Abstract
While the use of oleaginous Rhodotorula glutinis as a feedstock for biodiesel production is an attractive idea, as it can avoid the pollutions associated with over-consumption of fossil fuels. Nevertheless, the related costs, including the energy required for sterilization, remain a barrier to commercialization. This study thus used a low-pH non-sterile medium, instead of a completely sterilized one, to grow R. glutinis in a 5-L airlift bioreactor. The results show that R. glutinis can grow well at a low pH level of 4.0 and without sterilization of the medium, producing a final biomass of 11.7 g/L. Nevertheless, such a low pH will lead to fewer total lipids accumulation, and so a two-stage process of pH control in a non-sterile batch was proposed. Even this two-stage pH operation was also able to produce a similar final biomass of 11.7 g/L. However, the batch with two-stage pH control had a far higher lipid content of 55 ± 4 % as compared to that of 21 ± 3 % in the batch grown at pH 4.0. This study shows the potential of the proposed non-sterile process with two-stage pH control applied to the growth of R. glutinis to enhance the total lipid accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental concerns have led to increasing interest in alternatives to fossil fuels, with biodiesel having many advantageous characteristics in this regard, such as being safer than other options, as well as non-toxic and containing no sulfur [1]. However, the use of plant oils as the feedstock for biodiesel production will cause problems with regard to competition for arable land, an issue that does not arise with microbial oils. Any species with a lipid content of microorganisms in excess of 20 % (g/g) is regarded as an oleaginous one [2]. Some yeast strains can accumulate intracellular lipids, accounting for as much as 70 % of their biomass dry weight [3]. The majority of the lipids are triacylglycerol (TAG) containing long-chain fatty acids that are comparable to conventional vegetable oils, and can be further used as the feedstock for biodiesel production. Among all potential oleaginous microorganisms, microalgae have been widely explored due to their carbon fixation ability through the process of photosynthesis. Unfortunately, the slow growth rate of microalgae and the difficultly in harvesting the resulting cell mass, due to its low density of cell mass, present barriers to their commercial cultivation for use as a biodiesel feedstock. In contrast, Rhodotorula glutinis, a red soil yeast, has the characteristics of rapid growth and the ability to utilize a range of carbon sources, which mean that it is an attractive candidate for microbial oil production [3–6].
Various carbon sources and fermentation strategies have been examined to achieve a cheap process for R. glutinis cultivation [4, 7]. Among the various substrates, crude glycerol had been widely used in the fermentation process. Crude glycerol is the main by-product of the biodiesel manufacturing process, and is produced at rate that is about 10 % (w/w) that of biodiesel [8]. Since the worldwide production of biodiesel is increasing, this has also led to a greater amount of crude glycerol being produced, and thus a fall in its price [7, 9]. Therefore, crude glycerol is regarded as a good carbon source being used in the fermentation industry [10]. In addition to the substrate, different fermentation strategies have also been explored to reduce costs, such as applying irradiation and microfiltration to enhance the resulting cell density [5, 11]. The use of airlift bioreactors instead of conventional agitation tanks is also another way to reduce costs, and the former also have the advantages of simple operation and low energy consumption, making them is suitable for the cultivation of R. glutinis [12]. The energy consumption during the sterilization process is also a crucial factor affecting the production cost. Steam sterilization is often adopted in the fermentation industry to provide aseptic conditions for pure cultivation. In this, the standard sterilization process will be set at 121 °C for 10–15 min to minimize the possibility of contamination, and this requires a consider amount of heat energy, thus raising costs. In literature, several studies had been performed for examining the effects of non-sterile conditions on the final products [10, 13–15]. The results indicated that the non-sterile conditions had the potential to be applied in the fermenter process, while the target microorganism can predominate in the microorganism consortium under the non-sterile environment.
Consequently, this study examines the potential of using non-sterile conditions for the growth of R. glutinis. Since it was expected that contamination would be inevitable in this non-sterile process, a low-pH control strategy was adopted to inhibit the growth of contaminants. The effects of a non-sterile and low-pH environment on the cell growth and total lipid accumulation were then explored. Furthermore, a two-stage pH control strategy was proposed to enhance cell growth under non-sterile conditions, without reducing the lipid accumulation.
