Abstract
A host’s first line of defense in response to the threat of parasitic infection is behavior, yet the efficacy of anti-parasite behaviors in reducing infection are rarely quantified relative to immunological defense mechanisms. Larval amphibians developing in aquatic habitats are at risk of infection from a diverse assemblage of pathogens, some of which cause substantial morbidity and mortality, suggesting that behavioral avoidance and resistance could be significant defensive strategies. To quantify the importance of anti-parasite behaviors in reducing infection, we exposed larval Pacific chorus frogs (Pseudacris regilla) to pathogenic trematodes (Ribeiroia and Echinostoma) in one of two experimental conditions: behaviorally active (unmanipulated) or behaviorally impaired (anesthetized). By quantifying both the number of successful and unsuccessful parasites, we show that host behavior reduces infection prevalence and intensity for both parasites. Anesthetized hosts were 20–39% more likely to become infected and, when infected, supported 2.8-fold more parasitic cysts. Echinostoma had a 60% lower infection success relative to the more deadly Ribeiroia and was also more vulnerable to behaviorally mediated reductions in transmission. For Ribeiroia, increases in host mass enhanced infection success, consistent with epidemiological theory, but this relationship was eroded among active hosts. Our results underscore the importance of host behavior in mitigating disease risk and suggest that, in some systems, anti-parasite behaviors can be as or more effective than immune-mediated defenses in reducing infection. Considering the severe pathologies induced by these and other pathogens of amphibians, we emphasize the value of a broader understanding of anti-parasite behaviors and how co-occurring stressors affect them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growing interest has recently focused on the parallel threats of predators and pathogens as overlapping classes of ‘natural enemies’ (Hall et al. 2008; Raffel et al. 2008). Recognizing their inherent similarities, the emerging study of ‘enemy ecology’ seeks to unite the historically disparate fields of host–parasite ecology and predator–prey ecology to explore how they can mutually inform one another. Despite differences in enemy size, relationship intimacy, and resource consumption, both predators and parasites represent significant threats to many organisms and can often be functionally equivalent (Lafferty and Kuris 2002). Both groups have direct lethal effects and can induce defensive responses in the prey or host (Werner and Peacor 2003; Raffel et al. 2008). Parasites are also increasingly being incorporated into studies of food webs, community structure, and ecosystem energetics (Wood et al. 2007; Kuris et al. 2008; Lafferty et al. 2008). Nevertheless, research in host–parasite ecology has historically lagged behind that of predator–prey ecology in exploring the diversity of ecological responses to enemy threat (Hart 1990). For example, while extensive research has examined how the threat of predation affects prey activity, body size, phenotype, palatability, migratory behavior, and resource allocation, most research on defenses against parasitism have focused on host immunity (Hart 1990; Relyea 2001a, b; Lass and Spaak 2003; Ezenwa 2004; Råberg et al. 2009).
Alongside host immunity, however, and in direct parallel with anti-predator behaviors, hosts also employ behavioral defenses against the threat of infection (e.g., Moore 2002). Some animals exhibit anti-parasite defensive behaviors such as grooming, tail flipping, migration, and selective foraging to help avoid parasites or reduce infection (Hart 1992, 1994; Mooring et al. 2004), which are qualitatively similar to the behavioral changes of prey in response to predation threats (e.g., Werner and Peacor 2003). For a behavior to be considered a viable anti-parasite defense, it must reduce or eliminate infection by a parasite that negatively influences host fitness (see Hart 1992). For example, rainbow trout (Oncorhynchus mykiss) actively avoid free-swimming larval trematodes that cause cataracts, helping to decrease the rate of infection and severity of pathology (Karvonen et al. 2004). Although behavioral responses are considered the ‘first line of defense’ against infection (Hart 1994), the significance of host behavior in reducing or mitigating the effects of parasite infection remains largely unquantified relative to immunological defenses (Hart 1990; Kiesecker et al. 1999; Ezenwa 2004; Råberg et al. 2009).
