that effect as the by-product spectrum in both fermentations in 1 L scale was comparable (See Fig. 2).
A proof of concept for this fermentation approach could successfully be achieved in 1,000 L scale as well. The fermentations in 200 and 1,000 L scales showed reduced lag phases compared to the fermentations in the 1 L bioreactor (See Fig. 2). The biomass concentration of pre-culture may explain this effect, as the optical density (OD650) was OD650 = 0.300 ± 20 at inoculation time for 200L fermentations whereby in 1 L scale only OD650 = 0.120 ± 20 was achieved at starting time.
Dietz and Zeng [36] showed the glycerol fermentation under non-sterile conditions applying microbial communities but using higher salt content media and NaOH as a titration agent. Furthermore, Dietz and Zeng [36] have reached higher PDO concentrations but the substrate-specific yield (0.56 mol/mol) was somewhat lower compared to the results presented here. Recently Wilkens et al. [29] achieved higher space time yields with C. buryricum and higher titers with the drawback of adding 5 g/L yeast extract in the fermentation medium and 40 g/L yeast extract in the pure glycerol feed, increasing raw material costs and impeding downstream processing due to color formation. A comparable performance to this work was achieved with C. butyricum VPI 3266 [30] using raw glycerol without yeast extract as a feed solution.
With C. pasteurianum so far only significantly lower fermentation yields were achieved in the literature. Due to the formation of relatively high amounts of the by-product n-butanol, Biebl [17] reached yields of Y = 0.37 mol/mol and Taconi et al. reached 0.51 mol/mol [42]. In our work, butanol formation was only observed in 1 m3-scale with a final butanol concentration of 5 g/L and a corresponding reduction of yield to 0.56 molPDO/molGly.
Cross-flow filtration
The ultrafiltration of fermentation broth with a molecular cut-off of 10 kDa was established in miniplant scale with a yield of 97 % of broth or PDO in batch filtration. The yield limiting factor here was the ratio of residual retentate volume to the apparatus hold up, impeding further processing. Determined flux rates were in the range of \( \dot{V} \) = 30–40 L/(m2h) at a trans-membrane pressure of ΔP = 2 bar. This is a typically satisfying value, proving the technical feasibility of this alternative to, e.g., centrifugation for biomass separation.
The advantage of ultrafiltration in comparison to alternative biomass removal units, such as centrifugation or micro-filtration was demonstrated in the following process step of evaporation. Here a total separation of biomass and proteins, which is achieved by ultrafiltration, is essential to avoid strong foam formation (See Fig. 3). Micro-filtration does not permit the total removal of proteins due to the relatively low membrane cut-off. Protein removal by centrifugation demands the addition of flocculation agents [26].
Evaporation
To minimize the energy consumption in the whole process, water is separated in an evaporator step before rectification. This evaporation step can be run with steam compression or with sequence of evaporators with pressure reduction for integrated steam utilization. In this way the energy demand for water evaporation can be reduced significantly [26]. As the boiling point of pure water (100 °C) and PDO (214 °C) deviate by more than 100 K, this option was not available, if all would be separated in a single rectification step.
To evaluate this option more deeply regarding PDO losses with the distillate, Miniplant evaporation experiments with fermentation broth were analyzed and compared to results with a pure mixture of water/PDO [43]. For evaluation of the experimental results it was assumed, that phase equilibrium was reached. The collected experimental equilibrium data led to a thermodynamic yield of Y Exp = 0.82 in a 3-step evaporation with a final concentration of 40 % PDO. The yield of this step calculated with estimated data in Apsen Plus is Y sim = 0.92 (3-step distillation at 1, 0.5, 0.3 bar and equal heat distribution), which is significantly higher than the experimentally determined yield. The reason for this deviation could be an influence of by-products on the equilibrium, which was not covered by simulations in Aspen Plus®. Another explanation was that thermodynamic equilibrium is not reached in the miniplant evaporator. To overcome the significant losses, either the separation efficiency could be improved or the distillate could be recycled into the bioreactor. Using a dephlegmator or a short rectification column separation the efficiency could be improved with the drawback of energy losses due to refluxes.