Materials and methods
Microorganism and medium
Freeze-dried R. glutinis BCRC 21418 was obtained from the Bioresource Collection and Research Center, Taiwan (BCRC). The seed medium composition and the cultivation methods followed the suggestions provided by the BCRC. The fermentation medium (per liter) comprised defined amounts of crude glycerol, 2 g of yeast extract, 2 g of (NH4)2SO4, 1 g of KH2PO4, 0.5 g of MgSO4·7H2O, 0.1 g of CaCl2 and 0.1 g of NaCl [16]. Sodium hydroxide at 1.0 N or hydrogen chloride at 1.0 N was used to adjust the pH. The crude glycerol was purchased from a local biodiesel manufacturing company, and was the by-product of the conventional base catalyst transesterification process. The compositions of crude glycerol were 45 ± 5 % of glycerol, 16 ± 8 % methanol and 29 ± 6 % of ash, which was strongly depending on the batch obtained from the biodiesel plan.
Fermentation in 5-L airlift bioreactor
Batch fermentation was carried out in a 5-L internal-loop glass airlift bioreactor (30 cm in height, with a 10-cm outer diameter and 7.7-cm inner tube diameter) with a working volume of 3 L. All experiments were controlled at 24 °C and the pH was controlled at 5.5 using 1 N NaOH solution. The aeration rate was set at 1.5 vvm to explore the effects of a non-sterile medium on cell growth and total lipid accumulation [12].
Analytical methods
An infrared balance (Denver Instrument, IR 35) was adopted to rapidly measure the biomass concentration. Five mL of broth was centrifuged at 7000 rpm for 10 min. After removing the supernatant, about an equal volume of distilled water was added to eliminate impurities. This washing procedure was performed several times, and the final liquor was dried using the infrared balance at 150 °C to evaporate the water content.
The total lipid analysis was based on a modification of the procedure used by Bligh and Dyer [17]. The dry biomass was first ground into a fine powder; 0.05 g of the powder was then blended with 5 ml chloroform/methanol (2:1), and subsequently agitated for 20 min at room temperature in an orbital shaker. The solvent phase was recovered by centrifugation at 7000 rpm for 10 min. The same process was repeated twice, and the whole solvent was evaporated and dried under vacuum conditions.
The glycerol concentration was measured by HPLC (Agilent series 1100, Agilent Technologies, Santa Clara, CA, USA) with a refractive index detector, while the analysis was performed in a C-18 column (Vercopak N5ODS, 250 mm × 4.6 mm, Taiwan). The mobile phase was composed of 0.01 N H2SO4 with a flow rate of 0.4 ml/min [18]. All shaker conditions were performed in triplicate to have the expression of mean ± standard deviation
Results and discussion
Effects of low-pH and non-sterile medium on cell growth in the shaker trials
The heat energy spent on the sterilization process is one of the primary sources of power consumption in the fermentation industry, and to make microbial oils more economically feasible for use as the feedstock for biodiesel production, this energy requirement must be reduced. Nevertheless, a non-sterile condition certainly led to the contamination occurred, which might further inhibit the growth of expected microorganism. Therefore, this study thus examined the potential of using low pH to control contamination instead of sterilization of the medium. Comparisons of non-sterile and medium-sterilized trials, both operated at pH 3.0, 4.0, 4.5 and 5.5, respectively, were made in the shaker tests, and the results are shown in Fig. 1. These indicate that R. glutinis can grow well at a low pH. At all pH levels, the trials with a sterilized medium produced slightly more biomass than the non-sterile trials. It implied that the contamination might thus have been restricting the growth of R. glutinis in the non-sterile trials.
Even when the pH was as low as 3.0, the growth of R. glutinis did not totally cease, and it has been reported that R. glutinis can tolerate a very low pH level. The high tolerance of low-pH conditions may be due to the thicker cell membrane envelopes at low-pH conditions [19], as observations with an electron microscope showed that these became wrinkled and thick as the pH of the media became lower. The cell membrane grown at pH 1.5 was about four times as thick as that grown at pH 6.0, and this is likely to play an important role in the ability of R. glutinis to grow under acid conditions [19].
In the trials with a pH less than 4.5, both non-sterile and sterilized conditions will yield a similar biomass (Fig. 1). However, as the pH rises, the difference between the sterilized and non-sterilized trials became more marked. At pH 5.5, which is regarded as the suitable level for R. glutinis growth [12], the trial with a non-sterile medium produced significantly less biomass than the trial with the medium. This may be due to contamination in the non-sterile batch, in which the bacteria competed for the carbon source with R. glutinis, and thus less biomass was produced. Although the current biomass analytical method cannot differentiate the amounts of R. glutinis and contaminants, contamination could be observed in the trials with a non-sterile medium using the agar-spraying method. However, the use of a lower pH level can inhibit the growth of contaminants without negatively impacting the growth of R. glutinis. The use of a low pH instead of heat sterilization is thus an attractive strategy to reduce the energy used when growing R. glutinis.
