Abstract
Management agencies are considering introducing the Suminoe oyster Crassostrea ariakensis into Chesapeake Bay, USA. It is unknown if the growth of feral populations of this non-native oyster would be regulated by the same predators that once controlled the abundance of the native eastern oyster C. virginica. In laboratory studies, we compared the relative susceptibility of juvenile diploids (shell height < 25 mm) of both oyster species to invertebrate predators of eastern oyster juveniles. Predators included four species of mud crabs [Rhithropanopeus harrisii (carapace width 7–11 mm), Eurypanopeus depressus (6–21 mm), Dyspanopeus sayi (8–20 mm), and Panopeus herbstii (9–29 mm)], the blue crab Callinectes sapidus (35–65 mm), and two sizes of polyclad flatworms (Stylochus ellipticus and possibly Euplana gracilis; planar area ≲5 mm2 and ∼14 to 88 mm2). All four species of mud crab and the blue crab preyed significantly (ANOVA, P ≤ 0.05) more on C. ariakensis than on C. virginica, but predation by flatworms of both sizes did not differ significantly between oyster species. The greater susceptibility of C. ariakensis to crab predation was likely due to its shell compression strength being 64% lower than that of C. virginica (P = 0.005). To test for predator-induced enhancement of shell strength, we held oysters of both species for 54 days in the presence of, but protected from, C. sapidus and R. harrisii. Crabs were fed congeneric oysters twice weekly within each aquarium. Compared to controls, shell strength of C. virginica exposed to R. harrisii increased significantly (P < 0.043), as did shell strength of both oyster species exposed to C. sapidus (P < 0.01). Despite the changes in shell strength by both oyster species in the presence of C. sapidus, the shell of C. ariakensis remained 57% weaker than C. virginica. We conclude that, because C. ariakensis exposed to predators continued to have a weaker shell relative to C. virginica, the natural suite of crab and flatworm predators in Chesapeake Bay will likely serve to control the abundance of feral C. ariakensis. We caution that the situation in the natural environment may be sufficiently different in some locations that C. ariakensis may be able to compensate for its greater vulnerability to crab predation and hence become a nuisance species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stocks of native eastern oysters, Crassostrea virginica, in Chesapeake Bay have been severely depleted by long-term over-harvesting and resultant habitat destruction (Kennedy and Breisch 1983) and ongoing epizootics of two protistan parasitic diseases Haplosporidium nelsoni and Perkinsus marinus (Ford and Tripp 1996). This loss of suspension-feeding oysters has diminished their crucial ecosystem role (Newell et al. 2005) and imposed economic hardships associated with the collapse of the once valuable oyster fishery (NRC 2004). In response to this decline in C. virginica stocks, scientists and managers began seeking an alternative disease-tolerant oyster species that might be suitable for Chesapeake Bay conditions (NRC 2004; Allen 2005). An Asian species, the Suminoe oyster Crassostrea ariakensis, was identified as a candidate species for establishing a self-recruiting population by the deliberate release of diploid oysters into Chesapeake Bay (NRC 2004). A central question that must be answered when evaluating the possible introduction of C. ariakensis is whether the species will undergo a rapid population increase resulting in adverse ecosystem changes. Such dramatic population increases have happened for non-native bivalves in North American freshwaters (e.g., Nalepa and Schloesser 1993; Lee et al. 2005) and estuaries (e.g., Cloern 1982; Carlton et al. 1990; Kimmerer et al. 1994) and some of these increases have caused severe and often adverse ecological consequences (e.g., Nalepa and Schloesser 1993; Carlton 1999; Strayer et al. 1999).
