Abstract
Parasites represent a ubiquitous threat for most organisms, requiring potential hosts to invest in a range of strategies to defend against infection—these include both behavioural and physiological mechanisms. Avoidance is an essential first line of defence, but this behaviour may show a trade-off with host investment in physiological immunity. Importantly, while environmental stressors can lead to elevated hormones in vertebrates, such as glucocorticoids, that can reduce physiological immunity in certain contexts, behavioural defences may also be compromised. Here, we investigate anti-parasite behaviour and immune responses against a trematode (flatworm) parasite by larval amphibians (tadpoles) exposed or not to a simulated general stressor in the form of exogenous corticosterone. Tadpoles that were highly active in the presence of the trematode infectious stage (cercariae) had lower infection loads, and parasite loads from tadpoles treated only with dechlorinated water were significantly lower than those exposed to corticosterone or the solvent control. However, treatment did not affect immunity as measured through white blood-cell profiles, and there was no relationship between the latter and anti-parasite behaviour. Our results suggest that a broad range of stressors could increase host susceptibility to infection through altered anti-parasite behaviours if they elevate endogenous glucocorticoids, irrespective of physiological immunity effects. How hosts defend themselves against parasitism in the context of multiple challenges represents an important topic for future research, particularly as the risk posed by infectious diseases is predicted to increase in response to ongoing environmental change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investment in adaptive behaviours is a key element of animal success across a wide range of circumstances. One example of adaptive behaviour is that which provides resistance to parasite or pathogen infection (hereafter parasites). Parasite avoidance and other anti-parasite behaviours form part of the “behavioural immune system” (BIS) that has become increasingly recognised as a vital first line of defence against infection (Schaller and Park 2011; Ackerman et al. 2018; Amoroso 2021). Anti-parasite behaviours have been reported in a variety of animals, including crustaceans, amphibians, fish, birds, and mammals (Kiesecker et al. 1999; Karvonen et al. 2004; Behringer et al. 2006; Bush and Clayton 2018; Hart and Hart 2018). Such behaviours include avoidance of infectious stages or infected individuals, self-medication, and behavioural thermoregulation (Hart 1990, 1997; Parker et al. 2011). The benefits of a BIS are substantial given the costs associated with physiological resistance and infection itself (Bonneaud et al. 2003; Curtis 2014), making it critical to identify circumstances under which anti-parasite behaviours may be enhanced or compromised.
Notably, various abiotic and biotic stimuli may represent a challenge for homeostasis (i.e. act as a stressor) and can cause a physiological response that increases levels of various hormones, including glucocorticoids (GCs) in vertebrates (Sapolsky et al. 2000; Bonier et al. 2009). The hypothalamic–pituitary–adrenal (HPA) axis controls such reactions to stressors in mammals, with the hypothalamic–pituitary–interrenal (HPI) axis doing so for fish, amphibians and reptiles (Romero 2004; Sapolsky et al. 2000; Romero et al. 2009; Sheriff et al. 2011). Specifically, exposure to stressors can activate the HPA/HPI axis, resulting in the secretion of GCs—this is primarily cortisol for many mammals and ray-finned fish, but corticosterone (CORT) for birds, amphibians, and reptiles (Sheriff et al. 2011). In turn, GCs mediate various behavioural and physiological changes to help cope with the stressor(s), including their actions as potent anti-inflammatory agents, but they are also involved in day‐to‐day functions (see reviews by Wingfield et al. 1998; Sapolsky et al. 2000; MacDougall-Shackleton et al. 2019). GCs have thus often been considered as stressor biomarkers (Narayan et al. 2019) even though acute elevations are now typically considered adaptive because they aid in maintaining homeostasis, and can even be immune-stimulating (e.g. MacDougall-Shackleton et al. 2019). However, chronic increases can cause reductions in immunity and other critical activities (Wingfield et al. 1998; Sapolsky et al. 2000; Romero 2004; Martin 2009). For instance, GCs can inhibit leukocyte (white blood-cell—WBC) access to sites of inflammation and induce eosinophil apoptosis (Cain and Cidlowski 2017; Strehl et al. 2019). This said, animals may escape the effects of chronically-elevated GCs by reducing their reaction to stressors through habituation and dampening the GC response (Bennett et al. 2016; Fraker et al. 2009; Bokony et al. 2021).
The concept of ‘eco-physiology’ or ‘conservation physiology’ is thus important when considering that environmental stressors may affect wildlife through a range of physiological effects with various repercussions (Busch and Hayward 2009; Dantzer et al. 2014; Cooke et al. 2021), including their susceptibility to infectious diseases (Blaustein et al. 2012; Kernbach et al. 2018). It has been suggested that individuals in suboptimal environments, or in poor condition, may still be able to maintain behavioural defences against infection in the absence of a robust immune response (Adelman and Martin 2009); however, stress-associated hormone changes may not only affect physiological resistance via effects on innate or acquired immunity, but also the BIS given that elevated GCs are known to influence vertebrate behaviours. Short-term increases in GCs can increase locomotor activity, exploratory behaviour, foraging activity and risk-taking behaviours (Sapolsky et al. 2000; Martin et al. 2005), but chronically high GC levels have been associated with depression-like behaviours (e.g. lethargy and lack of reaction to stimuli in experimental animals—see Kim and Han 2006; Myers et al. 2014; Chen et al. 2016).
The effects of GCs on vertebrate behavioural responses to threats posed by natural enemies, such as predators, have reported varied findings such as reduced foraging and courtship that depend on the prey and predator species involved, as well as cue type (see review by Harris and Carr 2016). For instance, administering exogenous GCs was associated with stronger responses to predator cues in tree lizards (Thaker et al. 2009) and in rats (Rosen et al. 2008), but other authors have reported inhibition of anti-predator behaviour, such as for newts (Neuman-Lee et al. 2015) and tadpoles (Middlemis Maher et al. 2013; Gabor et al. 2019). In addition, fish with higher baseline cortisol levels were better able to maintain anti-predator displays when faced with repeated stressors (Cull et al. 2015). Sapolsky et al. (2000) suggested that higher baseline circulating GCs may better prepare animals to face certain challenges, such as predator attacks, but no clear trend has emerged regarding behavioural defences and activation of the HPA/HPI axis (Harris and Carr 2016).
Given the many similarities between predators and parasites as natural enemies with respect to victim defence strategies (Raffel et al. 2008; Buck et al. 2018), GC alterations could also affect anti-parasite behaviours in manners similar to those reported for predator avoidance. One such pathway would be if GCs directly suppress behaviours involved in parasite avoidance or removal, thereby reducing host resistance to infection. Such effects are critical to consider because behavioural resistance precedes physiological resistance during a host’s interaction with a single parasite (Amoroso 2021), and resistance precedes tolerance, be it behavioural or physiological (Råberg et al. 2007, 2009). Notably, behavioural changes in response to parasites are often mechanistically driven by physiology (Thompson and Kavaliers 1994), so could potentially be influenced by GCs. Changes in physiological resistance (innate and acquired immunity) could also indirectly affect behavioural resistance because these two types of defence are not independent, and are expected to interact with one another (Amoroso and Antonovics 2020; Amoroso 2021), thus representing a second pathway by which GC alterations could affect host anti-parasite behaviours. Both positive and negative covariation between behavioural and immunological traits may be seen, with either type of resistance driving the relationship, or both may be simultaneously affected by a third factor that links them together (see review by Lopes 2017).