A comparison of total lipid accumulation in the non-sterile batches (at pH 3.0, 4.0, 4.5 and 5.5) and the control sterilized trials (at pH 5.5) indicates that a neutral pH might be required to enhance total lipid accumulation. As seen in Fig. 2, a lower pH level led to a lower lipid content. The control batch had the highest lipid content of 36 %, and this was similar to the 32 % seen in the non-sterile batch that was also grown at pH 5.5. Nevertheless, as pH level decrease to less than 5.5, the lipid content was reduced to only about 20 % in the non-sterile batch at pH 3.0. The similar results were reported by Johnson et al., which stated that at pH 3, 5 and 6 the lipid contents were 12, 48 and 44 %, respectively, in the cultivation of R. glutinis using glucose as the carbon source [20]. They also reported that there was only a small change in the fatty acid profile over this wide pH range. Therefore, to achieve the ultimate goal of using a non-sterile medium with pH control instead of sterilized one, it is first necessary to overcome the problem of the low lipid content measured in the trials with a low pH.
The growth of R. glutinis in an airlift bioreactor with non-sterilized crude glycerol
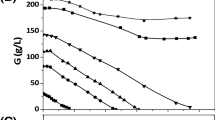
To examine the effects of using non-sterile conditions in the cultivation of R. glutinis on total lipid accumulation, trials of non-sterile batches at pH 4.0 and 5.5 in a 5-L airlift bioreactor were performed, and the results compared to those obtained with a control batch of sterilized medium at pH 5.5. The results are presented in Fig. 3, and indicate that the control batch (medium sterilized and pH 5.5) had the highest biomass, and also the highest total lipid accumulated. It is noted that the growth of the non-sterile batch with pH 5.5 would stop after about 40 h of cultivation. The likely reason for this is the serious contamination that occurred due to the non-sterile conditions, which impeded the growth of cells. Moreover, the non-sterile batch with a low pH of 4.0 also saw limited cell growth, at just 0.12 g/L h compared to the 0.25 obtained in the control batch, again indicating that a low pH would lead to less lipid accumulation. The non-sterile batch with pH 4.0 had a lipid content of only 20 ± 5 %, as compared to the 53 ± 6 % obtained in the control batch. Then, earlier study reported that when the yeast of Metschnikowia pulcherrima was cultured in a non-sterile glycerol-based media in two 500 L raceway ponds situated in a temperature-controlled glasshouse, this led to only about 2 g/L of biomass being obtained after 12 days of cultivation, as compared to about 15–20 g/L obtained under sterilized conditions [13]. It is thus clear that the contamination that occurred in the non-sterile conditions greatly impeded and inhibited the growth of microorganisms, and thus low-pH control is probably required at the beginning while the medium is not sterilized. Thus, the problem remaining for the non-sterile medium operation is how to enhance lipid accumulation under low-pH conditions.
The growth of R. glutinis using a two-stage pH control strategy
As described previously, a low pH is required to reduce contamination in the non-sterile process, but this then inhibits total lipid accumulation. Therefore, a two-stage pH control strategy was adopted to increase lipid accumulation and simultaneously avoid significant contamination under non-sterile conditions. This strategy consisted of pH controlled at 4.0 for the initial 48 h, followed by the second stage with pH set at 5.5 to enhance total lipid accumulation. The results of this two-stage process as compared to the use of a non-sterile batch at the constant pH of 4.0 are shown in Fig. 4. It can be seen that the biomass profiles are not significantly different between these two batches. However, the total lipids are clearly higher in the two-stage batch than the pH 4.0 batch, which are 55 ± 4 and 21 ± 3 %, respectively. In addition to the rapid lipid accumulation after the pH was increased to 5.5 after 48 h of cultivation, the glycerol consumption profiles also indicate that the two-stage pH control strategy was able to enhance lipid accumulation.
The detail of several key parameters in the airlift operations is shown in Table 1, which indicates that the strategy of two-stage pH control has good potential for use in oleaginous R. glutinis cultivation. Although R. glutinis can tolerate a pH as low as 4.0, the lipid content at this would fall to about 21 ± 3 % from the 52 ± 4 % seen with the control batch of conventional sterilized batch (pH 5.5), and thus a pH level of 5.5 might be required to enhance total lipid accumulation. The proposed two-stage pH control strategy could thus not only avoid contamination, but also enhance total lipid accumulation. The maximum biomass in the non-sterile batch with two-stage pH control can reach 11. 7 g/L (about 66 % of the biomass in control batch), with a high lipid content of 55 ± 4 %.
