Abstract
The prey naiveté hypothesis suggests that native prey may be vulnerable to introduced predators because they have not evolved appropriate defenses. However, recent evidence suggests that native prey sometimes exhibit induced defenses to introduced predators, as a result of rapid evolution or other processes. We examined whether Olympia oysters (Ostrea lurida) display inducible defenses in the presence of an invasive predator, the Atlantic oyster drill (Urosalpinx cinerea), and whether these responses vary among oyster populations from estuaries with and without this predator. We spawned oysters from six populations distributed among three estuaries in northern California, USA, and raised their offspring through two generations under common conditions to minimize effects of environmental history. We exposed second-generation oysters to cue treatments: drills eating oysters, drills eating barnacles, or control seawater. Oysters from all populations grew smaller shells when exposed to drill cues, and grew thicker and harder shells when those drills were eating oysters. Oysters exposed to drills eating other oysters were subsequently preyed upon at a slower rate. Although all oyster populations exhibited inducible defenses, oysters from the estuary with the greatest exposure to drills grew the smallest shells suggesting that oyster populations have evolved adaptive differences in the strength of their responses to predators. Our findings add to a growing body of literature that suggests that marine prey may be less likely to exhibit naiveté in the face of invasive predators than prey in communities that are more isolated from native predators, such as many freshwater and terrestrial island ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inducible prey defenses occur when organisms exhibit plastic changes in phenotype in response to cues from a predator (Harvell 1990; Tollrian and Harvell 1999; Freeman and Byers 2006). Inducible defenses tend to be favored over constitutive defenses when predation pressure is variable, predator cues can be detected, and defenses are energetically costly (Harvell 1990; Tollrian and Harvell 1999). Upon detection of predator cues, prey can exhibit chemical, behavioral, and/or morphological defenses (Tollrian and Harvell 1999; Ferrari et al. 2010; Bourdeau et al. 2013).
To date, most studies in animal predator–prey systems have examined inducible defenses in response to native predators (Ferrari et al. 2010; Carthey and Banks 2014). Invasive predators, however, are spreading at rapid rates, resulting in novel and often detrimental interactions with native prey (Strauss et al. 2006; Salo et al. 2007). The prey naiveté hypothesis proposes that native prey fail to respond to introduced predators with appropriate antipredator mechanisms because they lack an evolutionary history with these predators and do not recognize them as threats (Cox and Lima 2006). However, native prey sometimes do respond to novel predators with inducible defenses (Sih et al. 2010). This can occur through a previously existing detection mechanism, learned responses, or rapid evolution of predator detection (Sih et al. 2010; Bourdeau et al. 2013). For example, Freeman and Byers (2006) found that mussels that co-occurred with the invasive crab Hemigrapsus sanguineus in the field developed thicker shells when exposed to chemical cues from this predator. In contrast, mussels from sites where the predator was absent did not exhibit inducible defenses. Conversely, mussels from both locations exhibited shell thickening in response to the long-established invasive green crab, Carcinus maenas, providing evidence for rapid evolution of inducible defenses in response to the newly introduced Hemigrapsus.
Clear documentation of rapid evolution in response to invasive species is relatively rare (Strauss et al. 2006; Carthey and Banks 2014). Moreover, although phenotypic plasticity and adaptation are often considered independently, it is likely that the strength of plastic responses to invasive predators is itself under strong selection (Strauss et al. 2006). For example, different histories of exposure to invasive predators may select for native prey populations that are locally adapted in their plastic responses to predator cues (Trussell and Nicklin 2002; Freeman and Byers 2006). Such patterns of adaptive differentiation in inducible defenses can be investigated by comparing responses of native prey from populations that differ in their historical exposure to an invasive predator (Freeman and Byers 2006; Large and Smee 2013; Carthey and Banks 2014; Freeman et al. 2014). For example, Large and Smee (2013) compared behavioral and morphological responses of native dogwhelks from different sites to invasive green crabs and found that dogwhelks from populations with a long history of interaction with the invasive predator exhibited the largest inducible responses.
Here, we raised Olympia oysters (Ostrea lurida) from six sites distributed among three estuaries (Fig. 1) through two generations under common conditions in the laboratory, and tested whether oysters from sites with and without Atlantic oyster drills (Urosalpinx cinerea) are adapted to respond to this invasive predator. Olympia oysters are the only native oyster on the west coast of the US and Canada and they serve as a foundation species that provides habitat and ecosystem services such as water filtration and nutrient cycling (Newell 2004; Kimbro and Grosholz 2006). Atlantic oyster drills are native to the east coast of North America and were introduced to the west coast in the late nineteenth century (Carlton 1992). Urosalpinx have decreased native oyster abundance in some California estuaries including Tomales and San Francisco Bays (Kimbro et al. 2009; Zabin et al. 2010), whereas invasive drills are absent from other California estuaries such as Elkhorn Slough (Wasson et al. 2001). Although Urosalpinx were reported in Elkhorn Slough in 1945, they never became established (Carlton 1979).
Field sites in California, USA, for collection of Olympia oyster (Ostrea lurida) broodstock (NC Nick’s Cove, M Marshall, LL Loch Lomond, B Berkeley, OP Oyster Point, and ES Elkhorn Slough). Tomales Bay (NC and M) has a large population of the oyster drill Urosalpinx cinerea. San Francisco Bay has isolated populations of Urosalpinx (at OP, but not at LL or B) and Urosalpinx are absent from Elkhorn Slough
We performed a laboratory experiment to answer the following questions: (1) Do Olympia oysters exhibit inducible defenses in response to invasive Atlantic oyster drills? (2) Do inducible responses vary among oyster populations in northern California estuaries depending on their evolutionary history of co-occurrence with the predator?