In contrast with the extreme population-expansion scenario, it is plausible that the abundance of C. ariakensis may be controlled by the same predators that once controlled C. virginica populations when that species was more abundant. Under such a scenario, C. ariakensis might become a naturalized species that fills an important ecological niche within Chesapeake Bay and in other estuaries along the Atlantic coast of North America. A suite of predatory species feeding on different sizes of C. virginica serves to control the native oyster’s overall abundance and distribution (Osman and Abbe 1994; White and Wilson 1996). In previous research (Newell et al. 2000), we found that the most critical post-settlement life stage for C. virginica in mesohaline Chesapeake Bay is during the first few months after metamorphosis; once oysters grow larger than ∼10 mm in shell height about 1–2 months post-metamorphosis, mortality rates decline. Major predators on juvenile oysters (=spat) are flatworms Stylochus ellipticus (Newell et al. 2000) and various species of crabs (McDermott and Flower 1953; Krantz and Chamberlin 1978; Bisker and Castagna 1987; Eggleston 1990; Abbe and Breitburg 1992). Juvenile oysters have relatively greater vulnerability to crabs than large oysters because crabs tend to concentrate their foraging on the individuals at the lower end of the size range that they can physically open (Eggleston 1990; Seed and Suchanek 1992; Seed and Hughes 1995).
Because C. ariakensis is almost identical in gross morphology to C. virginica (Zhou and Allen 2003), we hypothesized that its abundance would also be governed by the same suite of predators that prey on comparably sized eastern oysters. We tested this hypothesis with a selection of different-sized predators that control the abundance of C. virginica at various shell sizes in Chesapeake Bay. We used the blue crab Callinectes sapidus and four species of mud crabs (Rhithropanopeus harrisii, Eurypanopeus depressus, Dyspanopeus sayi, and Panopeus herbstii). These crabs are common on oyster beds in mesohaline and polyhaline regions of Chesapeake Bay and their strong claws allow them to feed on oysters by crushing or chipping the shell edge (Milke and Kennedy 2001). As a contrast to the crabs in terms of feeding behavior, we also used polyclad flatworms S. ellipticus and possibly Euplana gracilis that enter oysters through their partially gaping valves (Loosanoff 1956). Flatworms then have access to the soft tissue, which they start to ingest; this attack eventually kills the oysters.
We found that all species of crabs selected C. ariakensis to a greater extent than C. virginica. Because the former species has a faster shell-growth rate than the latter species (Newell, unpublished observation), we hypothesized that species-specific differences in shell characteristics rendered C. ariakensis more susceptible to shell-crushing predators. Further, juvenile and actively growing adults of some bivalve species can modify their shell characteristics in ways that reduce an individual’s vulnerability to predators. These defenses, induced in response to “infochemicals” either from the predator itself (enemy-avoidance kairomones; Ruther et al. 2002) or from damaged and ingested conspecifics (alarm pheromones; Smith 1992; Stabell et al. 2003), can become manifest in a matter of weeks. Such induced phenotypic changes are superimposed upon genetic-based changes in defenses that develop over evolutionary time scales between a predator and its prey (Vermeij 1987; Prezant et al. 2006). For example, Leonard et al. (1999), Smith and Jennings (2000), and Reimer and Harms-Ringdahl (2001) have all observed that blue mussels Mytilus edulis exposed to green shore crabs Carcinus maenas increase the thickness and weight of their shell, a response interpreted as rendering the individual less susceptible to predatory attack. Thus, we tested the hypothesis that diploid juveniles of the two species of oyster grown in the presence of crustacean predators would exhibit differential changes in shell composition that enhanced shell strength.
Methods
Experimental oysters
The C. ariakensis larvae used to produce spat were provided by Taylor Shellfish Farms at Quilcene, Washington. The C. virginica larvae came from the Virginia Institute of Marine Science Eastern Shore hatchery at Wachapreague, Virginia. Species identification for 50 spat originating from each brood of larvae was confirmed by PCR amplification of a mitochondrial 16S gene segment using primers 16sar and 16sbr and restriction digestion with MseI (Hare et al. 2000).