Anti-parasite behaviors may be particularly important for hosts confronted by free-living infectious stages in the external environment, which are used by a variety of trematodes, cestodes, nematodes, fungi, and protists. In freshwater environments, larval amphibians are attacked by a diverse assemblage of free-living parasitic stages, including several groups that cause severe pathology (Sutherland 2005; Skerrat et al. 2007; Szuroczki and Richardson 2009). Ribeiroia and Echinostoma are ecologically similar groups of trematodes that can induce mortality and morbidity in amphibians (Johnson and McKenzie 2008; Szuroczki and Richardson 2009; Rohr et al. 2010). In tadpoles, Echinostoma infection can reduce survival, inhibit renal function, and cause edema (Fried et al. 1997; Schotthoefer et al. 2003; Holland et al. 2007). Infection by Ribeiroia ondatrae (hereafter Ribeiroia) reduces survival and induces severe malformations, including missing limbs, skin webbings, and extra limb elements (Sessions and Ruth 1990; Johnson et al. 1999, 2001, 2006; Kiesecker 2002; Stopper et al. 2002; Johnson and Hartson 2009). Because the severity of pathology depends on the number of parasites a host supports (“intensity-dependent pathology”; Johnson et al. 1999, 2001; Schotthoefer et al. 2003; Holland et al. 2007), behaviors that reduce infection may be important mechanisms to reduce disease (e.g., Kiesecker and Skelly 2000; Taylor et al. 2004; Koprivnikar et al. 2006). One important advantage of working with macroparasites, which do not reproduce directly within a host, is that the fate of each administered parasite can be quantified, helping to provide direct estimates of the effectiveness of anti-parasite behavior in reducing infection.
To explicitly quantify the importance of behavior in reducing parasite infection success, we experimentally inhibited the anti-parasite behavior of larval amphibians before confronting them with one of two pathogenic trematodes (Ribeiroia and Echinostoma), which vary in size, mode of entry, and pathology. In response to the free-swimming infectious stages (cercariae) of these trematodes, tadpoles exhibit bursts of activity, typically involving periods of fast swimming, rapid twisting, and aggressive turning that differ markedly from unexposed tadpoles (Daly, unpublished data); these behaviors can successfully dislodge parasites before they have a chance to enter the host’s body (Taylor et al. 2004). Using a vertebrate-specific anesthetic, we impaired the short-term avoidance behaviors of Pacific chorus frog (Pseudacris regilla) larvae and assessed the effects of behavior on each parasite’s transmission. Because we sought to disentangle the effects of host immunity and host behavior in influencing infection, we independently quantified both the number of parasites successful in finding a host as well as the number of unsuccessful parasites. The use of two metrics of transmission success enhanced the reliability of our findings and helped identify the mechanisms underlying interactions among infection, host behavior, and parasite species. In light of ongoing declines and deformities in amphibians associated with pathogenic infections (e.g., Collins and Crump 2009), our study has implications for understanding the fundamental processes of host defenses and for informing conservation.
Materials and methods
To quantify the contribution of host behavior in mediating parasite transmission, we administered trematode cercariae of Ribeiroia or Echinostoma to larval amphibians (Pseudacris regilla) that were either (1) capable of anti-parasite behavior, or (2) anesthetized to eliminate behavioral responses. We collected Pacific chorus frog (Pseudacris regilla) egg masses from the Eel River, California, and maintained hatching larvae on a 1:1 diet of crushed TetraMin and TetraVeggie (Tetra, Blacksburg, VA) fish food administered ad libitum. Upon reaching Gosner (1960) stages 31–33, tadpoles were randomly assigned to one of the following conditions: anesthetized + Ribeiroia (n = 31), unanesthetized + Ribeiroia (n = 31), anesthetized + Echinostoma (n = 20), and unanesthetized + Echinostoma (n = 20). We anesthetized tadpoles in a 0.125% solution of neutral-buffered MS-222 (tricaine methanesulfonate) for 10 min before transferring them to a plastic container (15.5 × 14.5 × 8 cm) with 0.5 L of clean water. Pilot experiments established that this volume of water allowed tadpoles to exhibit a full range of anti-parasite behaviors and that the designated anesthetic exposure was effective at suppressing tadpole behavior for 30 min without adverse effects (i.e., all tadpoles recovered and resumed normal behavior within 45 min) (see also Koprivnikar et al. 2006). As a vertebrate neurotoxin, MS-222 is unlikely to influence trematode cercariae, and we validated this assumption through observation. Larvae in the unanesthetized conditions were treated identically, with the exception that no MS-222 was placed in the solution.
We harvested cercariae from infected Helisoma spp. snails collected at field sites in California by placing individual snails in 325 mL of water and allowing them to release trematode cercariae, which we identified as Ribeiroia or Echinostoma based on standard characteristics (see Johnson and McKenzie 2008; Detwiler and Minchella 2009; Szuroczki and Richardson 2009). Within 4 h of release, cercariae were isolated, counted, and transferred into plastic containers with 0.5 L of water. We added individual tadpoles (anesthetized or unanesthetized) into containers with 25 cercariae of either parasite. After 20 min, we transferred hosts into separate containers filled with 1 L of water and allowed 48 h for cercarial encystment before euthanizing, measuring [mass and snout-vent-length (SVL)], and preserving each host. This short time frame was chosen to minimize parasite loss due to acquired immunity (Johnson and Hartson 2009).