Based on previous work which found that some bivalve species exhibit inducible defenses in response to predators, including crabs and snails (Smee and Weissburg 2006; Newell et al. 2007; Lord and Whitlatch 2012; Robinson et al. 2014; Scherer et al. 2016), we hypothesized that Olympia oysters would develop smaller, thicker, and harder shells when exposed to cues from predatory drills as compared to control seawater. We also hypothesized that the strength of these inducible defenses would depend on the prey items offered to drills. In particular, we expected cues from drills consuming conspecific oysters to signal an imminent threat, either through conspecific alarm cues or predator diet cues or both, and, therefore, to elicit a stronger response than cues from drills consuming barnacles (Turner 2008; Shaffery and Relyea 2016). We hypothesized that these inducible defenses would slow rates of predation by Atlantic oyster drills. Finally, we hypothesized that second-generation oysters originating from Tomales and San Francisco Bays would exhibit inducible defenses in the presence of drills, whereas those from Elkhorn Slough would not, because they lack an evolutionary history of co-occurrence with this predator.
Materials and methods
General approach
We chose six sites with Olympia oyster populations and differing densities of U. cinerea (C. Kwan, unpublished data; Fig. 1). To obtain broodstock, we collected adult Olympia oysters (n = 60–170 site−1) in Fall 2011 from approximately 0.0 m mean lower low water and transported them to Bodega Marine Laboratory (Bodega Bay, California, USA). Oysters from each site were evenly distributed among ten 19-L tanks (6 sites × 10 tanks = 60 tanks). We visually checked each tank daily for release of brooded larvae. Larvae were collected from multiple tanks (i.e., multiple females) from each site within a 16-day period and were pooled by site into 100-L cylinders that were bubbled with air and lined with 10 × 10 cm polyvinyl chloride (PVC) tiles for settlement. When settled oysters were approximately 6 weeks old, oysters were culled to approximately 10–20 oysters tile−1 to avoid overgrowth interactions, and the tiles (n = 50–76 tiles site−1) were transferred to 75-L tanks for long-term growth in common garden conditions (detailed in supplementary material online resource 1). After 16 months, we spawned first-generation oysters to obtain second-generation (F2) oysters, which were raised under common garden conditions in the laboratory for 3 months before beginning the experiment (supplementary material online resource 1). Using F2 oysters reared in a common laboratory environment allowed us to minimize the potential influences of environmental history and parental effects, to better test for phenotypic differences among populations associated with local adaptation (Kawecki and Ebert 2004; Sanford and Kelly 2011).
Experimental protocol and response variables
To test whether oysters from different estuaries and from different populations within a single estuary varied in their responses to the introduced predator, U. cinerea, we performed a laboratory experiment (Aug–Oct 2013) with F2 oysters from our six sites (Fig. 1). We used 3-month-old oysters (mean area ± SEM = 0.33 ± 0.01 mm2), an age at which oysters generally grow rapidly (Cheng et al. 2015). Two–four tiles were grouped together to achieve 12–13 oysters group−1. Groups of tiles were then randomly assigned to one of three treatments: seawater with cues from Urosalpinx eating Olympia oysters, seawater with cues from Urosalpinx eating barnacles (Chthamalus dalli) or control seawater (no animals). Urosalpinx were collected from Tomales Bay, CA and were fed Olympia oysters for 1 week and then starved for 1 week prior to the beginning of the experiment.
We used mesocosms, each of which consisted of an upstream treatment container (7 L) that held either control seawater, Urosalpinx eating barnacles, or Urosalpinx eating oysters. The upstream containers delivered water to six 4-L oyster containers (one per population) that were nested within that treatment. Tiles with juvenile oysters were attached with cable ties to mesh on the inside of each container. The full design consisted of 3 treatments × 6 populations × 6 replicate mesocosms = 108 oyster containers (n = 1375 oysters). Replicate mesocosms were distributed among three water tables. Within each replicate mesocosm, the positions of the oyster containers were re-randomized twice throughout the experiment to minimize the potential for position effects. The experiment ran for 6 weeks and predators were fed their respective food type ad libitum. See supplementary material online resource 1 for additional details regarding oyster husbandry during the experiment.
Oyster tiles were photographed at the beginning and end of the experiment. Approximately one-third of oysters on each tile (n = 429 oysters; 19–24 per treatment combination) were randomly selected for proportional growth measurements [calculated as (final area−initial area)/initial area] using ImageJ software (version 1.48, National Institutes of Health, USA). These oysters were then dissected for tissue and shell weight measurements. For each oyster, the top (right) valve and soft tissue were collected (leaving the lower valve cemented to the tile) and were dried in a laboratory oven at 80 °C for 24–48 h. Subsequently, shells were weighed using a microbalance (model XP2U, Mettler-Toledo LLC, Columbus, OH, USA). Then, with the aid of a dissecting microscope, we removed all tissue from the shells and re-weighed them to obtain shell-only weights. From these measurements, we calculated shell-to-tissue weight ratios to examine whether oysters from different populations or treatments allocated more energy towards their shells versus their soft tissue. As an index of shell thickness, we also examined shell weight-to-area ratios (Lord and Whitlatch 2012).
To test for differences in shell hardness, a subsample of five replicate oysters was selected from each of four groups [2 treatments (control and Urosalpinx consuming oysters) × 2 populations (Elkhorn Slough and Marshall)]. We chose this subset of treatment × population combinations because they were the most likely to exhibit differences in hardness based on preliminary growth data. The top shell of each oyster was first mounted and polymerized in epoxy resin. Samples were then ground down to expose the outermost layer of shell. Each shell was then polished and subsequently tested for microhardness using methods modified from Beniash et al. (2010). Five–ten indentations were made in each shell using a Micromet 2004 (Buehler, Lake Bluff, IL, USA) with a 0.2-kg load and 15 s of drilling time. These values were averaged for each sample and the indent diameter was converted to gigapascals (GPa). See supplementary material online resource 2 for additional methods.