For experiments with flatworms as predators, we compared the susceptibility of the youngest and smallest oysters because these life stages of C. virginica are most vulnerable to flatworm predation (Newell et al. 2000). These small (<5 mm shell height) oysters are very difficult to enumerate when they are attached to oyster shell, the natural settlement material for oysters. Thus, for all studies with flatworms we allowed oyster larvae to metamorphose on slate plates because their flat and uniform surface makes the small spat more visible (Newell et al. 2000). For our studies of predation by crabs, we allowed diploid oyster larvae to metamorphose and grow on large pieces of eastern oyster shell.
Plates and shell were suspended in static containers containing eyed pediveliger larvae (∼10,000 l−1) of one oyster species or the other that were fed a mixture of cultured microalgae species until metamorphosis. After diploid C. ariakensis and C. virginica larvae had metamorphosed, plates and shell were transferred to flow-through ambient Choptank River, Maryland, water (salinity 9–11). All effluent water was subject to continuous chlorination to prevent the possible release to the estuary of any C. ariakensis gametes. Spat were held for various periods so that they grew to the desired sizes for use with the different-sized predators (Table 1). For the plates, all spat on one horizontal surface were removed and those on the other horizontal surface were culled to a density of about 1 spat cm−2. For the shell material, spat density was not excessive and so we did not find it necessary to remove spat from either surface of the shell.
The location of spat on each uniquely numbered plate and piece of shell was recorded by photographing with a digital camera just before the plate or shell was haphazardly allocated to either a predation or control treatment. This record of the position and number of spat was used to monitor survivorship over the course of the experiments. The shell size of each oyster (either planar shell area of the top valve or shell height [=the maximum linear dimension from umbo to distal margin]) was measured directly from these photographs with image analysis software (ImageJ®) linked to a digitizing pen (Graphire3 by Wacom®).
Predation experiments
Our experimental design involved comparisons of the mortality of similarly sized C. ariakensis and C. virginica held within the same aquarium and exposed to either the same groups of flatworms or an individual crab predator. Each aquarium was dosed daily with a mixture of cultured microalgae species to provide oysters with a maintenance ration. Groups of the same oysters were held as controls in identical conditions, but without added predators, to quantify mortality due to factors other than predation.
Due to natural variability in spat abundance on the various substrates, we could only place approximately the same number of oysters of each species within each aquarium. To prevent resulting small variations in number of spat eaten from complicating data analysis, we expressed the numbers of oysters eaten as a percentage of the number of that oyster species initially placed into each aquarium. These percentages were arcsine transformed (Sokal and Rohlf 1995) before being analyzed with one-way ANOVA to compare the relative susceptibility of C. ariakensis and C. virginica to each species of predator.
Crab predation studies
We collected two species of mud crabs, R. harrisii and E. depressus, from the mesohaline Choptank River and held them for a few days to a few weeks at ambient salinities (∼10 salinity) and temperatures (∼22 to 25°C) while feeding them ad libitum on eastern oyster spat. We collected two species of mud crabs, P. herbstii and D. sayi, and the blue crab C. sapidus from polyhaline environments (salinity 24–28 and temperatures 23–25°C) near Ocean City, Maryland and acclimated over a 3-day period to holding conditions of 18 salinity and 25°C, while feeding them ad libitum on eastern oyster spat. Crab carapace width and total live wet weights were measured at the beginning of each experiment (Table 2).
Predation studies were performed in aquaria (21 l) maintained at ambient salinity and temperature. Before each feeding study, each crab was transferred to its respective aquarium and starved for 24 h before oysters were added and the studies begun. Sufficient pieces of shell were added haphazardly to each aquarium to provide ∼10 comparably sized spat of each oyster species of the required size-range. Digital photographs were taken of all spat at the start and end of each 30-day predation study and examined to determine mortality over the period.
Flatworm predation studies
We collected flatworms of different sizes from the Choptank River. There are two species of the Order Polycladida in Chesapeake Bay, S. ellipticus and E. gracilis, with the main distinction being that eyespots on the anterior margin of S. ellipticus are not present on E. gracilis. Because these markings are not apparent in microscopic S. ellipticus, we could not identify some flatworms to species and so in this paper we refer to all microscopic flatworms by the common name; the macroscopic flatworms were identified as S. ellipticus. Microscopic flatworms (surface area ≲5 mm2) were collected by holding juvenile eastern oysters in the river within mesh cages (400 μm mesh size) that allowed microscopic flatworms but not large predators to access the spat (Newell et al. 2000). Cages were retrieved after 72 h, and the contents, including any microscopic flatworms, were rinsed with ambient saltwater into a 4 l beaker.