For each exposed host, we quantified both the number of parasites that encysted successfully as well as the number that failed to infect the tadpole. This provided us with two independent measurements of parasite infection success and allowed us to simultaneously evaluate the effects of host behavior and innate immunity on our results. Immediately following tadpole removal, we filtered the remaining water through a glass fiber filter (pore size 0.7 μm, diameter 47 mm) using a vacuum pump to quantify any leftover cercariae. We stirred the water continuously during filtration and rinsed the filter manifold three times with deionized water to ensure all cercariae were recovered. We stained filters with a 0.01% Light Green (Fisher Scientific, Pittsburgh, PA) solution in 2% acetic acid to enhance the visibility of remaining cercariae. Following Prentice (1984), we examined filters within 24 h under a stereodissecting microscope and quantified remaining parasites. Concurrently, we necropsied preserved tadpoles to assess infection prevalence (percentage of infected hosts) and infection abundance (number of encysted parasites or metacercariae per host) (Bush et al. 1997). Necropsy methods followed Johnson and Hartson (2009), focusing on epithelial tissue around the limbs, base of the tail, and mandibles to locate Ribeiroia metacercariae, and in the kidneys and gill resorption areas to locate echinostome metacercariae.
We performed the statistical analyses using JMP Statistical Discovery software ver. 7.0.1® (SAS Institute, Cary, NC). To evaluate the effect of anti-parasite behavior on infection prevalence for each parasite, we used χ2 contingency analyses. We evaluated variation in the number of successful (i.e., encysted metacercariae) or unsuccessful cercariae using generalized linear models with a Poisson distribution and a log-link function. We also used generalized linear models to examine differences in the total number of recovered parasites (both encysted and unsuccessful) between anesthetized and unanesthetized treatments. Host mass was included as a covariate in the analyses of parasite counts because of its expected influence on host–parasite encounter likelihood. Finally, we used χ2 contingency analysis to compare the relative success of the two trematode species.
Results
Host behavior and infection success
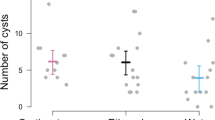
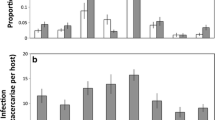
Behaviorally impaired (anesthetized) hosts exhibited higher infection prevalence and greater infection intensities relative to unanesthetized hosts. In experiments with Ribeiroia, 100% of anesthetized tadpoles became infected compared to 80.7% of unanesthetized individuals (χ2 = 6.643, df = 1, P = 0.01; Fig. 1). For Echinostoma, 90% of anesthetized and 55% of unanesthetized hosts supported metacercariae (χ2 = 6.144, df = 1, P = 0.013; Fig. 1). Similarly, anesthetized tadpoles exhibited significantly higher Ribeiroia infection abundances relative to unanesthetized hosts (generalized linear model with Poisson distribution; anesthetic χ2 = 99.58, P < 0.0001; Fig. 2a). On average, behaviorally impaired hosts had 2.8-fold more metacercariae than active individuals (9.2 vs. 3.3 cysts in the anesthetized and unanesthetized treatments, respectively) (Fig. 2a). Treatments involving exposure to Echinostoma yielded highly similar results, also with 2.8-fold greater encystment in anesthetized hosts (Anesthetic χ2 = 24.99, P < 0.0001; anesthetized mean 3.6 cysts; unanesthetized mean 1.3 cysts; Fig. 2a). Host mass was a significant and positive covariate in predicting Ribeiroia infection abundance among anesthetized hosts (χ2 = 44.17, P < 0.0001) but not among active hosts (χ2 = 1.11, P = 0.29) (Fig. 3). Mass was not a significant predictor of Echinostoma infection, regardless of behavior treatment (χ2 = 1.96, P = 0.16).