Looking at the composition of the evaporation product more in detail, important effects regarding the separation of fermentation by-products become clear. Depending on the base used for titration in fermentation (to neutralize the by-products acetic and butyric acids) the amount of organic acids in the concentrated broth changed (See Table 2). To illustrate this effect more clearly, Fig. 4 shows the amount of residual organic acids in relation to the PDO concentration for the three evaporation steps. In the diagram for broth titrated with NaOH (Fig. 4b) it can be noticed that the ratio of organic acids to PDO stayed constant in all concentration steps. This means that no organic acids were separated with the distillate. However, in case of the titration with ammonia (Fig. 4a), a different behavior was observed. The ratio of organic acids to PDO decreases step by step which corresponds to a separation of the organic acids from the PDO. So, the titration with ammonia can enhance the separation of by-product during the evaporation step. As the boiling point of ammonia is lower than the boiling point of both organic acids, ammonia tends to evaporate in the distillation. This reduces the pH value and shifts the dissociation equilibrium of the organic acids towards the non-dissociated form. Non-dissociated butyric and acetic acids also have a vapor pressure in contrast to their anions. Thus, due to the evaporation of ammonia, more non-dissociated acid molecules become available for evaporation and get into the distillate.
To validate this point, simulations were carried out with Aspen Plus® and the included “flash” model. In general the simulations confirmed the experimental values (See Fig. 4). Only in case of butyric acid the separation from broth titrated with ammonia, significant deviations between calculation and experimental results are observed. These deviations may be explained by the level of modeling detail. Some components of the fermentation broth, such as phosphate buffer or sulfate were not included in the simulation. Furthermore, thermophysical property data from prediction models might not reflect real values sufficiently. However, the simulation results show the same effects and tendencies as observed in the miniplant experiments and thus support the experimental results.
For the overall production process these results are of importance. It is not only due to the fact that the proper selection of fermentation process control can enhance the downstream processing. Applying NH3 for pH titration can also significantly reduce the costs for raw material, as its market prize is much lower than that for NaOH [27]. In this case approximately 50 % of the organic acids could be separated and this would reduce the amount of salt in the broth causing problems in later rectification.
Rectification of concentrated broth
Stepwise filtration and concentration of fermentation broth by evaporation led to sufficient amounts of concentrated PDO (38 % w/w, see Table 3 for detail) for further rectification to a high purity in miniplant scale. In a first rectification step mainly water and free organic acids were separated from high boiling PDO. The bottom product of this step contained PDO with x > 90 % w/w. In a second rectification step afterwards, PDO was separated as distillate from high boiling residuals like salts. Also residual glycerol may be separated in this step if the conversion in fermentation is not complete [26].
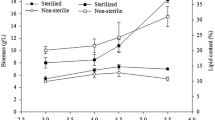
The first rectification step was carried out by heating the column with a constant mass reflux ratio of R = 0.5. After reaching a constant temperature of 154 °C at 105 mbar, a continuous feeding was stated. A continuous decrease of the temperature in the middle of the column can be observed, after starting continuous feeding of the column, until the condenser temperature is nearly reached (See Fig. 5a). Applying real fermentation broth, a clear temperature profile throughout the column is formed. This profile is indicated by the temperature difference from the column’s middle section to the top as well as the bottom of the column (Fig. 4, lower panel). Furthermore, at the column top, the temperature remained higher than 47 °C (the boiling point of water at 100 mbar). In conclusion, as the temperature of the model mixture and the real fermentation broth differed, fermentation broth components not reflected by the model mixture. The by-products (acetic and butyric acid) and fermentation medium might also have a strong influence on the separation efficiency in the rectification step. This hypothesis is strongly supported by flowchart simulations with Aspen Plus®. The separation was simulated in the “RadFrac” Model using n = 10 theoretical stages, being the separation efficiency of the miniplant rectification column at an apparent f factor of f = 0.5 Pa−0.5. In case of the model mixture, the separation is very sharp, reaching purities x > 99 % at a reflux ratio of R = 0.5. The separation takes place nearly completely in the lower half of the column and only pure water reaches the top. In contrast to this, from the temperature profile with fermentation broth (including the by-products acetic and butyric acid) it can be observed that the whole column is required for a good separation. Furthermore, a sharp separation even over the total column height is not possible. In the same way as what is measured in the miniplant column, the condenser temperature raises, if the sump product is set to be free of water. Unfortunately, the observed acid dissociation or precipitation effects could not be tackled by the available Aspen Plus® model. For this reason, the simulation results reflect only qualitatively but not exactly quantitatively the experimental results. So, an exact dimensioning of the rectification step and for clarification of the theoretical stages demand more information is necessary. Either an improved thermophysical model or more miniplant experiments with varying number of stages and reflux ratio are to be made.