Importantly, trade-offs between behavioural and physiological resistance to parasites may be expected because both have associated costs (Hall et al. 2017; Gassen et al. 2018; Gibson and Amoroso 2022). Individuals investing in a robust physiological (immune) response to infection risk should thus exhibit reduced anti-parasite behaviours to compensate for this cost. Conversely, individuals unable to mount an effective physiological response may instead invest more in putatively less-costly behavioural resistance (Curtis 2014). Such negative relationships between behavioural and physiological resistance have been demonstrated at the individual (e.g. Zylberberg et al. 2013, 2014; Stephenson 2019) and population levels (Klemme et al. 2020), but other studies have found a positive relationship (Klemme and Karvonen 2016), or even both positive and negative, depending on the circumstances (Schreier and Grindstaff 2020). Importantly, although physiological mechanisms underlying any behavioural–physiological resistance trade-offs have not been definitively demonstrated to date, GCs represent one potential link (Zylberberg et al. 2014; Schreier and Grindstaff 2020). Altered GCs could thus lead to concomitant changes in host resistance through behaviour or physiology, or both (Lopes 2017).
Here, we investigated whether larval amphibians (tadpoles) experimentally exposed to exogenous CORT were able to maintain effective anti-parasite behaviours, and if these were related to immune measures. Exogenous CORT is a well-established technique to raise endogenous CORT levels in larval amphibians (reviewed by Belden et al. 2005), and here this served as a proxy for a stressor that would result in the latter. In nature, exogenous exposure can also occur via ambient/environmental CORT that can affect aquatic animals (Tornabene et al. 2021) and should be considered in examining effects on host parasitism. Acute and chronic increases in tadpole CORT levels in response to various abiotic and biotic stressors are well-documented, including predation risk (e.g. Middlemis Maher et al. 2013; Bennett et al. 2016) and other challenges such as pond-drying, high conspecific density, pollution, and pathogen infection (e.g. Hopkins et al. 1997; Belden et al. 2007; Denver 2009; Gabor et al. 2013; Bókony et al. 2021). However, CORT production can be downregulated during chronic exposure to stressors (Fraker et al. 2009; Bennett et al. 2016; Bókony et al. 2021), so will not necessarily remain elevated.
As CORT is known to affect larval amphibian behaviour, including anti-predator defences, elevations could also influence anti-parasite behaviours. CORT generally stimulates tadpole locomotion and foraging (e.g. Crespi and Denver 2004, 2005), but elevated CORT enhances or reduces anti-predator behaviours such as freezing depending on the context (e.g. Fraker et al. 2009; Kulkarni and Gramapurohit 2017), and may not even play a role in some circumstances (Hossie et al. 2010). In contrast, bursts of high activity consisting of rapid swimming and twisting are key to the highly effective anti-parasite behaviours displayed by tadpoles from many taxa in the presence of free-swimming infectious stages (cercariae) from several species of trematode (parasitic flatworms). These movements are necessary for cercariae evasion and removal, and their experimental suppression leads to greater infection (e.g., Koprivnikar et al. 2006; 2014; Daly and Johnson 2011; Sears et al. 2013). Such behaviours are important as many trematodes can be pathogenic to larval amphibians (see review by Koprivnikar et al. 2012a). However, tadpole anti-parasite behaviour also shows significant intra- and interspecific variation (Koprivnikar et al. 2012b, 2014), and could be driven by infection risk or other factors, including stressor exposure.
Exogenous CORT could also affect anti-parasite behaviours in tadpoles by influencing their physiological immunity given that these components of resistance are unlikely to be independent of one another (Amoroso and Antonovics 2020; Amoroso 2021). Links between the HPI axis and immunity in larval amphibians are well-established, as well as with metamorphosis, with elevated endogenous GCs having various effects on WBC profiles (Rollins-Smith 2001, 2017; Rollins-Smith and Blair 1993). Critically, exogenous-CORT exposure of tadpoles has been shown to increase trematode infections while also altering WBC profiles (Belden and Kiesecker 2005; LaFonte and Johnson 2013), which are a key component of the larval amphibian immune response to these parasites (Martin and Conn 1990; Holland et al. 2007).
These attributes of our model system allowed us to experimentally expose hosts to a stressor-induced hormone (CORT) known to compromise the immune response against a parasite infection for which there is also effective behavioural resistance. We hypothesised that CORT-exposed tadpoles would exhibit a reduced immune response (via altered WBC profiles) to trematode infection but would correspondingly engage in greater anti-parasite behaviours to compensate if there is an inherent negative trade-off between behavioural and physiological resistance. Conversely, reductions in the BIS owing to elevated GCs would be problematic given the multiple stressors increasingly faced by wildlife that are capable of doing so (Munns 2006), with potentially important consequences in the context of infectious disease dynamics.
Materials and methods
Tadpole rearing and maintenance
Northern leopard frog (Rana (Lithobates) pipiens) egg masses were obtained from a commercial supplier (Boreal Science) and kept at room temperature (~ 22 °C) with a 14:10 h light:dark cycle until tadpoles had hatched. Tadpoles were then housed in ten 5 L tanks with ~ 100 tadpoles in each. Water was changed every second day using dechlorinated water, i.e. DW (tap water containing Nutrafin Aquaplus, A7928 Hagen®, and Nutrafin Cycle, A7906 Hagen®), and tadpoles were fed ad libitum with store-bought organic spinach softened with boiled DW as well as rabbit food pellets (Hagen®). Tadpoles were housed for approximately 3 weeks until a sufficient number reached at least Gosner developmental stage 25 (Gosner 1960); tadpoles at this stage are known to survive exposure to the trematode and exogenous-CORT doses used here (Belden and Kiesecker 2005; Daly and Johnson 2011; LaFonte and Johnson 2013; Koprivnikar et al. 2014). Animals were used in accordance with the Canadian Council on Animal Care guidelines, and with institutional approval from the animal care committee (AUP 633).