The two-thirds of oysters that were not dissected for tissue and shell weights were used for a subsequent experiment to test vulnerability of treatment groups to predation. We collected a fresh group of Urosalpinx from Tomales Bay, starved them for 1 week, and then placed two oyster drills in each 4-L oyster container (8–9 oysters container−1; 3 treatments × 6 populations × 6 replicate mesocosms = 108 oyster containers). At the conclusion of the 10-day experiment, we counted the number of oysters drilled in each container to determine whether predation rate differed among populations and treatments.
Statistical analyses
To assess differences in growth, thickness, and shell-to-tissue weight ratios, we used linear mixed effects models with population, treatment, and their interaction as fixed effects and water table, replicate nested within water table, container nested within replicate, and tile nested within container as random effects. To assess whether predation rates differed, we used a similar linear mixed effects model but with only table and replicate nested within table as random effects. To evaluate assumptions of normality and homogeneity of variances, we examined probability plots and model predictions against residuals for all response variables. Proportional growth data were square-root transformed to improve homogeneity of variances. Because of nonlinear components of the linear models for thickness and shell-to-tissue weight ratios, we ran a generalized additive model that revealed a quadratic relationship between the response variables and final shell area. Therefore, final shell area and the square of final shell area were included as covariates for these two response variables. To assess hardness differences, we used a two-way ANOVA with treatment and population as fixed factors.
Significance of variables was assessed using Satterthwaite’s approximation for degrees of freedom. All post hoc multiple comparisons were carried out using general linear hypothesis tests. All analyses were conducted using R (version 3.1.0) and the lme4, lmerTest, multcomp, gamm4, and ggplot2 packages (supplementary material online resource 3).
Results
Proportional growth differed among treatments (F 2,84.3 = 210.70, P < 0.0001) and populations (F 5,83.7 = 4.98, P = 0.00049; Fig. 2; supplementary material online resource 4). Overall, oysters grew the most when held under control conditions and grew 81% less when exposed to Urosalpinx consuming oysters (P < 0.001). There was also a significant interaction between population and treatment (F 10,83.6 = 2.52, P = 0.010). In the Urosalpinx consuming oysters treatment, F2 oysters from populations with the greatest exposure to drills in the field (Nick’s Cove and Marshall) grew significantly less (51–61%) than oysters from Loch Lomond and Elkhorn Slough (where Urosalpinx are absent). In the Urosalpinx consuming barnacles treatment, oysters from Nick’s Cove grew significantly less than oysters from all other sites (P < 0.05). In the control treatment, there were no differences among populations.
Proportional growth (least squares means ± SEM, which have been back-transformed to the original scale) of six oyster populations (Fig. 1) following a 6-week exposure to one of three cues: control seawater (Control), seawater with cues from Urosalpinx consuming barnacles (Drills–barnacles), or seawater with cues from Urosalpinx consuming oysters (Drills–oysters) (n = 108 oyster containers; six per treatment combination). Oysters from all populations grew more in the control treatment than the Urosalpinx consuming oysters treatment (P < 0.001). In the Urosalpinx consuming barnacles treatment, oysters from Nick’s Cove grew less than oysters from all other sites (P < 0.05). In the Urosalpinx consuming oysters treatment, oysters from Nick’s Cove and Marshall grew less than oysters from Loch Lomond and Elkhorn Slough (P < 0.05)
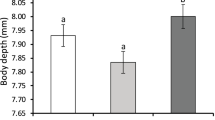
Shell thickness differed among treatments (F 2,13.5 = 4.00, P = 0.043) and populations (F 5,138.1 = 2.75, P = 0.021; Fig. 3). Olympia oysters exposed to cues from Urosalpinx consuming oysters were 6.9% thicker than those in the control treatment (Z value = 2.82, P = 0.013). Oysters originating from Loch Lomond were 11% thicker than oysters originating from Elkhorn Slough (Z value = 3.56, P = 0.0051). There was no interaction between treatment and population (F 10,136.9 = 0.93, P = 0.51).
Shell thickness index (top shell weight-to-area ratio in g cm−2) for oysters (mean ± SEM) following a 6-week exposure to one of three cues: control seawater (Control), seawater with cues from Urosalpinx consuming barnacles (Drills–barnacles), or seawater with cues from Urosalpinx consuming oysters (Drills–oysters) (n = 108 oyster containers; six per treatment combination). Since there was no population-by-treatment interaction, results for all six populations were pooled for this graph. Shared letters above bars indicate treatments that did not differ (Tukey–Kramer, P > 0.05)
Shell hardness also differed among treatments (F 1,16 = 4.93, P = 0.041). Oysters that had been exposed to Urosalpinx consuming oysters had 20% harder shells (mean ± SEM = 1.94 ± 0.11 GPa) than those from the control treatment (mean ± SEM = 1.61 ± 0.10 GPa). Shell hardness did not vary among populations (F 1,16 = 1.76, P = 0.20) and there was no interaction between treatment and population (F 1,16 = 0.054, P = 0.82).
Shell-to-tissue weight ratios did not differ significantly among treatments (F 2,12.9 = 3.074, P = 0.081; supplementary material online resource 5), but there was a trend for oysters from the Urosalpinx consuming oysters treatment to have higher shell-to-tissue weight ratios than oysters from the control treatment. There was no significant difference in shell-to-tissue weight ratios among populations (F 5,76.4 = 1.50, P = 0.20). The interaction term was significant at α = 0.1 (F 10,75.2 = 1.89, P = 0.059). In control conditions, oysters from Nick’s Cove had a higher investment in shell versus tissue than oysters from Loch Lomond. In the treatment with Urosalpinx consuming barnacles, oysters from Nick’s Cove and Marshall had a higher investment in shell versus tissue than oysters from Berkeley.