Another general aspect worth mentioning here is the non-linearity of the temperature profile (See Fig. 5b). It shows that the temperature control of the column is challenging. In the reboiler of the column hardly coalescing bubbles were formed and salts precipitated. Due to the foam formation, a continuous rectification could not be brought in steady state. This points to another difficulty of the process and the handling of fermentation broth. Foam formation and precipitation occur often in downstream processing of fermentation broth and demand special apparatus which tolerate those effects. In terms of the experiments performed on the PDO process here, the salt precipitation is mainly caused by NaCl, which is an impurity of raw glycerol. This emphasizes importance of the desalination of raw glycerol before the fermentation process. Application of concentrated fermentation broth from fermentation with desalinated raw glycerol in laboratory distillation could reduce precipitation to less than 1 % w/w of the input concentrate. Ammonia, butyric and acetic acids were recovered in the distillate and in this way separated from PDO.
A second rectification (at P Col = 30 mbar with a reflux ratio of R = 1) of the sump product of the described first rectification step led to a clear liquid top product containing 99 % of PDO. The condenser temperature maintained constant at T Top = 124, 5 °C higher than the calculated boiling point of PDO of T Top,sim = 119.1 °C at 30 mbar. In the dark brown column sump the temperature increased slightly over the batch time from T Sump = 128 °C to T Sump = 132 °C, potentially caused by increased concentration of residual by-products. Due to the minimal level of the rectification column evaporator, the experimental yield of this step was limited 71 %. Thus, a yield of 98 %, according to Aspen Plus simulations was assumed for overall process evaluation.
Integrated process analysis
Experimental results obtained in this study from raw material pretreatment (glycerol desalination), fermentation and downstream processing led to the suggestion of an alternative process flow sheet as shown in Fig. 6. The key differences to the approaches reported in the literature are: (1) reduction of salt input into the process by pretreatment of the raw glycerol, (2) avoidance of complex media components (yeast extract) to prevent potential problems in the downstream processing, (3) non-sterile and efficient fermentation using a newly isolated C. pasteurianum strain, (4) pH control of fermentation with ammonia to reduce the salt content of the fermentation broth in the downstream steps, especially by enhancing the separation of the by-products organic acids in the evaporation step, (4) proper ultrafiltration to avoid foam formation in the evaporation step.
Conventional fermentation broth from raw glycerol fermentation contains large amounts of salts (See Fig. 7). These salts accumulate from fermentation medium, raw glycerol and fermentation by-products, such as butyrate and acetate and from pH control. Assuming a fermentation broth containing 50 g/L PDO, this broth would also contain approximately 33 g/L of salts. As discussed above, these salts can cause heavy precipitation, if downstream approaches including distillation in which PDO and salts are concentrated due to their high boiling points. Based on the results of this work, the amount of salt to handle in distillation could be reduced significantly. Desalination of glycerol, minimal fermentation media and titration with ammonia favoring separation of ammonia salts in evaporation can reduce the total amount of salts entering rectification by a factor of about three.
Unsterile fermentation with C. pasteurianum is another feature reducing production costs due to reduced investment and energy costs.
The use of ultrafiltration supports foam reduction in evaporation and avoids addition of flocculation agents and offer implementation of alternative fermentation strategies. Reimann [20] e.g. has shown that process intensification by continuous fermentation with cell recycle by cross-flow filtration can lead to space time yields of STY > 10 g/(Lh).
The yield of every process step in our improved process is above 90 % except for the fermentation. The overall process yield is estimated to be 0.41 gPDO/gGly (see Fig. 7). Taking into account the glycerol content of 80 % w/w in raw glycerol, about 3 t of raw glycerol is required for 1 t of 99 % pure PDO.
Beside the fermentation and separation strategies, also energetic efficiency of the process plays an important role for the overall performance of a process. The energy demand for all unit operations was calculated based on our findings (See Fig. 8) for a scenario of C PDO = 50 g/L in fermentation. As no aeration in fermentation is necessary, a low specific-energy input of the stirrer of 0.5 kW/m3 was assumed. For the ultrafiltration a specific energy demand of 2.5 kWh/m3 according to Melin and Rautenbach [44] was used for calculation. Energy demand for the thermal separations was calculated from enthalpy of evaporation. A reduction of the energy demand due to a 3-step evaporation and corresponding energy recovery by a factor of 3 was assumed. The results indicate that the evaporation step has the highest contribution to the overall energy demand (See Fig. 8). This step itself is mainly dependent on the final PDO concentration in the fermentation which determines the amount of water to be evaporated. Thus, the total energy demand is highly dependent on the fermentation performance as well.