Parasite collection
Echinostomatid trematodes have a complex life cycle, whereby the first intermediate host is an aquatic snail within which asexual reproduction by the parasites produces free-swimming cercariae. Many echinostomatids infect tadpoles as their second intermediate host, with cercariae forming cysts in the nephric system that can substantially compromise renal function and decrease survival (Schotthoefer et al. 2003; Szuroczki and Richardson 2009; Koprivnikar et al. 2012b). Previous studies have demonstrated tadpole behavioural evasion and removal of echinostome cercariae (e.g., Koprivnikar et al. 2006, 2012a, 2014). This is a response to initial contact by the cercariae, which often creep down the tadpole’s exterior to reach the cloaca where they must enter to reach the nephric system. For this study, aquatic snails (Helisoma sp.) were collected locally in Ontario from two ponds and screened in the lab for trematode infection using established methods (see Szuroczki and Richardson 2009) to stimulate cercariae emergence by exposing snails to heat and light from a lamp. Emerged cercariae were identified as a species of Echinostoma using a standard key (Schell 1985); snails infected with this trematode were housed separately in DW and fed ad libitum with raw spinach. Whilst the exact identification of Echinostoma sp. would require molecular tools, cercariae of this genus readily infect many amphibians, and tadpoles behaviourally respond in the same manner to different echinostomatid cercariae (e.g. Koprivnikar et al. 2006, 2014). Prior to the tadpole behaviour experiments, Echinostoma sp. cercariae were collected as described above, pooled, and then divided into 1.5 mL microcentrifuge tubes so that each contained 15 cercariae in DW. Cercariae were no older than 4 h before use to avoid reductions in infectivity (McCarthy 1999).
Exogenous corticosterone exposure
We haphazardly removed 9 tadpoles that had reached at least Gosner stage 25 (based on visual inspection) from each of the 10 group housing containers, for a total of 90 tadpoles that were exposed to one of three solution treatments (30/treatment): 1) DW; 2) DW containing the solvent ethanol (0.2 mL of 80% ethanol per 1 L); and, 3) DW containing CORT (Sigma-Aldrich® ≥ 98.5%) at 0.1 µM, made by dissolving 0.0003464 g of CORT in 0.2 mL of 80% ethanol before adding it to 1 L of DW. Treatments 2 and 3 followed similar protocols for exogenous CORT-induced immunosuppression of tadpoles from previous studies (Belden and Kiesecker 2005; LaFonte and Johnson 2013). Exogenous-CORT exposure was used here to simulate a general stressor that elevates GCs because this is a well-established approach which results in higher levels of endogenous CORT for several amphibian species (e.g. Belden et al. 2005, 2010; Fraker et al. 2021). Tadpoles were individually housed in 1 L plastic containers (diameter ~ 10 cm) holding 500 mL of their respective treatment for 12 days. Treatments were renewed every second day by replacing the appropriate treatment solution, and tadpoles were fed boiled organic spinach ad libitum.
Behavioural trials
At the end of the 12-day period, behavioural trials were conducted by exposing each tadpole to 15 Echinostoma sp. cercariae which were collected as described above for snail screening and counted using a dissecting microscope. To assess tadpole anti-parasite behaviour, we followed a methodology employed by similar studies (e.g. Koprivnikar et al. 2012b, 2014). Briefly, tadpoles were first acclimatised in individual experimental arenas (500 mL containers of DW) for 15 min during which their behaviour in the absence of cercariae was recorded. Recordings took place using three digital camera and tripod stations that were separated from each other and the experimenters by cardboard blinds. Individual tadpoles were assigned to each camera using a random number generator. After the initial 15 min of recording, cercariae were then added by emptying the contents of each microcentrifuge tube into the container, followed by recording for another 15 min. Following the behavioural recordings, tadpoles were left in their individual experimental containers and euthanised 24 h later using a buffered solution of 1% tricaine methanesulfonate (MS-222). When viewing the recordings, we considered tadpole activity at 45 time points that were separated by 20-s intervals (i.e. 15 min total). Tadpole behaviour was recorded as active/inactive at each time point.
Immune profiles
We chose to focus on the relative abundance of white blood cells (WBCs, i.e. leukocytes) as our measure of tadpole physiological (immunological) resistance because these play a key role in vertebrate defences against macroparasites and are specifically involved in tadpole responses to infection by echinostomatids (e.g. Martin and Conn 1990; Holland et al. 2007). Eosinophils play a particularly important role in this context (Kiesecker 2002; Belden and Kiesecker 2005; LaFonte and Johnson 2013). Importantly, WBC profiles in amphibians are not only affected by various stressors, including parasitic infection and contaminants (Davis et al. 2004, 2010; Marcogliese et al. 2009), but also by elevated GCs (e.g. Burraco et al. 2017), including that resulting from exogenous-CORT exposure (Belden and Kiesecker 2005; LaFonte and Johnson 2013).
Following euthanasia, tadpoles were weighed, and blood smears were prepared for each tadpole before freezing them in individually labelled plastic bags for follow-up parasite examinations. To collect blood smears from individual tadpoles, a slit was made on the tail, with blood collected using a 1 mL plastic pipette. A drop of blood was placed on a glass slide, and a smear was prepared using a second glass slide (Hadji-Azimi et al. 1987). The blood smear slide was left to air dry for approximately 30 min before fixation by dipping it for 1 min into a methanol solution. Slides were then immersed in Giemsa stain for 30 s, rinsed twice by dipping for 10 s into each of two consecutive jars containing distilled water, and left to air dry. Blood cells were identified and counted under a dissecting microscope following standard methods (Davis 2009). Due to their small size, it was not possible to obtain a standardised amount of blood from each tadpole. We therefore quantified the ratio of WBCs to red blood cells for each individual tadpole rather than total number, as measured on each slide up to the point when either 100 total WBCs were counted, or 150 fields of view had been examined. Given their importance for tadpole immune responses to trematodes, we also calculated the proportion of eosinophils making up the WBCs present.
Parasite enumeration
After the frozen tadpoles were thawed, their Gosner developmental stage was determined using a stereomicroscope. Tadpoles were then dissected, with their nephric system examined to assess the number of Echinostoma sp. cysts present. Given the need for necropsies to assess parasite infection, this excluded the possibility of assessing whole-body CORT levels in the tissues of individual tadpoles, with previous studies involving exogenous CORT and trematode exposure facing the same constraint (Belden and Kiesecker 2005; LaFonte and Johnson 2013). Whilst assays using water-borne CORT as a proxy for endogenous CORT levels in larval amphibians are a valuable tool for non-invasive measurement (Narayan et al. 2019), the relationship between the two is not consistent among species and development stages (McClelland and Woodley 2021). Notably, water-borne CORT may not be a reliable way to assess endogenous CORT for premetamorphic (Gosner stage 25–35) northern leopard frog tadpoles (McClelland and Woodley 2021).
Data analysis
All data analyses were carried out using R version 3.3.1 (R Core Team 2021) in RStudio (RStudio Team 2021) with the package lme4 (Bates et al. 2015) for GLMMs. Due to mortalities during the treatment and experimental periods, the final numbers of tadpoles in each of the 3 treatments were 14, 17, and 12 in the DW, ethanol solvent, and CORT treatment groups, respectively. All tadpoles used in this study were Gosner developmental stages 27–30 at the time the experiment ended, with stage positively correlated with tadpole mass. A generalised linear model using the Poisson family was used to test for any effect of treatment on Gosner stage and no differences were found between any of the three treatments (CORT/DW: z = −0.239, p = 0.811; ethanol/DW: z = −0.046, p = 0.963; CORT/ethanol z = −0.206, p = 0.837) (Electronic Supplementary Material: Table ESM1). However, given that previous studies have reported changes in immunocompetence with Gosner stage (e.g. Davis 2009), all of our models included this as a random effect variable rather than mass. To analyse the effect of treatment on parasite load, we first used a generalised linear mixed-effects model (GLMM) that included parasite load as the response variable and treatment as the predictor variable using the Poisson family.