Oyster vulnerability to predation differed among treatments (F 2,88 = 3.96, P = 0.023; Fig. 4). Predators consumed 35–45% fewer oysters when those oysters had been previously exposed to cues from Urosalpinx consuming oysters. This predation rate was significantly less than that for oysters that had been exposed to cues from Urosalpinx consuming barnacles (Z value = 2.82, P = 0.014). There was also an effect of population (F 5,88 = 2.43, P = 0.041) with a post hoc Tukey–Kramer test revealing a trend for oysters from the site lacking Urosalpinx (Elkhorn Slough) to be preyed upon at a higher rate than oysters from Nick’s Cove (Z = 2.72, P = 0.072). There was no interaction between treatment and population (F 10,88 = 0.75, P = 0.68).
Predation rates (mean number of oysters consumed ± SEM) by two Urosalpinx placed in containers for 10 days with oysters previously exposed to one of the three cues: control seawater (Control), seawater with cues from Urosalpinx consuming barnacles (Drills–barnacles), or seawater with cues from Urosalpinx consuming oysters (Drills–oysters) (n = 108 oyster containers; six per treatment combination). Since there was no population-by-treatment interaction, results for all six populations were pooled for this graph. Shared letters above bars indicate treatments that did not differ (Tukey–Kramer, P > 0.05)
Discussion
Inducible defenses in response to an invasive predator
Although we expected oyster populations that do not co-occur with Atlantic oyster drills to be naive to this invasive predator, we found that oysters from all populations detected and responded to Urosalpinx in the laboratory. Oysters from all populations grew smaller, thicker, and harder shells when exposed to cues from invasive drills consuming oysters. Interestingly, oysters also grew smaller shells when exposed to cues from drills consuming barnacles. Thus, oysters changed their morphology in the presence of Urosalpinx regardless of predator diet, demonstrating that oysters responded to more than just a conspecific cue (i.e. alarm cue or predator diet cue).
There are multiple mechanisms that could explain why prey respond to a completely novel predator. In our study, Olympia oysters might have evolved the ability to respond to this invasive predator during the 80–100 years that these two species have co-occurred in some California estuaries. However, while rapid evolution might explain the occurrence of inducible defenses in oysters from Tomales and San Francisco Bays, it cannot explain their presence in oysters from Elkhorn Slough, where Urosalpinx are absent. Rapid evolution in one estuary coupled with dispersal and gene flow among estuaries (e.g., Carson 2010) could lead to inducible defenses in locations that do not encounter drills. However, there is evidence based on microsatellite data (Stick 2011) and asynchronous recruitment (K. Wasson, unpublished data) that Olympia oyster larval dispersal and connectivity among estuaries in this region may be restricted.
Another potential explanation for the lack of prey naiveté is that the invasive predator might release chemical cues similar to those of a native predator. These similarities might enable prey to detect invasive predators and respond with antipredator defenses (Sih et al. 2010; Bourdeau et al. 2013). Although this is possible in our study system, we were unable to compare oyster responses to Urosalpinx with those elicited by native predatory snails; native whelks (Acanthinucella spirata, Nucella canaliculata) did not prey on Olympia oysters, even when held with no other prey in the laboratory for 3–4 weeks (J. Bible, unpublished data). To our knowledge, A. spirata is the only native drilling predator that preys on Olympia oysters in northern California estuaries (Kimbro et al. 2009). However, despite overlap in habitat with Olympia oysters, Acanthinucella preys primarily on barnacles (Perry 1987). N. canaliculata is an outer coast dogwhelk that does not co-occur with Olympia oysters and consumes primarily mussels and barnacles (Sanford et al. 2003). The moon snail Neverita lewisii may co-occur with Olympia oysters in some locations (Baker 1995), but was not considered in this study since its diet consists primarily of infaunal bivalves (Cook and Bendell-Young 2010). Although selective pressure from native drilling predators appears weak, it cannot be ruled out as a possible factor in the oyster’s response to Urosalpinx. Native predators may have imposed sufficient selection on oysters to favor the capacity to detect drilling predators, including, perhaps, novel species like Urosalpinx.
Alternatively, oysters might detect a very general chemical cue (e.g., sulfurous compounds generated during metabolism) that enables them to respond to predation risk from many different sources (e.g., both native predators such as snails, crabs, and sea stars as well as introduced predatory drills) (Sih et al. 2010; Carthey and Banks 2014). In a supplementary experiment (supplementary material online resource 6), we found that all six oyster populations also responded with inducible defenses to cues from another invasive drill (Ocenebra inornata). Because this invasive drill is found in Tomales Bay but not in San Francisco Bay or Elkhorn Slough, the uniform response across estuaries is consistent with a response to either a drill-related cue or a more general predation cue.
Inducible defenses can also be a learned response (i.e., cue association; Chivers and Smith 1998), which can enable prey to detect introduced predators (Scherer and Smee 2016). However, most evidence of learned responses comes from studies of vertebrates rather than invertebrates (Chivers and Smith 1998; Ferrari et al. 2010) and is associated with behavioral responses (Chivers et al. 2002; Brown 2003; Batabyal et al. 2014) rather than morphological ones. In this experiment, oysters were raised for two generations under common conditions (detailed in supplementary material online resource 1) and were never previously exposed to the predator; so, cue association cannot explain our results.
Finally, oysters could also be responding to conspecific or heterospecific alarm cues rather than cues from the predator itself (Ferrari et al. 2010; Bourdeau et al. 2013). Our results revealed a greater response when conspecifics were eaten, but oysters still responded with decreased growth when predators were eating barnacles (Fig. 2). Many studies have demonstrated prey detection of heterospecific alarm cues (reviewed by Ferrari et al. 2010). In freshwater and marine invertebrates, studies support the idea that prey response to heterospecific cues is likely limited by phylogenetic relatedness (Hazlett and McLay 2005; Dalesman et al. 2007; Turner 2008; Ferrari et al. 2010). For example, mussels demonstrated the ability to detect alarm cues from cockles (another bivalve), but did not detect cues from a gastropod (Fässler and Kaiser 2008). Although it is possible that oysters might detect alarm cues from barnacles given that they live in the same habitat and are preyed upon by the same predator (Scherer and Smee 2016), the phylogenetic distance between these taxa makes this possibility unlikely. Therefore, it seems more likely that the oyster response to Urosalpinx consuming barnacles is a response to the predator itself or its digestive cues. Thus, available evidence suggests that Olympia oysters are most likely reacting to both a general predator cue and a conspecific cue associated with the predator’s diet or an alarm signal.