Because microscopic flatworms are essentially invisible to the naked eye and very difficult to locate and catch, we did not collect and add an exact number of these flatworms to each treatment. Instead, we added aliquots of the water containing flatworms from the cages to aquaria held at ambient conditions (salinity 10; temperature 25°C) in the dark. Although this procedure will not result in exactly the same number of flatworms being added to each replicate, this was not a concern because we were only interested in the relative mortality of each oyster species and not absolute rates of mortality. Oysters of both species attached to slate plates were placed in approximately equal numbers and of various sizes (Table 1) into these aquaria. At intervals after the start of the experiment, each plate was examined under a dissecting microscope mounted such that the pan contents were not disturbed. We could clearly discern microscopic flatworms feeding on these spat but no other predatory species were observed feeding on oysters. Feeding trials lasted for ∼30 days, with photographs taken at the start and end of the experiment being used to estimate oyster mortality.
Macroscopic S. ellipticus (∼14 to 88 mm2) were collected individually by hand sorting through juvenile eastern oysters obtained from the Choptank River. These flatworms were photographed and their body area measured using image analysis software. Body area is the best indicator of flatworm size because of their amorphous shape. The experimental protocol was identical to that described for microscopic flatworms except that a known number of flatworms were added to each aquarium containing the two species of oysters set on slate plates.
Shell strength experiments
We tested the hypothesis that both species of oysters would respond to the presence of the effluent (infochemicals) from crab predators feeding on congeneric oysters by altering the composition of their shell and thereby increasing shell strength. Oysters were held from June 22 through August 14, 2005 in 6 l aquaria supplied with flowing (3.6 l h−1) ambient Choptank River water (salinity ∼10; temperature 22–25°C). Eighteen aquaria held individual C. ariakensis (range of shell height 13–27.8 mm, mean = 17.2 mm, SD = 2.6) and 18 aquaria held individual C. virginica (range of shell height 9.2–27.6 mm; mean = 17.2 mm, SD = 3.7) protected within plastic-mesh cages (3.2 mm mesh opening) from crab predators.
Because we were studying diploid oysters, these experiments were conducted under laboratory quarantine conditions rather than in the field, where oysters of both species would have been exposed to natural abundances of crustacean predators. In these laboratory studies, we did not try to approximate a natural field abundance of predators per unit area. Instead, we compared the response between oyster species when exposed to a similar crab biomass. To each of six of the aquaria holding C. ariakensis and six holding C. virginica we added a single small blue crab C. sapidus (carapace width 26–51 mm). Another six of the C. ariakensis aquaria and six of those holding C. virginica had R. harrisii mud crabs (carapace width 8–13 mm) added, ranging from 3 to 5 crabs per aquaria depending on crab size (i.e., five smaller mud crabs or three larger mud crabs). Finally, six aquaria of C. ariakensis and six of those holding C. virginica were maintained as controls with no added predators. Twice a week, the crabs were fed ad libitum by adding to each aquarium C. ariakensis or C. virginica tissue, to match the oyster species being tested in that treatment.
After 54 days of experimental treatment, some oysters from each aquarium were narcotized in seawater that had been bubbled for 12 h with carbon dioxide. The adductor muscle of gaping oysters was cut and each oyster placed individually in Petri dishes and sufficient dilute hydrogen peroxide (15% H2O2 in deionized water) added to cover the oyster. The procedure completely digested the tissue within 12 h, after which each shell was rinsed with deionized water, dried at 80°C for 24 h, and weighed. Each valve was photographed and image analysis was used to measure the planar surface area of the upper (right) valve. We then calculated the shell density (valve surface area/valve total dry weight; mg cm−2) for each top valve.