Ribeiroia and Echinostoma infection prevalence in Pacific chorus frog (Pseudacrisregilla) larvae as a function of behavior treatment (anesthetized vs. active). Different lowercase letters indicate significant differences among groups (P < 0.05). Data are presented as the percentage of individuals in each treatment with at least one metacercariae of a given parasite
Number [mean +1 standard error (SE)] of successful Ribeiroia and Echinostoma recovered as metacercariae (a) and unsuccessful Ribeiroia and Echinostoma cercariae recovered (b) following parasite exposures with anesthetized (white bars) and active (black bars) larval Pacific chorus frogs (Pseudacris regilla). Note differences in Y-axis scale
Correspondingly, we recovered significantly fewer unsuccessful cercariae (i.e., leftover) for both parasites from water that had contained anesthetized tadpoles (Ribeiroia: anesthetic χ2 = 14.26, P = 0.0002, n = 62; Echinostoma: anesthetic χ2 = 13.99, P = 0.0002, n = 40; Fig. 2b). Again, host mass was a significant covariate only for trials with Ribeiroia (Ribeiroia: χ2 = 20.89, P < 0.0001; Echinostoma: χ2 = 1.61, P = 0.21). There were no differences in host size (SVL) or mass between treatments for experiments with Ribeiroia (Mass t ratio −0.39, P = 0.69; SVL t ratio 1.18, P = 0.24, n = 62) or those with Echinostoma (Mass t ratio −1.26, P = 0.22; SVL t ratio −1.70, P = 0.010, n = 40).
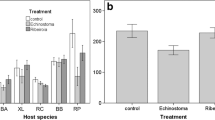
Combining the data for recovered metacercariae (successful parasites) and unsuccessful cercariae, our overall recovery rate of administered parasites was high for both Ribeiroia (89.6%) and Echinostoma (84.4%) (Fig. 4). To evaluate whether active hosts might be consuming cercariae and thereby reducing their total infection, we examined differences in total cercariae recovery (metacercariae and unsuccessful cercariae) between anesthetized and unanesthetized tadpoles (Fig. 3a). If tadpoles consumed cercariae, more total parasites should be recovered from anesthetized treatments than from unanesthetized treatments. In treatments with Ribeiroia, we recovered more (93.7%) total parasites from anesthetized hosts than from active hosts (t test, t = 4.47, P < 0.0001, n = 62; Fig. 4), consistent with the hypothesis that active tadpoles consume cercariae. Conversely, however, in experiments involving Echinostoma, we recovered more parasites (89.4%) from active tadpoles (t test, t = −3.05, P = 0.0041, n = 40; Fig. 4).
Total number of parasites recovered (mean +1 SE) for Ribeiroia and Echinostoma as a function of host behavior treatment (anesthetized vs. active). Total number of parasites is the sum of successful parasites (recovered as metacercariae in necropsied hosts) and unsuccessful parasites (recovered as failed cercariae). Initial exposures involved 25 cercariae of either parasite
Differences between parasites in response to host behavior
Among active hosts, Ribeiroia had a significantly higher infection prevalence than Echinostoma (80.7 vs. 55.5%, χ2 = 3.851, P = 0.049, df = 1), whereas we found no differences in infection prevalence between anesthetized hosts (100 vs. 90%, χ2 = 3.227, P = 0.725, df = 1). On average, hosts exposed to Ribeiroia exhibited 2.5 × more metacercariae than those exposed to Echinostoma, regardless of behavior treatment (Unanesthetized treatment: parasite χ2 = 21.57, P < 0.0001, n = 51; Anesthetized treatment: parasite χ2 = 58.301, P < 0.0001, n = 51; Fig. 2a). There was no significant difference in the number of unsuccessful Ribeiroia and Echinostoma cercariae for anesthetized tadpoles (parasite χ2 = 2.923, P = 0.087). However, for active hosts, we recovered a greater number of unsuccessful Echinostoma cercariae compared with Ribeiroia (parasite χ2 = 5.96, P = 0.015, n = 51; Fig. 2b).
Discussion
Our results highlight the importance of host behavior in mediating parasite infection success. Although epidemiological research concerned with host defenses has traditionally emphasized immunity, behavioral avoidance and resistance represent significant defense mechanisms against infection (Hart 1990, 1994; Barber et al. 2000; Moore 2002). Examples of behavioral defenses include grooming, habitat selection, selective foraging, predation, and local- or large-scale migrations, but the efficacy of such responses in reducing infections often goes unquantified. By experimentally manipulating the behavior of larval amphibians, we have demonstrated the effectiveness of anti-parasite behavior in reducing infections by two pathogenic trematodes. In response to identical levels of exposure, active hosts were 20–39% less likely than anesthetized hosts to become infected. Tadpoles exposed to cercariae exhibited intermittent periods of rapid swimming in conjunction with multiple turns and spins, consistent with those behaviors described by Taylor et al. (2004). Among infected individuals, active hosts also exhibited 64% fewer parasites than individuals with impaired behavioral responses. For Ribeiroia, host mass was a positive predictor of infection success, consistent with epidemiological theory (e.g., Anderson and May 1982), but this relationship weakened considerably for active hosts (Fig. 3), highlighting the mediating influence of host behavior. Considering that trematode infection success is often stage dependent and may decrease with tadpole growth (Schotthoefer et al. 2003; Holland et al. 2007), the relative importance of behavioral resistance may vary throughout the development of larval amphibians, possibly with particular importance for younger, more vulnerable individuals.