Divergent defensive responses among oyster populations
As predicted, oysters grew the smallest shells in the presence of predators consuming conspecifics when the oysters originated from the estuary with the highest predation pressure by invasive drills (Fig. 2). This is consistent with the hypothesis that oyster populations differ in response to predation cues. Because we used oysters raised through two common garden generations in the laboratory with no previous exposure to these predators, the differences documented here very likely reflect evolutionary differences among populations. Given that Urosalpinx predation causes oyster mortality in Tomales Bay (Kimbro et al. 2009) whereas this predator is absent in Elkhorn Slough (Wasson 2010), it is possible that differential selection imposed by Urosalpinx has favored the rapid evolution of more pronounced responses to predation cues in Tomales Bay populations. Alternatively, oyster populations may have evolved different responses to this invasive predator due to selection imposed by unknown variation in the intensity of native predation (e.g., Acanthinucella, Cancer crabs). Although we do not have quantitative data comparing overall predation rates across sites, predation appears to be minimally important in regulating Elkhorn Slough oyster populations (Wasson 2010). In some cases, prey from lower risk environments may be less likely to respond to both native and invasive predators (Large and Smee 2013). Because we were unable to test responses to a native predator (e.g., Freeman and Byers 2006), we cannot conclude definitively whether the observed differences among oyster populations reflect spatially varying selection imposed by the invasive drill or native predators.
Although the role of selective pressure from native versus invasive predators remains to be determined, our results indicate that oyster populations are locally adapted to variation in predation pressure, including that imposed by invasive drills. Local adaptation has been understudied in marine ecosystems (reviewed by Conover et al. 2006; Baums 2008; Sanford and Kelly 2011; Sotka 2012), yet recent evidence suggests that adaptive differentiation among marine populations may be common (Sanford and Kelly 2011) and can have critical implications for conservation (Baums 2008; Bible and Sanford 2016). Here, differential responses to Urosalpinx emphasize that not all prey populations are equivalent in their vulnerability to invasive predators.
Implications for inducible defenses in the field
Although inducible defenses can reduce vulnerability to predation, they often result in costs or trade-offs, especially those associated with reproduction (Trussell and Smith 2000; Creel and Christianson 2008; Ferrari et al. 2010; Sih et al. 2010). Our results revealed a trend for oysters exposed to Urosalpinx consuming oysters to increase energy investment in hard shell relative to tissue (supplementary material online resource 5). However, it is unclear whether this resulted from active re-allocation of energy or as a by-product of decreased feeding when exposed to predator cues, as has been observed in some marine snails (Bourdeau 2010). Alternatively, reduced growth in the presence of predators might reflect increased investment in reproduction (Reznick and Endler 1982). However, since our juvenile oysters were not yet reproductive, the slower growth rate in the presence of Urosalpinx might be unrelated to reproductive investment and might, in fact, lead to longer time to sexual maturity (Mazón-Suástegui et al. 2011), with implications for fitness. In addition, the striking changes in shell size and morphology we observed might make oysters more vulnerable to other stressors. For example, due to the high surface-area-to-volume ratio, small oysters may be more vulnerable to desiccation at low tide (Denny 1993). Such associated costs may favor defenses that are inducible rather than constitutive (Tollrian and Harvell 1999).
Increasingly, studies have demonstrated that inducible defenses lead to the predicted increases in survival (Mirza and Chivers 2002; Robinson et al. 2008; Smee and Weissburg 2006; Robinson et al. 2014). We found that predation rate was reduced on oysters that had been exposed to cues from Urosalpinx consuming oysters (Fig. 4), consistent with induced morphological shell changes such as thickening and hardening that provide defensive benefits against drilling. Reduced growth itself might also be an effective defense against predation, since smaller prey are often less preferred (Paine 1981; Lively et al. 2000).
Prey naiveté in marine ecosystems
Although the prey naiveté hypothesis is increasingly tested, its generality remains unclear (Carthey and Banks 2014). Results from some studies support prey naiveté in the presence of invaders (Edgell and Neufeld 2008; Kindinger 2015), whereas results from other studies demonstrate prey ability to recognize novel predators (Losos et al. 2004; Large and Smee 2013; Freeman et al. 2013; Black et al. 2014). Our findings join a growing body of published evidence suggesting that the prey naiveté hypothesis is often not supported in marine systems (Table 1). Although this literature is currently limited in the number of studies and range of taxa, available evidence suggests that it is more common for marine prey to respond to invasive predators (via various mechanisms) than for them to exhibit naiveté. This is consistent with theoretical arguments that marine species, like those in contiguous terrestrial systems, are often exposed to biotic interchanges and pervasive predation (Paine 1994) and, therefore, may be less likely to display prey naiveté than many freshwater and terrestrial island species (Cox and Lima 2006). Freshwater systems are often isolated and can have striking variation among sites in predator abundance (e.g., Reznick and Endler 1982), increasing the likelihood of naiveté and vulnerability to invasive predators in some prey populations (Cox and Lima 2006). Similarly, most evidence of terrestrial prey naiveté stems from island ecosystems (e.g., Griffiths et al. 1998; Losos et al. 2004), where geographic isolation often results in reduced exposure to native predators (Blackburn et al. 2004).