These same top valves used to determine dry weight were then analyzed for total organic content using the weight-loss-on-ignition procedure (Goulletquer and Wolowicz 1989; Prezant et al. 2006) to determine if there were differences between C. ariakensis and C. virginica. Clean glass beakers (50 ml) were heated at 550°C for 1 h to burn off any residual matter, cooled in a desiccator, and weighed. An upper valve was placed in each beaker, which was then dried at 80°C for 1 h, cooled in a desiccator, and reweighed. Beakers containing top valves were then heated at 550°C for 9 h, after which they were cooled in a desiccator, and reweighed. The loss in weight between valves at 80°C and at 550°C was used as an estimate of shell organic content.
We used an Instron® load compression instrument to determine the shell breaking strength for four live oysters of each species from each aquarium, measured as the force (Newtons) required to just penetrate the upper valve by a blunt metal point (2 mm2 surface area). Oysters of both species grown individually on slate plates were used for this test because the flat undersurface of the plate facilitated holding them securely in the instrument. Also, the shells of oysters grown on flat slate are more symmetrical and uniform than those grown on the uneven surface of oyster shell. This uniformity of shell shape allowed us to position the metal point consistently on the widest (=highest) part of the shell which was located just anteriorally of the center of the shell. The area of the top valve of each oyster was measured from a digital photograph.
The shell density, compression strength, and percent organic content for the two species of oysters exposed to either C. sapidus or R. harrisii was compared statistically to their appropriate controls by analysis of variance. Each of the six aquaria for the two crab treatments and the six control aquaria maintained without crabs were independent from the others in the experiment but the oysters within an aquarium were not independent. Data for individual oysters in each aquarium for the individual treatments were first analyzed to determine equality of variances using nested analysis of variance with the Bonferroni multiple-comparison test. Individual aquaria within treatments found to have unequal variances were removed (never more than two) from further analysis. A nested analysis of variance was then used to compare variance among treatments, with aquaria nested within their respective treatments. Because the data on percent shell organic content were not normally distributed, all values were arcsine transformed before statistical analysis (Sokal and Rohlf 1995) and back-transformed to percentage values for interpretation.
Results
Selective predation studies
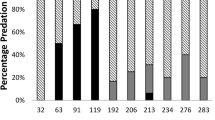
All five crab species preyed on a range of sizes of both species of oysters (Table 1). Crabs ate oysters both larger and smaller than the mean oyster size made available as prey items for each species, with no consistent pattern of predation on different sizes within one species of oyster compared with the other. All five crab species fed to a significantly (Table 3; P < 0.05) greater degree on C. ariakensis compared to C. virginica when offered similarly sized spat (Table 1). By contrast, there were no significant differences in predation by microscopic and macroscopic flatworms, which fed with similar intensity on both species of oysters (Table 3). For the control oysters, maintained in identical conditions but without added predators, there were no significant differences (ANOVA; P = 0.05) in species-specific mortality during these experiments.
Shell strength study
Crassostrea virginica unexposed to crab predators had a mean shell compression strength of 51.1 N and shell density of 131.6 mg cm−2 (Table 4), both of which were significantly greater (Tables 4, 5; P = 0.005 and P < 0.0001, respectively) than the values (18.5 N and shell density of 64.2 mg cm−2) for similarly sized C. ariakensis. C. ariakensis exposed to C. sapidus for 54 days, but not those exposed to R. harrisii, showed a significant increase (P < 0.039) in shell density compared to the controls. This increase in density translated into a significant increase (P = 0.002) in compression strength, which doubled from 18.5 to 35.5 N (Tables 4, 5). This pattern was different in C. virginica, where individuals exposed to either R. harrisii or C. sapidus did not exhibit significant changes in shell density compared to the control oysters (Tables 4, 5). Despite this lack of increase in shell density, individual C. virginica exposed to either C. sapidus or R. harrisii exhibited significantly enhanced shell strength of 83.4 N (P = 0.01) and 71.4 N (P = 0.04), respectively, compared to 51.1 N for controls.