A separate GLMM using the binomial family was used to investigate the effect of parasite exposure on tadpole activity across the three treatments. The number of times spent active out of a total of 45 timepoints both in the presence and absence of trematode cercariae was the binomial response variable with treatment and parasite exposure as predictor variables. To further examine the relationship between activity and parasite load, a GLMM using the Poisson family was carried out which contained the number of trematode cysts as the response variable, with two predictor variables—the centred variable of activity in the presence of parasites (number of time points during which a tadpole was active), and the categorical variable of treatment. In both models, an interaction between the two response variables, parasite exposure and treatment, and tadpole activity and treatment, respectively, was included. After examining the corrected akaike information criterion (AICc) value for these models, using the package MuMin (Bartoń 2018), the simpler model was chosen in both cases based on a lower AICc (AICc 606.87, 233.99 respectively), compared to the model which included the interaction (AICc 609.66, 236.08 respectively).
Finally, two linear regression mixed models were used to examine tadpole immune measures. First, tadpole immune measures were analysed in response to parasite load and the three treatment conditions. The response variable was the ratio of white to red blood cells (WBC: RBC) after a square-root transformation, with the predictor variables represented by the number of trematode cysts and treatment. The second analysis examined WBC: RBC (square-root transformed) in response to the centred predictor variable of tadpole activity in the presence of parasites. We did not analyse the eosinophil data specifically as the number of eosinophils as a proportion of the total WBCs was the exact same for all three treatments at 0.01.
Results
Mean parasite loads (number of Echinostoma sp. cysts) were 6.17, 6.06, and 3.93 for the CORT, ethanol, and DW-treated tadpoles, respectively; of the tadpoles surviving until the end of the experiment, 95% were successfully infected with cercariae. Parasite loads from tadpoles treated only with DW were significantly lower than those treated with either CORT (z = 2.163, p = 0.031) or the vehicle control ethanol (z = 2.644, p = 0.008) and there was no significant difference in parasite load between CORT and ethanol treated tadpoles (z = −0.305, p = 0.760) (Fig. 1; Electronic Supplementary Material (ESM), Table ESM2).
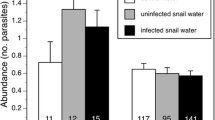
When considering tadpole activity as a measure of their behavioural resistance, tadpoles showed increased activity in the presence of trematode cercariae (z = 5.551, p = < 0.001; Fig. 2; Table ESM3), and there was an overall negative relationship between individual tadpole activity during exposure to the live trematode cercariae and the resultant parasite loads, i.e. less active tadpoles had higher loads of Echinostoma sp. cysts (z = −2.289, p = 0.022; Table ESM4). When comparing overall activity between treatments, that of tadpoles exposed to CORT was significantly lower than for tadpoles exposed to DW (z = −2.888, p = 0.004) or to ethanol (z = −2.054, p = 0.040) (Fig. 2). There was no significant difference in activity between tadpoles exposed to ethanol or DW (z = −0.853, p = 0.394) (Table ESM3). On average, CORT-treated tadpoles were active during 6.42 of the 45 time points at which activity (yes/no) was assessed, and ethanol- and DW-treated tadpoles were active for an average of 11.71 and 14.07 time points out of 45, respectively.
With respect to physiological immune measures, the WBC: RBC of tadpoles showed no significant relationship with their parasite load (t = −1.614, p = 0.107), and was not significantly affected by prior CORT exposure (t = 1.152, p = 0.249) (Table ESM5). In addition, tadpole WBC: RBC showed no significant relationship with their activity level in the presence of parasites (t = −0.026, p = 0.979) (Table ESM6). Gosner stage accounted for close to zero variance in models examining tadpole WBC: RBC (Table ESM2–6).
Discussion
Here we used exogenous glucocorticoid (GC) exposure of larval amphibians to simulate an aversive environmental stimulus that is capable of elevating endogenous GCs as part of a general physiological response to stressors which is seen in many vertebrates, finding that this negatively impacted the anti-parasite behaviours exhibited by tadpoles. Tadpoles exposed to exogenous CORT were the least active overall, including in the presence of free-swimming parasite infectious stages (cercariae of the trematode Echinostoma sp.), and concomitantly had high parasite loads. However, CORT exposure had no effect on immunity as measured by WBC profiles, and there was no relationship between the latter and host anti-parasite behaviour. Contrary to our expectations, this suggests that exposure to a stressor which elevates GCs over a similar period can increase host susceptibility to parasite or pathogen infection by compromising the BIS alone. Our results highlight the importance of adaptive host behavioural responses to the risk of parasite infection, and thus the broad potential of environmental stressors to influence infectious disease dynamics if they negatively affect anti-parasite behaviours by elevating endogenous GCs.
Anti-parasite behaviours have been reported for many host taxa and are a key component in avoiding infection by various parasites and pathogens (Behringer et al. 2018; Bush and Clayton 2018; Hart and Hart 2018). Our findings confirm the negative relationship between tadpole activity in the presence of cercariae and infection loads as reported by previous studies (e.g. Daly and Johnson 2011; Koprivnikar et al. 2014), with tadpoles in the water control having the fewest cysts. However, the number of cysts in CORT-exposed individuals was unexpectedly similar to the solvent control, and we posit that altered anti-parasite behaviours may still have played a role even though the overall activity of tadpoles in ethanol was similar to those only exposed to water. Notably, while tadpoles in the CORT treatment were consistently less active than those in the ethanol or water treatments, extreme activity levels were most commonly seen in ethanol-exposed tadpoles, especially when cercariae were present (see Fig. 2). This matches what has been reported for activity by ethanol-exposed R. pipiens tadpoles in response to a novel stimulus (Durbin et al. 2021), as well as overall activity in larval zebrafish after ethanol exposure (Chen et al. 2011). Critically, ethanol-exposed fish were not only prone to hyperactivity, but they also exhibited simplified swimming paths, i.e. fewer sharp twists and turns. Whilst we were not able to evaluate this, we believe it also occurred in our experiment, with important implications because the sharp flicks and twists of tadpoles are key to preventing cercariae establishment (Taylor et al. 2004; Koprivnikar et al. 2006; Szuroczki and Richardson 2012; Sears et al. 2013). Future studies should thus consider the effects of stressors and elevated GCs on specific components of anti-parasite behaviours, not just overall activity.