In theory, individual prey that detect and respond to invasive predators with inducible defenses are expected to have higher fitness (Sih et al. 2010). However, even in the absence of prey naiveté, many marine populations are heavily impacted by invasive predators (Grosholz 2002). For example, although some damselfish species display antipredator behavior in the presence of invasive predatory lionfish (Black et al. 2014), these predators have caused extensive mortality in reef fish populations (Green et al. 2012). Similarly, although we have found that Olympia oysters respond to Urosalpinx with appropriate changes in shell morphology, these invasive predators can still cause extensive mortality in oyster populations (Kimbro et al. 2009; Zabin et al. 2010). The recognition that induction of defenses does not necessarily mean escaping high impacts from invasive predators (Banks and Dickman 2007) is an important and underappreciated consideration in predicting the effects of invasive predators. Although inducible defenses often slow predation, they seldom dissuade predation entirely. Thus, although our results demonstrate that some oyster populations have evolved a greater capacity to respond with inducible defenses than others, even the most well-defended prey populations may be impacted by large populations of invasive predators.
References
Anton A, Cure K, Layman CA, Puntila R, Simpson MS, Bruno JF (2016) Prey naiveté to invasive lionfish Pterois volitans on Caribbean coral reefs. Mar Ecol Prog Ser 544:257–269. doi:10.3354/meps11553
Baker P (1995) Review of ecology and fishery of the Olympia oyster, Ostrea lurida with annotated bibliography. J. Shellfish Res. 14:501–518
Banks PB, Dickman CR (2007) Alien predation and the effects of multiple levels of prey naiveté. Trends Ecol Evol 22:229–230. doi:10.1016/j.tree.2007.02.006
Batabyal A, Gosavi SM, Gramapurohit NP (2014) Determining sensitive stages for learning to detect predators in larval bronzed frogs: importance of alarm cues in learning. J Biosci 39:701–710. doi:10.1007/s12038-014-9455-7
Baums IB (2008) A restoration genetics guide for coral reef conservation. Mol Ecol 17:2796–2811. doi:10.1111/j.1365-294X.2008.03787.x
Beniash E, Ivanina A, Lieb NS, Kurochkin I, Sokolova IM (2010) Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar Ecol Prog Ser 419:95–108. doi:10.3354/meps08841
Bible JM, Sanford E (2016) Local adaptation in an estuarine foundation species: implications for restoration. Biol Cons 193:95–102. doi:10.1016/j.biocon.2015.11.015
Black AN, Weimann SR, Imhoff VE, Richter ML, Itzkowitz M (2014) A differential prey response to invasive lionfish, Pterois volitans: prey naiveté and risk-sensitive courtship. J Exp Mar Biol Ecol 460:1–7. doi:10.1016/j.jembe.2014.06.002
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958. doi:10.1126/science.1101617
Bourdeau PE (2010) An inducible morphological defence is a passive by-product of behaviour in a marine snail. Proc R Soc B 277:455–462. doi:10.1098/rspb.2009.1295
Bourdeau PE, Pangle KL, Reed EM, Peacor SD (2013) Finely tuned response of native prey to an invasive predator in a freshwater system. Ecology 94:1449–1455. doi:10.1890/12-2116.1
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234. doi:10.1046/j.1467-2979.2003.00132.x
Carlton JT (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific coast of North America. PhD Dissertation, University of California
Carlton JT (1992) Introduced marine and estuarine mollusks of North America: an end-of-the-20th-century perspective. J Shellfish Res 11:489–505
Carson HS (2010) Population connectivity of the Olympia oyster in southern California. Limnol Oceanogr 55:134–148. doi:10.4319/lo.2010.55.1.0134
Carthey AJR, Banks PB (2014) Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biol Rev 89:932–949. doi:10.1111/brv.12087
Cheng BS, Bible JM, Chang AL, Ferner MC, Wasson K, Zabin CJ, Latta M, Deck A, Todgham AE, Grosholz ED (2015) Testing local and global stressor impacts on a coastal foundation species using an ecologically realistic framework. Global Change Biol 21:2488–2499. doi:10.1111/gcb.12895
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Chivers DP, Mirza RS, Johnston JG (2002) Learned recognition of heterospecific alarm cues enhances survival during encounters with predators. Behaviour 139:929–938. doi:10.1163/156853902320387909
Conover DO, Clarke LM, Munch SB, Wagner GN (2006) Spatial and temporal scales of adaptive divergence in marine fishes and the implications for conservation. J Fish Biol 69:21–47. doi:10.1111/j.1095-8649.2006.01274.x
Cook N, Bendell-Young L (2010) Determining the ecological role of Euspira lewisii: part I: feeding ecology. J Shellfish Res 29:223–232. doi:10.2983/035.029.0119
Coverdale TC, Axelman EE, Brisson CP, Young EW, Altieri AH, Bertness MD (2013) New England salt marsh recovery: opportunistic colonization of an invasive species and its non-consumptive effects. PLoS One 8(8):e73823. doi:10.1371/journal.pone.0073823
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680. doi:10.1016/j.tree.2006.07.011
Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201. doi:10.1016/j.tree.2007.12.004
Dalesman S, Rundle SD, Bilton DT, Cotton PA (2007) Phylogenetic relatedness and ecological interactions determine antipredator behavior. Ecology 88:2462–2467. doi:10.1890/07-0403.1
Denny M (1993) Air and water: the biology and physics of life’s media. Princeton University Press, Princeton
Edgell TC, Neufeld CJ (2008) Experimental evidence for latent developmental plasticity: intertidal whelks respond to a native but not an introduced predator. Biol Lett 4:385–387. doi:10.1098/rsbl.2008.0204