Overall confidence in these results is bolstered by the use of two independent methods to assess infection success; we quantified both the number of successful metacercariae recovered within host tissues and the number of unsuccessful parasites left at the end of each exposure trial, each of which provided complementary findings. If we assume that all unrecovered parasites (i.e., the difference between the sum of both metrics and the total parasites administered) were lost due to innate immunity, which is likely an overestimate of immune-mediated defenses, our results suggest that anti-parasite behavior was approximately 25% more effective in reducing infection relative to innate immunity, although the short-time scale of our experiment precluded a comparison that also incorporated acquired immunity.
These results are broadly consistent with those obtained in earlier studies focused on anti-parasite behaviors in amphibians. For example, Koprivnikar et al. (2006) found that Echinostoma infection prevalence (but not infection intensity) increased when tadpoles were maintained at cold temperatures or anesthetized. Similarly, based on an observational study, Taylor et al. (2004) suggested that short periods of erratic movement by tadpoles functioned to reduce trematode encystment success (but see Thiemann and Wassersug 2000). How and in response to what stimuli amphibians initiate a behavioral response remains conjectural, however. Taylor et al. (2004) suggested that physical contact with cercariae is needed to induce a response, whereas Rohr et al. (2009) reported that larval amphibians show behavioral avoidance in response to cercarial chemical cues, indicating that physical contact may not be required. The latter researchers suggested that elevated activity occurs in response to chemical or vibrational cues released by cercariae. Thus, while the majority of evidence supports the hypothesis that increased tadpole activity functions effectively as an anti-parasite defense mechanism (Taylor et al. 2004; Koprivnikar et al. 2006; Rohr et al. 2009), more research is needed to understand whether this acts primarily to prevent initial parasite attachment, to dislodge parasites already in contact with the host, or both.
Despite the generally consistent effects of host behavior in reducing infection by both Ribeiroia and Echinostoma, our results revealed intriguing differences in the transmission patterns of the two parasites. Regardless of experimental treatment, Echinostoma consistently exhibited lower infection intensities in amphibian hosts. On average, hosts exposed to Echinostoma had 40% fewer metacercariae than those exposed to Ribeiroia. Based on our comparisons of successful and failed parasites across treatments and between parasites, we suggest that two mechanisms contribute to this pattern. First, the anti-parasite behavior of tadpoles was apparently more effective in reducing Echinostoma contact with hosts relative to Ribeiroia. In support of this hypothesis, we recovered significantly more unsuccessful Echinostoma cercariae within active tadpole treatments than we did Ribeiroia cercariae. This may suggest the two parasites, while ecologically similar, use different mechanisms or behaviors to find and attach to hosts, a notion supported by the differing influence of host mass on the transmission success of each parasite. In addition, because Ribeiroia cercariae are larger than those of Echinostoma, they may be more active or more persistent swimmers, perhaps contributing to their relatively high infection success. Second, Echinostoma parasites experienced lower encystment success than Ribeiroia even following host entry. Correspondingly, we found fewer Echinostoma metacercariae in anesthetized hosts relative to Ribeiroia, even though there was no significant difference in the number of unsuccessful cercariae between the two parasites. This suggests that a greater fraction of administered Echinostoma failed to migrate to the host kidneys and establish successfully as metacercariae, although we cannot eliminate the possibility that systematic differences in our ability to recover the two parasites contributed to this pattern.
These differences may also explain the apparently contradictory findings involving total parasite recovery (successful and unsuccessful) between the two parasites as a function of treatment. We hypothesized that, in addition to swimming behavior, active tadpoles might be more likely to consume cercariae either actively or inadvertently while respiring, providing another mechanism whereby behavior could reduce infection (assuming cercariae were killed in the process). Correspondingly, we recovered fewer total Ribeiroia (successful + unsuccessful) in the active host treatments than in the anesthetized treatments. In the Echinostoma study, however, we noted the opposite pattern, with more parasites recovered in the anesthetized treatments, but given that this treatment involved a greater fraction of administered cercariae finding the tadpole host, after which they had to migrate successfully to the kidneys, this finding could simply result from proportionally greater losses of Echinostoma cercariae during the encystment process. More work is clearly needed to explicitly quantify the potential roles of host consumption and intra-host mortality in driving these patterns.