Median tadpole activity (with upper and lower quartiles shown) as measured by the number of times an individual was active (yes/no) during 45 timepoints in 15 min either in the absence (grey) or presence (pink) of trematode cercariae after being subjected to one of three treatments for 12 days before parasite exposure
It is important to identify environmental circumstances in which behavioural defences against infection may be reduced. For instance, both synchronous and asynchronous exposure to predation cues can interfere with tadpole anti-parasite behaviours (Koprivnikar and Penalva 2015; Koprivnikar and Urichuk 2017). In addition, elevated trematode infection loads as a result of tadpole exposure to aquatic pollutants, such as road salts, have been attributed to reduced behavioural rather than physiological immunity (Milotic et al. 2017). Whilst GC levels were not considered in these studies that demonstrated how various aversive stimuli can negatively affect the BIS, our results suggest that elevation of endogenous GCs in response to environmental stressors could be a common underlying factor which warrants further study given the capacity for these hormones to influence host behaviour.
Whilst our study is the first to consider how a general stressor could affect anti-parasite behaviours in aquatic vertebrates through GCs, others have examined anti-predator responses. The majority of these investigations reported that exogenous-CORT exposure reduced tadpole anti-predator behaviours by increasing their activity levels (e.g. Fraker et al. 2009; Middlemis Maher et al. 2013). This is the opposite response of most larval amphibians to predation risk (e.g. Hossie et al. 2017), thus elevated CORT could increase activity in a way that makes tadpoles more likely to be seen by visual predators (Gabor et al. 2019). In contrast, Kulkarni and Gramapurohit (2017) found that larval frog exposure to 5, 10 or 20 μg/L CORT enhanced their anti-predator response in a concentration-dependent manner by reducing their activity. In addition, Sullivan et al. (2021) reported that chronic, but not short-term, CORT elevations reduced exploratory behaviours in adult salamanders. However, the nature of the predator stimulus (e.g. conspecific alarm cues or larval dragonfly kairomones) used varies amongst studies and may contribute to the inconsistent effects of CORT on tadpole behavioural responses. It is thus unclear whether elevated GCs in amphibians generally decrease or increase their activity levels, as well as their responses to natural enemies. In mammals, chronically-elevated GCs may induce depression-like behaviours such as lethargy and helplessness (reviews by Erickson et al. 2003; Chen et al. 2016).
It is important to note that GCs have a wide range of physiological, behavioural and cognitive functions; elevated GCs in several contexts are thus not necessarily “stressful” in a negative sense and are important for maintaining homeostasis (Sapolsky et al. 2000; Erickson et al. 2003; MacDougall-Shackleton et al. 2019; Romero and Beattie 2022). Short-term increases in GC levels can aid in early responses to aversive stimuli (Erickson et al. 2003; Wingfield and Sapolsky 2003; Romero and Beattie 2022); however, chronic elevations are known to suppress various behaviours (e.g. reproduction as in Woodley and Lacy 2010). In the face of chronically-elevated GCs, diminished-behavioural output as a strategy to redirect critical resources may be advantageous in many situations (Myers et al. 2014), especially as sustained-GC elevations can result in homeostatic overload (Romero et al. 2009). Such a mechanism may help to explain why CORT-exposed tadpoles here showed the least activity overall.
Whilst infection loads were higher in our CORT- and ethanol-exposed tadpoles compared to the water control, there was no difference in their physiological immune response as measured by WBC: RBC, nor was there a difference in their eosinophil ratios. This is despite the fact that previous studies clearly demonstrated immunosuppressive effects of exogenous-CORT exposure on larval amphibians, including decreased eosinophils, that were associated with greater trematode infection (Belden and Kiesecker 2005; LaFonte and Johnson 2013). It thus appears that chronic exposure to exogenous CORT affected host susceptibility to parasite infection in our study through changes in the behavioural, and not physiological, immune system. Although exogenous CORT and trematode exposure should have opposing effects by being behaviour and immune-suppressing vs. behaviour and immune-stimulating, respectively, tadpoles in the water treatment would have had the capacity to mount a response via WBCs. As such, exogenous-CORT exposure either did not affect the post-infection immune response or plasma-WBC profiles are not appreciably altered as a result of echinostome infection, as seen for another trematode, Ribeiroia ondatrae (Koprivnikar et al. 2019). It is also possible that the 24 h period between parasite exposure and tadpole euthanasia here was insufficient for WBC changes to have occurred. In addition, plasma-WBC profiles provide information on only one aspect of the potential immune response to trematode cysts. For instance, Holland et al. (2007) found that eosinophils and macrophages developed directly around echinostome cysts in infected tissue. Future investigations should thus consider additional measures of immunity when assessing responses to elevated GCs. It would also be helpful to evaluate physiological immunity in a subset of tadpoles before parasite exposure, as well as measure whole-body CORT to quantify individual levels although we had no reason to presume that exogenous-CORT exposure failed to raise endogenous levels here given that this is a well-documented approach to achieve this outcome in larval amphibians (review by Belden et al. 2005). For similar studies, further refinement and validation of water-borne CORT assays may also be useful. Finally, measuring CORT in tadpoles while in the presence of parasite infectious stages such as cercariae would also be informative to understand CORT regulation in response to an acute stressor.
We had predicted that individual tadpoles exhibiting the greatest anti-parasite activity in the presence of trematode infectious stages (cercariae) would show the least robust-induced immune response post-infection, i.e. display a trade-off between behavioural and physiological immunity. However, this was not seen despite various reports of a negative relationship between different types of defences against natural enemies. For example, crucian carp with a morphological phenotype providing defence against predators have reduced immune function (Vinterstare et al. 2019). More specifically, trade-offs have also been reported with respect to host defences against parasites (review by Lopes 2017). For instance, Klemme et al. (2020) demonstrated significant genetic variation in parasite defence traits across populations of salmonid fish, as well as negative associations between traits, with the most resistant populations showing the weakest parasite avoidance behaviours, and also the lowest infection tolerance. At the individual level, male house finches that avoided sick conspecifics invested less in their immune response to a pathogen—in contrast, males which spent time associating with, or near, sick conspecifics had stronger immune responses (Zylberberg et al. 2013). This being said, trade-offs may be dependent on the immune measures employed, especially if these have different costs (Zylberberg et al. 2014), with antibody responses most commonly considered (e.g. Zylberberg et al. 2013; Schreier and Grindstaff 2020). It is possible that investment into WBC-mediated immune responses, such as those considered here, are not affected by that in the BIS. This trade-off might also only occur in specific circumstances or may not be induced by changes in GCs. Future investigations should consider the specific circumstances under which trade-offs between behavioural and physiological immunity may be observed (e.g. dual stressors such as predators and parasites), and also include a suite of immune measures to examine both standing and induced immunity.
It is critical to understand the extent to which potential hosts can engage in anti-parasite behaviours given their central roles in defence (Hart 1990, 1997), especially because the threat posed by parasites and pathogens is predicted to increase in response to various environmental stressors (Oppliger et al. 1998; Byers 2020). Our study not only demonstrates the key role of behavioural immunity but also highlights the potential for interruption of this front line of defence by any environmental stressor that could chronically elevate endogenous GCs, including ambient/environmental CORT (e.g. Gabor et al. 2018; Tornabene et al. 2021), as we simulated here with exogenous GC exposure. The field of eco-physiology, which considers how anthropogenic disturbances may affect wildlife through physiology, including their susceptibility to infectious diseases (Busch and Hayward 2009; Hing et al. 2016), should therefore consider behavioural effects as well. Behavioural responses to multiple stressors are also important to understand (Hale et al. 2016). We recommend that future studies continue to explore various aspects of parasite avoidance, resistance, and tolerance, and how these are affected by physiological responses to aversive stimuli. In this way, we stand to gain a greater understanding of how both physiological and behavioural defences will play roles in mitigating the risks and costs of parasitism in an ever-changing environment.