Edgell TC, Lynch BR, Trussell GC, Palmer AR (2009) Experimental evidence for the rapid evolution of behavioral canalization in natural populations. Am Nat 174:434–440. doi:10.1086/603639
Fässler SMM, Kaiser MJ (2008) Phylogenetically mediated anti-predator responses in bivalve molluscs. Mar Ecol Prog Ser 363:217–225. doi:10.3354/meps07459
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724. doi:10.1139/z10-029
Freeman AS, Byers JE (2006) Divergent induced responses to an invasive predator in marine mussel populations. Science 313:831–833. doi:10.1126/science.1125485
Freeman AS, Hamer CE (2009) The persistent effect of wave exposure on TMIIs and crab predation in Nucella lapillus. J Exp Mar Biol Ecol 372:58–63. doi:10.1016/j.jembe.2009.02.002
Freeman AS, Wright JT, Hewitt CL, Campbell ML, Szeto K (2013) A gastropod’s induced behavioral and morphological responses to invasive Carcinus maenas in Australia indicate a lack of novelty advantage. Biol Invasions 15:1795–1805. doi:10.1007/s10530-013-0409-z
Freeman AS, Dernbach E, Marcos C, Koob E (2014) Biogeographic contrast of Nucella lapillus responses to Carcinus maenas. J Exp Mar Biol Ecol 452:1–8. doi:10.1016/j.jembe.2013.11.010
Green SJ, Akins JL, Maljkovic A, Côté IM (2012) Invasive lionfish drive Atlantic coral reef fish declines. PLoS One 7(3):e32596. doi:10.1371/journal.pone.0032596
Griffiths RA, Schley L, Sharp PE, Dennis JL, Román A (1998) Behavioural responses of Mallorcan midwife toad tadpoles to natural and unnatural snake predators. Anim Behav 55:207–214. doi:10.1006/anbe.1997.0596
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17:22–27. doi:10.1016/s0169-5347(01)02358-8
Harvell CD (1990) The ecology and evolution of inducible defenses. Q Rev Biol 65:323–340. doi:10.1086/416841
Hazlett BA, McLay C (2005) Responses of the crab Heterozius rotundifrons to heterospecific chemical alarm cues: phylogeny vs. ecological overlap. J Chem Ecol 31:671–677. doi:10.1007/s10886-005-2054-1
Hooks AP, Padilla DK (2014) Prey responses to the presence of a native and nonnative predator. J Exp Mar Biol Ecol 461:209–215. doi:10.1016/j.jembe.2014.07.022
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241. doi:10.1111/j.1461-0248.2004.00684.x
Kimbro DL, Grosholz ED (2006) Disturbance influences oyster community richness and evenness, but not diversity. Ecology 87:2378–2388. doi:10.1890/0012-9658(2006)87[2378:DIOCRA]2.0.CO;2
Kimbro DL, Grosholz ED, Baukus AJ, Nesbitt NJ, Travis NM, Attoe S, Coleman-Hulbert C (2009) Invasive species cause large-scale loss of native California oyster habitat by disrupting trophic cascades. Oecologia 160:563–575. doi:10.1007/s00442-009-1322-0
Kindinger TL (2015) Behavioral response of native Atlantic territorial three spot damselfish (Stegastes planifrons) toward invasive Pacific red lionfish (Pterois volitans). Environ Biol Fishes 98:487–498. doi:10.1007/s10641-014-0279-y
Large SI, Smee DL (2010) Type and nature of cues used by Nucella lapillus to evaluate predation risk. J Exp Mar Biol Ecol 396:10–17. doi:10.1016/j.jembe.2010.10.005
Large SI, Smee DL (2013) Biogeographic variation in behavioral and morphological responses to predation risk. Oecologia 171:961–969. doi:10.1007/s00442-012-2450-5
Large SI, Torres P, Smee DL (2012) Behavior and morphology of Nucella lapillus influenced by predator type and predator diet. Aquat Biol 16:189–196. doi:10.3354/ab00452
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14. doi:10.2307/176976
Lively CM, Hazel WN, Schellenberger MJ, Michelson KS (2000) Predator-induced defense: variation for inducibility in an intertidal barnacle. Ecology 81:1240–1247. doi:10.1890/0012-9658(2000)081[1240:pidvfi]2.0.co;2
Lord JP, Whitlatch RB (2012) Inducible defenses in the eastern oyster Crassostrea virginica Gmelin in response to the presence of the predatory oyster drill Urosalpinx cinerea Say in Long Island sound. Mar Biol 159:1177–1182. doi:10.1007/s00227-012-1896-7
Losos JB, Schoener TW, Spiller DA (2004) Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432:505–508. doi:10.1038/nature03039
Marsh-Hunkin KE, Gochfeld DJ, Slattery M (2013) Antipredator responses to invasive lionfish, Pterois volitans: interspecific differences in cue utilization by two coral reef gobies. Mar Biol 160:1029–1040. doi:10.1007/s00227-012-2156-6
Mazón-Suástegui JM, Ruíz-García MC, Chávez-Villalba J, Rodríguez-Jaramillo C, Saucedo PE (2011) Analysis of growth and first reproduction of hatchery-reared juvenile Cortez oyster (Crassostrea corteziensis) in northwestern Mexico: proposal of a minimal fishing size. Aquacult Res 42:1558–1568. doi:10.1111/j.1365-2109.2010.02748.x
Mirza RS, Chivers DP (2002) Behavioural responses to conspecific disturbance chemicals enhance survival of juvenile brook charr, Salvelinus fontinalis, during encounters with predators. Behaviour 139:1099–1109. doi:10.1163/15685390260437272
Newell RIE (2004) Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: a review. J Shellfish Res 23:51–61
Newell RIE, Kennedy VS, Shaw KS (2007) Comparative vulnerability to predators, and induced defense responses, of eastern oysters Crassostrea virginica and non-native Crassostrea ariakensis oysters in Chesapeake Bay. Mar Biol 152:449–460. doi:10.1007/s00227-007-0706-0
Paine RT (1981) Barnacle ecology: is competition important? The forgotten roles of disturbance and predation. Paleobiology 7:553–560
Paine RT (1994) Marine rocky shores and community ecology: an experimentalist’s perspective. In: Kinne O (ed) Excellence in ecology, vol 4. Ecology Institute, Germany, pp 1–152