Conclusion
In this study, a new approach on the process optimization for microbial production of PDO was worked out. PDO was produced at a purity of 99 % from raw glycerol and a technical proof of concept was achieved by the demonstration in miniplant scale.
Unconventional optimization potential of the glycerol fermentation, such as the avoidance of sterilization or complex fermentation media components in connection with the strain C. pasteurianum K1, was successfully tackled.
In contrast to other approaches, the work presented here concentrated on an integrated analysis of the process concept and the interaction of different unit operations. The advantage of this integrated process analysis became more obvious from a further aspect of the medium optimization for fermentation: the minimization of the salt content combined with the desalination of raw glycerol turned out to be a possibility to avoid salt precipitation in the later evaporation and distillation steps. Here also the use of ammonia in the fermentation was found to play an important role, as it does not only reduce the cost for the fermentation step, but also enhances separation efficiency of the fermentation by-products acetic acid and butyric acids. So, substrate pretreatment or fermentation parameter changes not only influence the fermentation itself, but may have a strong impact on the downstream process, too. Additionally, also interactions between individual downstream steps, such as the dependence of foam formation in the evaporation on the way of biomass separation were discovered.
Overall, these facts emphasize that process optimization might not lead to the optimal concept, if unit operations are only optimized separately. Thus, an integral analysis and optimization is of high importance in developing bioproduction systems.
References
Ulber R, Soyez K (2004) 5000 Jahre Biotechnologie: vom Wein zum Penicillin. Chem unserer Zeit 38(3):172–180
Weizmann C (1919) Production of acetone and alcohol by bacteriological processes. US patent 1315585
Chiao J, Sun Z (2007) History of the acetone–butanol–ethanol fermentation industry in China: development of continuous production technology. J Mol Microbiol Biotechnol 13(1–3):12–14
Soetaert W, Vandamme EJ (2010) Industrial Biotechnology. WILEY-VCH, Weinheim
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotech 104(1–3):155–172
Lunt J (1998) Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stabil 59(1–3):145–152
Sin LT, Rahmat AR, Rahman WAWA (2012) Polylactic acid: PLA biopolymer technology and applications. Elsevier, Oxford
Scholten E, Renz T, Thomas J (2009) Continuous cultivation approach for fermentative succinic acid production from crude glycerol by Basfia succiniciproducens DD1. Biotechnol Lett 31(12):1947–1951
BASF SE (2012) Press release. http://www.basf.com/group/pressrelease/P-12-444
BASF SE (2012) Press release. http://www.basf.com/group/pressrelease/P-12-363
Kurian JV (2005) A new polymer platform for the future—Sorona from corn derived 1,3-propanediol. J Polym Environ 13(2):159–167
Biebl H, Menzel K, Zeng A-P, Deckwer W-D (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27(6):895–913
Forsberg CW (1987) Production of 1,3-propanediol from glycerol by Clostridium acetobutylicum and other Clostridium species. Appl Environ Microbiol 53(4):639–643
Xiu Z-L, Zeng A-P (2008) Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl Microbiol Biotechnol 78(6):917–926
Zeng A-P, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Biebl H (2001) Fermentation of glycerol by Clostridium pasteurianum—batch and continuous culture studies. J Ind Microbiol Biot 27:18–26
Deckwer W-D (1995) Microbial conversion of glycerol to 1,3-propanediol. FEMS Microbiol Rev 16(2–3):143–149
Willke T, Vorlop K (2008) Biotransformation of glycerol into 1,3-propanediol. Euro J Lipid Sci Tech 110(9):831–840
Reimann A, Biebl H, Deckwer W-D (1998) Production of 1,3-propanediol by Clostridium butyricum in continuous culture with cell recycling. Appl Microbiol Biotechnol 49:359–363
Malinowski JJ (1999) Evaluation of liquid extraction potentials for downstream separation of 1,3-propanediol. Biotechnol Tech 13:127–130
Malinowski JJ (2000) Reactive extraction for downstream separation of 1,3-propanediol. Biotechnol Prog 16(1):76–79