Variation in parasite success as a function of species and host behavior is likely due to differences in parasite mode of entry and migration through host tissue. The larger Ribeiroia cercariae generally attach to tadpole skin and actively migrate to the area where the host tail connects to the body before burrowing into the skin using chemical proteases (Szuroczki and Richardson 2009; Rohr et al. 2010). This process often causes substantial pathology, including mortality and limb malformations, but it also entails a relatively short migratory path. In contrast, following attachment, Echinostoma cercariae crawl along a host’s skin to the cloaca, which they must enter and follow to the kidneys before encysting as metacercariae (Schotthoefer et al. 2003; Rohr et al. 2010). The highly specific point of required entry (cloaca) could incur a longer period of opportunity for tadpoles to dislodge cercariae, while the prolonged intra-host migration (without a protective cyst wall) may increase the risk the parasite exhausts its limited energy reserves or is eliminated via host immunity.
If anti-parasite behaviors are important determinants of infection susceptibility, any factor that reduces these responses would function to increase infection success in natural environments. Environmental stressors often affect rates of parasite infection (Kiesecker 2002; Lafferty and Holt 2003; Johnson et al. 2007; Koprivnikar et al. 2007). Some stressors, such as chemical contamination and predatory threat, reduce larval amphibian activity (Bridges 1999; Relyea 2001a, b; Relyea and Mills 2001; Rohr et al. 2009), potentially increasing the risk of infection. For example, tadpoles exposed to environmentally relevant concentrations of nitrate or nitrite swim less vigorously and exhibit paralysis (Marco et al. 1999). Concurrently, pesticide exposure can immunosuppress hosts and lead to increased infection (Taylor et al. 1999; Kiesecker 2002; Rohr et al. 2008a, b). It follows that in wetlands with significant contaminant runoff, chemically altered behavioral responses may act in concert with impaired immune function to weaken hosts’ major defenses against infection and disease. Increased infection by Ribeiroia and Echinostoma enhances the risk of detrimental disease effects, including edema, renal failure, limb malformations, and mortality (Holland et al. 2007; Johnson and McKenzie 2008). Stressors that reduce behavior and impair immunity therefore have the potential to negatively affect host resistance and increase disease risk in amphibians, which have become one of the most threatened classes of vertebrates worldwide (Wake and Vredenburg 2008; Collins and Crump 2009).
There is growing appreciation in ecology of the similarities (and differences) between parasitism and predation as analogous ‘natural enemies’ (e.g., Holt and Dobson 2006; Hall et al. 2008; Raffel et al. 2008). For instance, alongside the anti-parasite behaviors discussed here, amphibian larvae exhibit anti-predator responses involving a reduction in activity in the presence of predators or their chemical cues (e.g., Relyea 2001a, b), helping to reduce prey conspicuousness and predation risk (Skelly 1994). When both parasites and predators are present, there exists a trade-off between anti-predator and anti-parasite behavioral defenses (Baker and Smith 1997; Decaestecker et al. 2002), particularly if defenses against one enemy increase the risk of the other. In this instance, hosts/prey will be forced between the proverbial rock and a hard place (Baker and Smith 1997). Decaestecker et al. (2002) found that, by migrating vertically to reduce predation risk from fishes, Daphnia increased their exposure to pathogens associated with lake sediment. Similarly, tadpoles exposed to fish predators reduced their activity, thereby exposing them to increased infections by Echinostoma cercariae (Thiemann and Wassersug 2000). Considering that the threat of predation can have community-structuring effects that are as important as predation itself (Werner and Peacor 2003; Preisser et al. 2005), more research is needed to identify and understand the direct and indirect ecological effects of parasitism, particularly in combination with predation as is common in natural environments. A broader understanding of the context-dependent interactions between anti-parasite responses and other stressors will also provide important information for management and conservation strategies.