Data availability
Data will be archived and available from the figshare repository using the following https://doi.org/10.6084/m9.figshare.25681758.
References
Ackerman JM, Hill SE, Murray DR (2018) 2018. The behavioral immune system: current concerns and future directions. Soc Personal Psychol 12:57–70
Adelman JS, Martin LB (2009) Vertebrate sickness behaviors: adaptive and integrated neuroendocrine immune responses. Integr Comp Biol 49:202–214
Amoroso CR (2021) Integrating concepts of physiological and behavioral resistance to parasites. Front Ecol Evol 9:47
Amoroso CR, Antonovics J (2020) Evolution of behavioural resistance in host–pathogen systems. Biol Lett 16:20200508
Bartoń K (2018) MuMIn: multi-model inference. CRAN. https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Behringer DC, Butler MJ, Shields JD (2006) Avoidance of disease by social lobsters. Nature 441:421–421
Behringer DC, Karvonen A, Bojko J (2018) Parasite avoidance behaviours in aquatic environments. Phil Trans R Soc B Biol Sci 373:20170202
Belden LK, Kiesecker JM (2005) Glucocorticosteroid hormone treatment of larval treefrogs increases infection by Alaria sp trematode cercariae. J Parasitol 91:686–688
Belden LK, Moore IT, Wingfield JC, Blaustein AR (2005) Corticosterone and growth in Pacific treefrog (Hyla regilla) tadpoles. Copeia 2005(2):424–430
Belden LK, Rubbo MJ, Wingfield JC, Kiesecker JM (2007) Searching for the physiological mechanism of density dependence: does corticosterone regulate tadpole responses to density? Physiol Biochem Zool 80:444–451
Belden LK, Wingfield JC, Kiesecker JM (2010) Variation in the hormonal stress response among larvae of three amphibian species. J Exp Zool Part A Ecol Genet Physiol 313(8):524–531
Bennett AM, Longhi JN, Chin EH, Burness G, Kerr LR, Murray DL (2016) Acute changes in whole body corticosterone in response to perceived predation risk: a mechanism for anti-predator behavior in anurans? Gen Comp Endocrinol 229:62–66
Blaustein AR, Gervasi SS, Johnson PT, Hoverman JT, Belden LK, Bradley PW, Xie GY (2012) Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Philos Trans R Soc B Biol Sci 367:1688–1707
Bókony V, Ujhegyi N, Hamow KÁ, Bosch J, Thumsová B, Vörös J, Aspbury AS, Gabor CR (2021) Stressed tadpoles mount more efficient glucocorticoid negative feedback in anthropogenic habitats due to phenotypic plasticity. Sci Total Environ 753:141896
Bonier F, Martin PR, Moore IT, Wingfield JC (2009) Do baseline glucocorticoids predict fitness? Trends Ecol Evol 24:634–642
Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G (2003) Assessing the cost of mounting an immune response. Am Nat 161:367–379
Buck JC, Weinstein SB, Young HS (2018) Ecological and evolutionary consequences of parasite avoidance. Trends Ecol Evol 33:619–632
Burraco P, Miranda F, Bertó A, Vazquez LA, Gomez-Mestre I (2017) Validated flow cytometry allows rapid quantitative assessment of immune responses in amphibians. Amphibia-Reptilia 38:232–237
Busch DS, Hayward LS (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142:2844–2853
Bush SE, Clayton DH (2018) Anti-parasite behaviour of birds. Philos Trans R Soc B Biol Sci 373:20170196
Byers JE (2020) Effects of climate change on parasites and disease in estuarine and nearshore environments. PLoS Biol 18:e3000743
Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17(4):233–247
Chen TH, Wang YH, Wu YH (2011) Developmental exposures to ethanol or dimethylsulfoxide at low concentrations alter locomotor activity in larval zebrafish: implications for behavioral toxicity bioassays. Aquat Toxicol 102(3–4):162–166
Chen J, Wang ZZ, Zuo W, Zhang S, Chu SF, Chen NH (2016) Effects of chronic mild stress on behavioral and neurobiological parameters-role of glucocorticoid. Horm Behav 78:150–159
Cooke SJ, Bergman JN, Madliger CL, Cramp RL, Beardall J, Burness G, Clark TD, Dantzer B, De La Barrera E, Fangue NA, Franklin CE (2021) One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conserv Physiol 9:coab009
Crespi EJ, Denver RJ (2004) Ontogeny of corticotropin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii). Horm Behav 46:399–410
Crespi EJ, Denver RJ (2005) Roles of stress hormones in food intake regulation in anuran amphibians throughout the life cycle. Comp Biochem Physiol A Mol Integr Physiol 141:381–390
Cull F, O’Connor CM, Suski CD, Shultz AD, Danylchuk AJ, Cooke SJ (2015) Puff and bite: the relationship between the glucocorticoid stress response and anti-predator performance in checkered puffer (Sphoeroides testudineus). Gen Comp Endocrinol 214:1–8
Curtis VA (2014) Infection-avoidance behaviour in humans and other animals. Trends Immunol 35:457–464
Daly EW, Johnson PTJ (2011) Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165:1043–1050
Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2:cou023
Davis AK (2009) Metamorphosis-related changes in leukocyte profiles of larval bullfrogs (Rana catesbeiana). Comp Clin Pathol 18:181–186
Davis AK, Cook KC, Altizer S (2004) Leukocyte profiles in wild house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. EcoHealth 1:362–373
Davis AK, Keel MK, Ferreira A, Maerz JC (2010) Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Pathol 19:49–55
Denver RJ (2009) Stress hormones mediate environment–genotype interactions during amphibian development. Gen Comp Endocrinol 164:20–31
Durbin MR, Pelcher LR, McClelland SJ, Woodley SK (2021) Effects of early ethanol exposure on Lithobates pipiens tadpole development. J Young Investig 39:38–44
Erickson K, Drevets W, Schulkin J (2003) Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev 27:233–246
Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J, Denver RJ (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Fraker ME, Ludsin SA, Luttbeg B, Denver RJ (2021) Stress hormone-mediated antipredator morphology improves escape performance in amphibian tadpoles. Sci Rep 11(1):1–9
Gabor CR, Fisher MC, Bosch J (2013) A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLoS ONE 8:e56054
Gabor CR, Davis DR, Kim DS, Zabierek KC, Bendik NF (2018) Urbanization is associated with elevated corticosterone in jollyville plateau salamanders. Ecol Ind 85:229–235
Gabor CR, Perkins HR, Heitmann AT, Forsburg ZR, Aspbury AS (2019) Roundup™ with corticosterone functions as an infodisruptor to antipredator response in tadpoles. Front Ecol Evol. https://doi.org/10.3389/fevo.2019.00114
Gassen J, Prokosch ML, Makhanova A, Eimerbrink MJ, White JD, Proffitt Leyva RP, Peterman JL, Nicolas SC, Reynolds TA, Maner JK, McNulty JK (2018) Behavioral immune system activity predicts downregulation of chronic basal inflammation. PLoS ONE 13:e0203961