Perry DM (1987) Optimal diet theory: behavior of a starved predatory snail. Oecologia 72:360–365. doi:10.1007/bf00377564
Reznick D, Endler JA (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177. doi:10.2307/2407978
Robinson BW, Januszkiewicz AJ, Koblitz JC (2008) Survival benefits and divergence of predator-induced behavior between pumpkinseed sunfish ecomorphs. Behav Ecol 19:263–271. doi:10.1093/beheco/arm133
Robinson EM, Lunt J, Marshall CD, Smee DL (2014) Eastern oysters Crassostrea virginica deter crab predators by altering their morphology in response to crab cues. Aquat Biol 20:111–118. doi:10.3354/ab00549
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc Lond 274:1237–1243. doi:10.1098/rspb.2006.0444
Sanford E, Kelly M (2011) Local adaptation in marine invertebrates. Annu Rev Mar Sci 3:509–535. doi:10.1146/annurev-marine-120709-142756
Sanford E, Roth MS, Johns GC, Wares JP, Somero GN (2003) Local selection and latitudinal variation in a marine predator–prey interaction. Science 300:1135–1137. doi:10.1126/science.1083437
Scherer AE, Smee DL (2016) A review of predator diet effects on prey defensive responses. Chemoecology 26:83–100. doi:10.1007/s00049-016-0208-y
Scherer AE, Lunt J, Draper AM, Smee DL (2016) Phenotypic plasticity in oysters (Crassostrea virginica) mediated by chemical signals from predators and injured prey. Invertebr Biol 135:97–107. doi:10.1111/ivb.12120
Shaffery HM, Relyea RA (2016) Dissecting the smell of fear from conspecific and heterospecific prey: investigating the processes that induce anti-predator defenses. Oecologia 180:55–65. doi:10.1007/s00442-015-3444-x
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. doi:10.1111/j.1600-0706.2009.18039.x
Smee DL, Weissburg MJ (2006) Clamming up: environmental forces diminish the perceptive ability of bivalve prey. Ecology 87:1587–1598. doi:10.1007/s00442-012-2450-5
Sotka EE (2012) Natural selection, larval dispersal, and the geography of phenotype in the sea. Integr Comp Biol 52:538–545. doi:10.1093/icb/ics084
Stick DA (2011) Identification of optimal broodstock for Pacific Northwest oysters. PhD dissertation, Oregon State University
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374. doi:10.1111/j.1461-0248.2005.00874.x
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, New Jersey
Trussell GC, Nicklin MO (2002) Cue sensitivity, inducible defense, and trade-offs in a marine snail. Ecology 83:1635–1647. doi:10.2307/3071984
Trussell GC, Smith LD (2000) Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc Natl Acad Sci 97:2123–2127. doi:10.1073/pnas.040423397
Turner A (2008) Predator diet and prey behaviour: freshwater snails discriminate among closely related prey in a predator’s diet. Anim Behav 79:1211–1217. doi:10.1016/j.anbehav.2008.06.005
Vadas RL Sr, Burrows MT, Hughes RN (1994) Foraging strategies of dogwhelks, Nucella lapillus (L.): interacting effects of age, diet and chemical cues to the threat of predation. Oecologia 100:439–450. doi:10.1007/bf00317866
Wasson K (2010) Informing Olympia oyster restoration: evaluation of factors that limit populations in a California estuary. Wetlands 30:449–459. doi:10.1007/s13157-010-0056-4
Wasson K, Zabin CJ, Bedinger L, Diaz MC, Pearse JS (2001) Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biol Conserv 102:143–153. doi:10.1016/s0006-3207(01)00098-2
Whitlow WL (2010) Changes in survivorship, behavior, and morphology in native soft-shell clams induced by invasive green crab predators. Mar Ecol 31:418–430. doi:10.1111/j.1439-0485.2009.00350.x
Zabin CJ, Attoe S, Grosholz ED, Coleman-Hulbert C (2010) Shellfish conservation and restoration in San Francisco Bay: opportunities and constraints. Final report for the Subtidal Habitat Goals Committee. University of California
Acknowledgements
We thank G. Baxter, J. Bean, M. Carroll, B. Cheng, A. Deck, E. Ernst, G. Fleener, F. Hayes, L. Heidenreich, P. Jones, C. Knight, J. Lankford, K. Laughlin, A. Nieto, A. Ninokawa, C. Norton, L. Rose, E. Seubert, C. Star, D. Stone, and P. Stull for their invaluable laboratory and field support. We thank members of the Bodega Marine Laboratory staff: J. Newman, K. Menard, D. Hall, and P. Smith. This manuscript was improved by helpful comments from B. Gaylord, E. Grosholz and two anonymous reviewers. This publication was developed under STAR Fellowship Assistance Agreement No. FP-917430 awarded by the US Environmental Protection Agency (EPA) to JMB. It has not been formally reviewed by EPA. The views expressed in this publication are solely those of the authors, and EPA does not endorse any products or commercial services mentioned in this publication. Additional funding was provided by National Science Foundation Grants OCE-1041089 and OCE-1220648 to ES, a UC Multicampus Research Programs & Initiatives Grant, and by the Pacific Coast Science and Learning Center, Point Reyes National Seashore. This is a contribution of the Bodega Marine Laboratory, University of California, Davis, USA.
Author contribution statement
JMB, ES, and KRG conceived and designed the experiments. JMB and KRG performed the experiments. JMB analyzed the data. JMB and ES wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Pablo Munguia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bible, J.M., Griffith, K.R. & Sanford, E. Inducible defenses in Olympia oysters in response to an invasive predator. Oecologia 183, 809–819 (2017). https://doi.org/10.1007/s00442-017-3811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3811-x