References
Anderson RM, May RM (1982) The population biology of infectious disease. Springer, Berlin
Baker RL, Smith BP (1997) Conflict between antipredator and anti-parasite behaviour in larval damselflies. Oecologia 109:622–628
Barber I, Hoare D, Krause J (2000) Effects of parasites on fish behaviour: a review and evolutionary perspective. Rev Fish Biol Fish 10:131–165
Bridges CM (1999) Effects of a pesticide on tadpole activity and predator avoidance behavior. J Herpetol 33:303–306
Bush AK, Lafferty D, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83: 575–583
Collins JP, Crump ML (2009) Extinction in our times: global amphibian decline. Oxford University Press, Oxford
Decaestecker E, De Meester L, Ebert D (2002) In deep trouble: habitat selection constrained by multiple enemies in zooplankton. Proc Natl Acad Sci USA 99:5481–5485
Detwiler JT, Minchella DJ (2009) Intermediate host availability masks the strength of experimentally-derived colonization patterns in echinostome trematodes. Int J Parasitol 39:585–590
Ezenwa VO (2004) Selective defecation and selective foraging: antiparasite behavior in wild ungulates? Ethology 110:851–862
Fried B, Pane PL, Reddy A (1997) Experimental infection of Rana pipiens tadpoles with Echinostoma trivolvis cercariae. Parasitol Res 83:666–669
Gosner N (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hall SR, Lafferty KD, Brown JM, Caceres CE, Chase JM, Dobson AP, Holt RD, Jones CG, Randolph SE, Rohani P (2008) Is infectious disease just another type of predator-prey interaction? In: Ostfeld RS, Keesing F, Eviner VT (eds) Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems. Princeton University Press, New Jersey
Hart BL (1990) Behavioral adaptations to pathogens and parasites: 5 strategies. Neurosci Biobehav Rev 14:273–294
Hart BL (1992) Behavioral adaptations to parasitism: an ethological approach. J Parasitol 78:256–265
Hart BL (1994) Behavioral defense against parasites: interaction with parasite invasiveness. Parasitology 109:S139–S151
Holland MP, Skelly DK, Kashgarin M, Bolden SR, Harrison LM, Cappello M (2007) Echinostome infection in green frogs (Rana clamitans) is stage and age dependent. J Zool 271:455–462
Holt RD, Dobson AP (2006) Extending the principles of community ecology to address the epidemiology of host-pathogen systems. In: Collinge SK, Ray C (eds) Disease ecology: community structure and pathogen dynamics. Oxford University Press, Oxford
Johnson PTJ, Hartson RB (2009) All hosts are not equal: explaining differential patterns of malformations in an amphibian community. J Anim Ecol 78:191–201
Johnson PTJ, McKenzie MJ (2008) Effects of environmental change on helminth infections in amphibians: exploring the emergence of Ribeiroia and Echinostoma infections in North America. In: Fried B, Toledo R (eds) The biology of echinostomes. Springer, New York, pp 249–280
Johnson PTJ, Lunde KB, Ritchie EG, Launer AE (1999) The effect of trematode infection on amphibian limb development and survivorship. Science 284:802–804
Johnson PTJ, Lunde KB, Haight RW, Bowerman J, Blaustein AR (2001) Ribeiroia ondatrae (Trematoda: digenea) infection induces severe limb malformations in western toads (Bufo boreas). Can J Zool 79:370–379
Johnson PTJ, Preu ER, Sutherland DR, Romansic J, Han B, Blaustein AR (2006) Adding infection to injury: synergistic effects of predation and parasitism on salamander limb malformations. Ecology 87:2227–2235
Johnson PTJ, Chase JM, Dosch KL, Gross J, Hartson RB, Larson D, Sutherland DR, Carpenter SR (2007) Aquatic eutrophication promotes pathogenic infection in amphibians. Proc Natl Acad Sci USA 104:15781–15786
Karvonen A, Seppala O, Valtonen ET (2004) Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitology 129:159–164
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian deformities in nature? Proc Natl Acad Sci USA 99:9900–9904
Kiesecker JM, Skelly DK (2000) The choice of oviposition site by gray treefrogs: the role of potential parasitic infection. Ecology 81:2939–2943
Kiesecker JM, Skelly DK, Beard KH, Preisser E (1999) Behavioral reduction of infection risk. Proc Natl Acad Sci USA 96:9165–9168
Koprivnikar J, Forbes MR, Baker RL (2006) On the efficacy of anti-parasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can J Zool 84:1623–1629
Koprivnikar J, Forbes MR, Baker RL (2007) Contaminant effects on host parasite interactions: atrazine, frogs, and trematodes. Environ Toxicol Chem 26:2166–2170
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518
Lafferty KD, Holt RD (2003) How should environmental stress affect the population dynamics of disease? Ecol Lett 6:654–664