Gibson AK, Amoroso CR (2022) Evolution and ecology of parasite avoidance. Annu Rev Ecol Evol Syst 53:47–67
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hadji-Azimi I, Coosemans V, Canicatti C, Perrenot N (1987) Atlas of adult Xenopus laevis laevis hematology. Dev Comp Immunol 11:807–874
Hale R, Piggott JJ, Swearer SE (2016) Describing and understanding behavioral responses to multiple stressors and multiple stimuli. Ecol Evol 7:38–47
Hall MD, Bento G, Ebert D (2017) The evolutionary consequences of stepwise infection processes. Trends Ecol Evol 32:612–623
Harris BN, Carr JA (2016) The role of the hypothalamus–pituitary–adrenal/interrenal axis in mediating predator-avoidance trade-offs. Gen Comp Endocrinol 230:110–142
Hart BL (1990) Behavioral adaptations to pathogens and parasites-5 Strategies. Neurosci Biobehav Rev 14:273–294
Hart BL (1997) Behavioural defence. In: Clayton DH, Moore J (eds) Host-parasite evolution: General principles and avian models. Oxford University Press, pp 59–77
Hart BL, Hart LA (2018) How mammals stay healthy in nature: the evolution of behaviours to avoid parasites and pathogens. Philos Trans R Soc Lond B Biol Sci 373:20170205
Hing S, Narayan EJ, Thompson RA, Godfrey SS (2016) The relationship between physiological stress and wildlife disease: consequences for health and conservation. Wildl Res 43:51–60
Holland M, Skelly DK, Kashgarian M, Bolden SR, Harrison LM, Cappello M (2007) Echinostome infection in green frogs (Rana clamitans) is stage and age dependent. Jzool 271:455–462
Hopkins WA, Mendonça MT, Congdon JD (1997) Increased circulating levels of testosterone and corticosterone in southern toads, Bufo terrestris, exposed to coal combustion waste. Gen Comp Endocrinol 108:237–246
Hossie TJ, Ferland-Raymond B, Burness G, Murray DL (2010) Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Écoscience 17:100–108
Hossie T, Landolt K, Murray DL (2017) Determinants and co-expression of anti-predator responses in amphibian tadpoles: a meta-analysis. Oikos. https://doi.org/10.1111/oik.03305
Karvonen A, Seppälä O, Valtonen ET (2004) Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitol 129:159–164
Kernbach ME, Hall RJ, Burkett-Cadena ND, Unnasch TR, Martin LB (2018) Dim light at night: physiological effects and ecological consequences for infectious disease. Integr Comp Biol 58:995–1007
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? PNAS 99:9900–9904
Kiesecker JM, Skelly DK, Beard KH, Preisser E (1999) Behavioral reduction of infection risk. PNAS 96:9165–9168
Kim KS, Han PL (2006) Optimization of chronic stress paradigms using anxiety-and depression-like behavioral parameters. J Neurosci Res 83(3):497–507
Klemme I, Karvonen A (2016) Vertebrate defense against parasites: interactions between avoidance, resistance, and tolerance. Ecol Evol 7:561–571
Klemme I, Hyvärinen P, Karvonen A (2020) Negative associations between parasite avoidance, resistance and tolerance predict host health in salmonid fish populations. Proc R Soc B Biol Sci 287:20200388
Koprivnikar J, Penalva L (2015) Lesser of two evils? Foraging choices in response to threats of predation and parasitism. PLoS ONE 10:e0116569
Koprivnikar J, Urichuk TMY (2017) Time-lagged effect of predators on tadpole behaviour and parasite infection. Biol Lett 13:20170440
Koprivnikar J, Forbes MR, Baker RL (2006) On the efficacy of anti-parasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can J Zool 84:1623–1629
Koprivnikar J, Marcogliese DJ, Rohr JR, Orlofske SA, Raffel TR, Johnson PT (2012a) Macroparasite infections of amphibians: what can they tell us? EcoHealth 9:342–360
Koprivnikar J, Gibson CH, Redfern JC (2012b) Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc R Soc B Biol Sci 279:1544–1550
Koprivnikar J, Redfern JC, Mazier HL (2014) Variation in anti-parasite behaviour and infection among larval amphibian species. Oecologia 174:1179–1185
Koprivnikar J, Hoye BJ, Urichuk TM, Johnson PT (2019) Endocrine and immune responses of larval amphibians to trematode exposure. Parasitol Res 118:275–288
Kulkarni PS, Gramapurohit NP (2017) Effect of corticosterone on larval growth, antipredator behaviour and metamorphosis of Hylarana indica. Gen Comp Endocrinol 251:21–29
LaFonte BE, Johnson PTJ (2013) Experimental infection dynamics: using immunosuppression and in vivo parasite tracking to understand host resistance in an amphibian-trematode system. J Exp Biol 216:3700–3708
Lopes PC (2017) Why are behavioral and immune traits linked? Horm Behav 88:52–59
MacDougall-Shackleton SA, Bonier F, Romero LM, Moore IT (2019) Glucocorticoids and “stress” are not synonymous. Integr Comp Biol 1:obz017
Marcogliese DJ, King KC, Salo HM, Fournier M, Brousseau P, Spear P, Champoux L, McLaughlin JD, Boily M (2009) Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat Toxicol 91:126–134
Martin LB (2009) Stress and immunity in wild vertebrates: timing is everything. Gen Comp Endocrinol 163:70–76
Martin TR, Conn DB (1990) The pathogenicity, localization, and cyst structure of echinostomatid metacercariae (trematoda) infecting the kidneys of the frogs Rana clamitans and Rana pipiens. J Parasitol 76:414–419
Martin LB, Gilliam J, Han P, Lee K, Wikelski M (2005) Corticosterone suppresses cutaneous immune function in temperate but not tropical house sparrows, Passer domesticus. Gen Comp Endocrinol 140:126–135
McCarthy AM (1999) The influence of temperature on the survival and infectivity of the cercariae of Echinoparyphium recurvatum (Digenea: Echinostomatidae). Parasitol 118(4):383–388
McClelland SJ, Woodley SK (2021) Water-borne corticosterone assay is a valid method in some but not all life-history stages in northern leopard frogs. Gen Comp Endocrinol 312:113858
Middlemis Maher J, Werner EE, Denver RJ (2013) Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc R Soc B Biol Sci 280:20123075
Milotic D, Milotic M, Koprivnikar J (2017) Effects of road salt on larval amphibian susceptibility to parasitism through behavior and immunocompetence. Aquat Toxicol 189:42–49
Munns J (2006) Assessing risks to wildlife populations from multiple stressors: overview of the problem and research needs. Ecol Soc 11:23
Myers B, McKlveen JM, Herman JP (2014) Glucocorticoid actions on synapses, circuits, and behavior: Implications for the energetics of stress. Front Neuroendocrinol 35:180–196
Narayan EJ, Forsburg ZR, Davis DR, Gabor CR (2019) Non-invasive methods for measuring and monitoring stress physiology in imperiled amphibians. Front Ecol Evol 7:431