Günzel B (1991) Mikrobielle Herstellung von 1,3-Propandiol durch Clostridium butyricum und adsorptive Aufarbeitung von Diolen. Dissertation Technische Universität Braunschweig, Braunschweig
Li Z, Hu T, Xiu Z (2011) Extraction of 1,3-propanediol from glycerol-based fermentation broths with methanol/phosphate aqueous two-phase system. Process Biochem 46(2):586–591
Aydoğan Ö, Bayraktar E, Mehmetoğlu Ü, Kaeding T, Zeng A-P (2010) Selection and optimization of an aqueous two-phase system for the recovery of 1,3-propandiol from fermentation broth. Eng Life Sci 10(2):121–129
Grothe E (2000) Konzeption und Wirtschaftlichkeit der industriellen Glycerinvergärung zu 1,3-Propandiol. Dissertation Technische Universität Braunschweig, Braunschweig
Propanergy Projektbericht (2011) Marketing concept. http://www.propanergy.eu/cms/Content/download/Deliverable6_MarketingconceptforPDO.pdf
Majer S, Mueller-Langer F, Zeller V, Kaltschmitt M (2009) Implications of biodiesel production and utilisation on global climate—a literature review. Euro J Lipid Sci Tech 111(8):747–762
Wilkens E, Ringel A, Hortig D, Willke T, Vorlop K-D (2012) High-level production of 1,3-propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl Microbiol Biotechnol 93(3):1057–1063
González-Pajuelo M, Andrade JC, Vasconcelos I (2004) Production of 1,3-propanediol by Clostridium butyricum VPI 3266 using a synthetic medium and raw glycerol. J Ind Microbiol Biotechnol 31(9):442–446
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki A, Nychas G-J, Zeng A-P (2011) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91(1):101–112
Mu Y, Teng H, Zhang D-J, Wang W, Xiu Z-L (2006) Microbial production of 1,3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol Lett 28(21):1755–1759
Dietz D (2013) 1,3-Propanediol production from raw glycerol by mixed cultures: from laboratory to pilot plant scales. Dissertation TU Hamburg-Harburg, Hamburg
Metsoviti M, Paraskevaidi K, Koutinas A, Zeng A-P, Papanikolaou S (2012) Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process Biochem 47(12):1872–1882
Metsoviti M, An-Ping Zeng A-P, Koutinas AA, Papanikolaou S (2013) Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J Biotech 163(4):408–418
Dietz D, Zeng A-P (2013) Efficient production of 1,3-propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioprocess Biosyst Eng. doi:10.1007/s00449-013-0989-0
Greß D, Hartmann H, Kaibel G, Seid B (1979) Einsatz von mathematischer Simulation und Miniplant-Technik in der Verfahrensentwicklung. Chem Ing Tech 51(6):601–611
Buschulte TK, Heimann F (1995) Verfahrensentwicklung durch Kombination von Prozeßsimulation und Miniplant-Technik. Chem Ing Tech 67(6):718–723
Deibele L, Dohrn R (2006) Miniplant-Technik. WILEY-VCH, Weinheim
Aspen Tech (2006) Aspen plus documentation
Chatzifragkou A, Dietz D, Komaitis M, Zeng A-P, Papanikolaou S (2010) Effect of biodiesel-derived waste glycerol impurities on biomass and 1,3-propanediol production of Clostridium butyricum VPI 1718. Biotechnol Bioeng 107(1):76–84
Taconi KA, Venkataramanan KP, Johnson DT (2009) Growth and solvent production by Clostridium pasteurianum ATCC 6013 utilizing biodiesel-derived crude glycerol as the sole carbon source. Environ Prog Sustainable Energy 28(1):100–110
Sanz MT, Blanco B, Beltrán S, Cabezas JL, Coca J (2001) Vapor liquid equilibria of binary and ternary systems with water, 1,3-propanediol and glycerol. J Chem Eng Data 46(3):635–639
Melin T, Rautenbach R (2007) Membranverfahren. Grundlagen der Modul- und Anlagenauslegung. Springer, Heidelberg
Acknowledgments
The work presented here was kindly supported within the research project “PROPANERGY” in the Sevens Framework Program of the European Union (grant agreement no. 212671). We acknowledge also the support of the project partner BKW for scale-up study in their bio-pilot plant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaeding, T., DaLuz, J., Kube, J. et al. Integrated study of fermentation and downstream processing in a miniplant significantly improved the microbial 1,3-propanediol production from raw glycerol. Bioprocess Biosyst Eng 38, 575–586 (2015). https://doi.org/10.1007/s00449-014-1297-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1297-z