The percentage organic content of the shell of control C. ariakensis, determined by weight loss on combustion at 550°C, was 2.95%, which was significantly higher (Tables 4, 5; P = 0.006) than the value of 1.35% for C. virginica (Tables 4, 5). The percent shell organic content of the shells of C. ariakensis exposed for 54 days to C. sapidus declined, although not significantly, to 2.01% (P = 0.067). For C. ariakensis exposed to R. harrisii, shell organic content declined significantly to 1.9% (P = 0.027), compared to the control oysters. This pattern of declining organic content was exactly the opposite for C. virginica exposed for 54 days to the two species of crabs. For C. virginica exposed to R. harrisii, the shell organic content increased significantly (P = 0.004) to 2.27% although the shell organic content of 1.78% for oysters exposed to C. sapidus did not differ significantly (P = 0.074) from the controls.
Discussion
Post-settlement predation is generally recognized as an important factor in regulating the abundance of C. virginica populations (e.g., Osman and Abbe 1994; White and Wilson 1996; Newell et al. 2000). We investigated if diploid C. ariakensis juveniles are as equally vulnerable as diploid C. virginica juveniles to predatory invertebrates native to mesohaline and polyhaline environments in the mid-Atlantic region of North America. This is an important consideration because managers in Chesapeake Bay are evaluating a series of options for increasing oyster stocks. One option involves the deliberate release of diploid C. ariakensis to establish a self-recruiting population (NRC 2004). A slightly more conservative option being considered involves aquaculture of triploid C. ariakensis (Allen 2005). Because triploid oysters do not produce gametes, they are reproductively sterile and some proponents suggest that their use will not lead to the establishment of feral C. ariakensis populations. Unfortunately, the procedure for breeding triploid oysters still allows ∼0.1% of the spat to be reproductively normal diploids (Allen 2005). Furthermore, as triploid oysters grow older, a small percentage can produce haploid gametes through the process of mosaic reversion (NRC 2004). Thus, if triploids from commercial aquaculture farms are left unharvested in nature, perhaps by being lost from storm-damaged cages, there is the potential for introducing diploid oysters by such reversion. A major concern associated with either option is that if C. ariakensis were to escape predator control it could become highly abundant, perhaps to the point of becoming a nuisance species.
We compared the relative susceptibility of C. ariakensis and C. virginica to predation from a time soon after metamorphosis until the spat had attained a shell height of ∼25 mm. For field populations of C. virginica in Chesapeake Bay, larval settlement occurs predominately from late June though early September and, under optimal conditions, spat can grow to a shell height of ∼25 mm by November (Kennedy 1996). In a comparative study of diploid C. ariakensis and C. virginica growth in quarantine mesocosm tanks supplied with flowing seawater (salinity ∼10), C. ariakensis grew in shell height at almost twice the rate of C. virginica between metamorphosis and about mid-November (Newell, unpublished observation). In autumn, when water temperatures declined below ∼ 8°C, C. virginica ceased to grow but C. ariakensis continued to increase in shell size until water temperatures declined below ∼ 4°C. This faster rate of shell growth will enable C. ariakensis to attain a size refuge from attack by the small predators we tested sooner than C. virginica. However, C. ariakensis will still be in the most vulnerable size category of <25 mm shell height for at least several months post-settlement. This period between summer and mid-autumn is a period when water temperatures are warm enough to permit vigorous foraging by the predators we studied.