Neuman-Lee LA, Stokes AN, Greenfield S, Hopkins GR, Brodie ED Jr, French SS (2015) The role of corticosterone and toxicity in the antipredator behavior of the rough-skinned Newt (Taricha granulosa). Gen Comp Endocrinol 213:59–64
Oppliger A, Clobert J, Lecomte J, Lorenzon P, Boudjemadi K, John-Alder H (1998) Environmental stress increases the prevalence and intensity of blood parasite infection in the common lizard Lacerta vivipara. Ecol Lett 1:129–138
Parker BJ, Barribeau SM, Laughton AM, de Roode JC, Gerardo NM (2011) Non-immunological defense in an evolutionary framework. Trends Ecol Evol 26:242–248
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Råberg L, Sim D, Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318(5851):812–814
Råberg L, Graham AL, Read AF (2009) Decomposing health: tolerance and resistance to parasites in animals. Philos Trans R Soc Lond B Biol Sci 364(1513):37–49
Raffel TR, Martin LB, Rohr JR (2008) Parasites as predators: unifying natural enemy ecology. Trends Ecol Evol 23:610–618
Rollins-Smith LA (2001) Neuroendocrine-immune system interactions in amphibians: implications for understanding global amphibian declines. Immunol Res 23:273–280
Rollins-Smith LA (2017) Amphibian immunity–stress, disease, and climate change. Dev Comp Immunol 66:111–119
Rollins-Smith LA, Blair PJ (1993) The effects of corticosteroid hormones and thyroid hormones on lymphocyte viability and proliferation during development and metamorphosis of Xenopus laevis. Differentiation 54(3):155–160
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Romero LM, Beattie UK (2022) Common myths of glucocorticoid function in ecology and conservation. J Exp Zool Part A Ecol Integr Physiol 337(1):7–14
Romero LM, Dickens MJ, Cyr NE (2009) The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav 55(3):375–389
Rosen JB, Donley MP, Gray D, West EA, Morgan MA, Schulkin J (2008) Chronic corticosterone administration does not potentiate unconditioned freezing to the predator odor, trimethylthiazoline. Behav Brain Res 194:32–38
RStudio Team (2021) RStudio: integrated development for R. RStudio, Boston, MA. http://www.rstudio.com/
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
Schaller M, Park JH (2011) The behavioral immune system (and why it matters). Curr Dir Psychol Sci 20:99–103
Schell SC (1985) Handbook of trematodes of North America north of Mexico. University Press of Idaho, Caldwell
Schotthoefer AM, Cole RA, Beasley VR (2003) Relationship of tadpole stage to location of echinostome cercariae encystment and the consequences for tadpole survival. J Parasitol 89:475–482
Schreier K, Grindstaff J (2020) Repeatable behavioural and immune defence strategies against infection are not traded off. Anim Behav 162:11–22
Sears BF, Snyder PW, Rohr JR (2013) Infection deflection: hosts control parasite location with behaviour to improve tolerance. Proc R Soc B Biol Sci 280(1762):20130759
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166(4):869–887
Stephenson JF (2019) Parasite-induced plasticity in host social behaviour depends on sex and susceptibility. Biol Lett 15:20190557
Strehl C, Ehlers L, Gaber T, Buttgereit F (2019) Glucocorticoids—all-rounders tackling the versatile players of the immune system. Front Immunol 10:1744
Sullivan AM, Kratzer IE, Jobe SC, Lewis JL (2021) Combined effects of experimentally elevated CORT and predation threat on exploratory and foraging behavior of Desmognathus ochrophaeus. J Herpetol 55:208–214
Szuroczki D, Richardson JML (2009) The role of trematode parasites in larval anuran communities: an aquatic ecologist’s guide to the major players. Oecologia 161:371–385
Szuroczki D, Richardson JM (2012) The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS ONE 7(11):e49592
Taylor CN, Oseen KL, Wassersug RJ (2004) On the behavioural response of Rana and Bufo tadpoles to echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Can J Zool 82:701–706
Thaker M, Lima SL, Hews DK (2009) Acute corticosterone elevation enhances antipredator behaviors in male tree lizard morphs. Horm Behav 56:51–57
Thompson SN, Kavaliers M (1994) Physiological bases for parasite-induced alterations of host behaviour. Parasitol 109:S119–S138
Tornabene BJ, Hossack BR, Crespi EJ, Breuner CW (2021) Evaluating corticosterone as a biomarker for amphibians exposed to increased salinity and ambient corticosterone. Conserv Physiol 9:coab049
Vinterstare J, Hegemann A, Nilsson PA, Hulthén K, Brönmark C (2019) Defence versus defence: are crucian carp trading off immune function against predator-induced morphology? J Anim Ecol 88:1510–1521
Wingfield JC, Sapolsky R (2003) Reproduction and resistance to stress: when and how. Jneuroendocrinol 15:711–724
Wingfield J, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD (1998) Ecological bases of hormone–behavior interactions: the “emergency life history stage.” Am Zool 38:191–206
Woodley SK, Lacy EL (2010) An acute stressor alters steroid hormone levels and activity but not sexual behavior in male and female ocoee salamanders (Desmognathus ocoee). Horm Behav 58:427–432
Zylberberg M, Klasing KC, Hahn TP (2013) House finches (Carpodacus mexicanus) balance investment in behavioural and immunological defences against pathogens. Biol Lett 9:20120856
Zylberberg M, Klasing KC, Hahn TP (2014) In house finches, haemorhous mexicanus, risk takers invest more in innate immune function. Anim Behav 89:115–122
Acknowledgements
The authors wish to thank the following for their contribution to lab work and project data discussions: Lucia Santos, Brad Jacobs and Tsukushi Kamiya.
Funding
This study was funded by a NSERC Discovery Grant to J.K.
Author information
Authors and Affiliations
Contributions
JK and KoD conceived and designed the experiments. KoD performed the experiments. KoD, DM and MM collected the data. KoD analysed the data. KoD and JK wrote the manuscript; other authors provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional and/or national guidelines for the care and use of animals were followed. Animals were used in accordance with the Canadian Council on Animal Care guidelines, and with institutional approval from the animal care committee (AUP 633).
Additional information
Communicated by Bryan Brown.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Dwyer, K., Milotic, D., Milotic, M. et al. Behave yourself: effects of exogenous-glucocorticoid exposure on larval amphibian anti-parasite behaviour and physiology. Oecologia 205, 95–106 (2024). https://doi.org/10.1007/s00442-024-05547-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-024-05547-6