Conclusions
From the perspective of cost considerations, a cheap process for the cultivation of R. glutinis should be the first priority to make microbial oils feasible for use as the feedstock for biodiesel production. The current study thus examined the use of a low pH instead of heat sterilization to reduce energy consumption. However, a lower pH would lead to less total lipid accumulation. To reduce the contamination that can occur with a non-sterile process, and also enhance total lipid accumulation, a two-stage pH control strategy was thus proposed. The results indicated that the use of this approach in the non-sterile process could successfully enhance lipids accumulation, which had the great potential being applied on the commercialized microbial oils production for the purpose of cost reduction.
References
Aransiola EF, Ojumu TV, Oyekola OO, Madzimbamuto TF, Ikhu-Omoregbe DIO (2014) A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 61:276–297. doi:10.1016/j.biombioe.2013.11.014
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227
Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M (2009) Biodiesel production from oleaginous microorganisms. Renewable Energy 34:1–5
Yen HW, Yang YC, Yu YH (2012) Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J Biosci Bioeng 114(4):453–456. doi:10.1016/j.jbiosc.2012.04.022
Yen HW, Zhang Z (2011) Enhancement of cell growth rate by light irradiation in the cultivation of Rhodotorula glutinis. Bioresour Technol 102(19):9279–9281
Yen HW, Zhang Z (2011) Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J Biosci Bioeng 112(1):71–74
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218
Hu S, Luo X, Wan C, Li Y (2012) Characterization of Crude Glycerol from Biodiesel Plants. J Agric Food Chem 60:5915–5921
Liang Y, Cui Y, Trushenski J, Blackburn JW (2010) Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 101(19):7581–7586
Chatzifragkou A, Papanikolaou S, Dietz D, Doulgeraki AI, Nychas GJ, Zeng AP (2011) Production of 1,3-propanediol by Clostridium butyricum growing on biodiesel-derived crude glycerol through a non-sterilized fermentation process. Appl Microbiol Biotechnol 91(1):101–112. doi:10.1007/s00253-011-3247-x
Yen H-W, Yang Y-C (2012) The effects of irradiation and microfiltration on the cells growing and total lipids production in the cultivation of Rhodotorula glutinis. Bioresour Technol 107:539–541
Yen H-W, Liu YX (2014) Application of airlift bioreactor for the cultivation of aerobic oleaginous yeast Rhodotorula glutinis with different aeration rates. J Biosci Bioeng 118:195–198
Santamauro F, Whiffin FM, Scott RJ, Chuck CJ (2014) Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechnol Biofuels 7:34–43
Xing D, Ren N, Wang A, Li Q, Feng Y, Ma F (2008) Continuous hydrogen production of auto-aggregative Ethanoligenens harbinense YUAN-3 under non-sterile condition. Int J Hydrogen Energy 33(5):1489–1495
Pattra S, Lay C-H, Lin C-Y, O-Thong S, Reungsang A (2011) Performance and population analysis of hydrogen production from sugarcane juice by non-sterile continuous stirred tank reactor augmented with Clostridium butyricum. Int J Hydrogen Energy 36(14):8697–8703
Kim BK, Park PK, Chae HJ, Kim EY (2004) Effect of phenol on β-carotene content in total carotenoids production in cultivation of Rhodotorula glutinis. Korean J Chem Eng 21:689–692
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Athalye SK, Garcia RA, Wen Z (2009) Use of biodiesel-derived crude glycerol for producing eicosapentaenoic acid (EPA) by the fungus Pythium irregulare. J Agric Food Chem 57:2739–2744
Nguyen VAT, Senoo K, Mishima T, Hisamatsu M (2001) Multiple tolerance of Rhodotorula glutinis R-1 to acid, aluminum ion and manganese ion, and its unusual ability of neutralizing acidic medium. J Biosci Bioeng 92:366–371
Johnson V, Singh M, Saini VS, Sista VR, Yadav NK (1992) Effect of pH on lipid accumulation by an oleaginous yeast: Rhodotorula glutinis IIP-30. World J Microbiol Biotechnol 8:382–384
Acknowledgments
The authors gratefully acknowledge the financial support this study received from Taiwan’s Ministry of Science and Technology (MOST) under grant number 103-2623-E-029-001-ET and 103-3113-E-006-006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yen, HW., Liao, YT. & Liu, Y.X. The growth of oleaginous Rhodotorula glutinis in an airlift bioreactor on crude glycerol through a non-sterile fermentation process. Bioprocess Biosyst Eng 38, 1541–1546 (2015). https://doi.org/10.1007/s00449-015-1396-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1396-5