Lafferty KD, Kuris AM (2002) Trophic strategies, animal diversity and body size. Trends Ecol Evol 17:507–513
Lafferty KD, Allesina S, Arim M, Cherie J, et al (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546
Lass S, Spaak P (2003) Chemically induced anti-predator defences in plankton: a review. Hydrobiologia 491:221–239
Marco A, Quilchano C, Blaustein AR (1999) Sensitivity to nitrate and nitrite in pond breeding amphibians from the Pacific Northwest, USA. Environ Toxicol Chem 18:2836–2839
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, New York
Mooring MS, Blumstein DT, Stoner CJ (2004) The evolution of parasite-defence grooming in ungulates. Biol J Linn Soc 81:17–37
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86:501–509
Prentice MA (1984) A field-evolved differential filtration method for recovery of schistosome cercariae. Ann Trop Med Parasitol 78:117–127
Råberg L, Graham AL, Read AF (2009) Decomposing health: tolerance and resistance to parasites in animals. Phil Trans Roy Soc B 364:37–49
Raffel TR, Martin LB, Rohr JR (2008) Parasites as predators: unifying natural enemy ecology. Trends Ecol Evol 23:610–618
Relyea RA (2001a) Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82:523–540
Relyea RA (2001b) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82:541–554
Relyea RA, Mills N (2001) Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles (Hyla versicolor). Proc Natl Acad Sci USA 98:2491–2496
Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR (2008a) Agrochemicals increase trematode infections in a declining amphibian species. Nature 455:1235–1239
Rohr JR, Raffel TR, Sessions SK, Hudson PJ (2008b) Understanding the net effects of pesticides on amphibian trematode infections. Ecol Appl 18:1743–1753
Rohr JR, Swan A, Raffel TR, Hudson PJ (2009) Parasites, info-disruption, and the ecology of fear. Oecologia 159:447–454
Rohr JR, Raffel TR, Sessions SK (2010) Digenetic trematodes and their relationship to amphibian declines and deformities. In: H Heatwole (ed) Amphibian biology, vol 8: amphibian decline: diseases, parasites, maladies, and pollution. J.W. Surrey Beatty & Sons, Chipping Norton
Schotthoefer AM, Cole RA, Beasley VR (2003) Relationship of tadpole stage to location of echinostome cercariae encystment and the consequences for tadpole survival. J Parasitol 89:475–482
Sessions SK, Ruth SB (1990) Explanation for naturally occurring supernumerary limbs in amphibians. J Exp Zool 254:38–47
Skelly DK (1994) Activity level and the susceptibility of anuran larvae to predation. Anim Behav 47:465–468
Skerrat LF, Berger L, Speare R (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Stopper GF, Hecker L, Franssen RA, Sessions SK (2002) How trematodes cause limb deformities in amphibians. J Exp Zool 294:252–263
Sutherland DR (2005) Parasites of North American Frogs. In: Lannoo MJ (ed) Amphibian declines: the conservation status of United States species. University of California Press, Berkeley, pp 109–123
Szuroczki D, Richardson JML (2009) The role of trematode parasites in larval anuran communities: an aquatic ecologist’s guide. Oecologia 161:371–385
Taylor SK, Williams ES, Mills KW (1999) Effects of malathion on disease susceptibility in Woodhouse’s toads. J Wildlife Dis 35:536–541
Taylor CN, Oseen KL, Wassersug RJ (2004) On the behavioural response of Rana and Bufo tadpoles to Echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Can J Zool 82:701–706
Thiemann GW, Wassersug RJ (2000) Patterns and consequences of behavioural responses to predators and parasites in Rana tadpoles. Biol J Linn Soc 71:513–528
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA105:11466–11473
Werner EE, Peacor SC (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100
Wood CL, Byers JE, Cottingham KL, Altman I, Donahue MJ, Blakeslee AMH (2007) Parasites alter community structure. Proc Natl Acad Sci USA 104:9335–9339
Acknowledgments
We thank D. Miller, S. Collinge, S. Paull, S. Orlofske, K. Dosch, and R. Jadin for their guidance and assistance in this project. S. Kupferberg and the Angelo Reserve generously provided experimental materials. EWD gratefully acknowledges funding support from the University of Colorado Undergraduate Research Opportunities Program. This project was supported by a grant from the National Science Foundation (DEB-0553768) and a fellowship from the David and Lucile Packard Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Carla Caceres.
Rights and permissions
About this article
Cite this article
Daly, E.W., Johnson, P.T.J. Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165, 1043–1050 (2011). https://doi.org/10.1007/s00442-010-1778-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-010-1778-y