Crab predation studies
Mud and blue crabs are important predators of C. virginica juveniles (see White and Wilson 1996). We found that five known crab predators of C. virginica, the euryhaline blue crab C. sapidus, the mesohaline mud crabs R. harrisii and E. depressus, and the polyhaline mud crabs P. herbstii and D. sayi, fed significantly (Table 3; P < 0.05) more on C. ariakensis than on C. virginica. Our experiments involved comparative choice studies with diploid C. ariakensis and C. virginica held within the same aquarium and exposed to the same individual crab. This design avoided possible complications (e.g., those caused by differences in degree of hunger, or phase of the crab molt cycle, etc.) that can occur in studies where prey species are tested separately. All spat tested were from larvae allowed to metamorphose and grow on large oyster shells to mimic the conditions on natural oyster bars if C. ariakensis were to start breeding in Chesapeake Bay. By rearing both species of oysters under the same conditions, we eliminated the possibility that environmental differences would alter their shell characteristics and thereby change their vulnerability to predators. We also found that the four species of mud crabs used in our experiments could open C. ariakensis that had a shell height greater than the crab’s carapace width (unpublished data). These results are consistent with studies by McDermott and Flower (1953) and Bisker and Castagna’s (1987) of P. herbstii feeding on a size range of C. virginica. We conclude that if C. ariakensis were introduced into Chesapeake Bay, juveniles would be highly vulnerable to the various mud crab species and the blue crabs that are common members of an oyster reef ecosystem.
We specifically avoided evaluating the vulnerability to crab predation of single oysters that are not attached to a large piece of oyster shell. Such “cultchless” oysters can suffer almost total mortality from blue crab predation because crabs can more easily crush the oyster’s shell in the absence of the protection afforded by a large piece of shell substrate (Krantz and Chamberlin 1978; Bisker and Castagna 1987). Bishop and Peterson (2006) used such “cultchless” oysters in their laboratory study of blue crab predation on larger (25–35 mm shell height) triploid C. ariakensis and diploid C. virginica. They recognized that oysters living in natural aggregations attached to shell might exhibit different susceptibility to predators than single “cultchless” oysters. As justification of their experimental protocol, they cited field observations and preliminary laboratory studies indicating that C. ariakensis typically lives as single individuals, in contrast with C. virginica whose gregarious settling behavior permits massive reef building. This justification is not supported by our own ongoing laboratory studies in mesohaline mesocosms in which we are comparing the reef-building characteristics of diploid C. virginica and C. ariakensis (Newell, unpublished observation). Over a 3-year period, we have found that cohorts of diploid larval C. ariakensis that were allowed to settle and metamorphose on the same eastern oyster shell (10 cm deep layer of shells with an overall area of 0.8 m2) form clusters that are essentially indistinguishable from those formed by C. virginica in the same study.
Shell strength study
One likely reason for the significantly greater consumption of C. ariakensis exhibited by all five species of crabs we tested is that the shells of these non-native oysters were significantly weaker (P = 0.005) than those of similarly sized C. virginica (Table 5). The compression force required to break the right valve of living oysters that had never been exposed to crab predators was 18.5 N for C. ariakensis, which was 64% lower than the force (51.1 N) to break the valves of C. virginica of a comparable size (Tables 4, 5). This differential shell strength between oyster species stems from the fact that C. ariakensis had a shell density of 64.2 mg cm−2, a value 51% lower (significant at P < 0.0001) than the density of 131.6 mg cm−2 for comparably sized C. virginica. Shell thickness is generally recognized to be a good predictor of strength in bivalves because a thick shell is an obvious means to thwart predation (e.g., Vermeij 1987; Leonard et al. 1999; Smith and Jennings 2000; Zuschin and Stanton 2001). A thinner shell means that crabs can more easily access the tissue of C. ariakensis than C. virginica by directly crushing the shell valves.
The right (upper) valve of specimens of C. ariakensis that had never been exposed to crab predators contained 2.95% organic matter, which was significantly greater (P = 0.006) than the 1.35% present in comparably sized C. virginica right valves. For consistency, we only analyzed the composition of the right valve, which is also the valve we used to determine compression strength. The right valve is the one mainly subject to crab attack because the left valve is generally protected by the large piece of shell that oysters typically attach to in the natural environment. Such consistency in analysis is important because, at least in C. virginica, the right valve has greater organic content than the more densely mineralized left valve (Carriker 1996). Our experimental protocol involved pre-treating shells with 15% hydrogen peroxide to digest and remove body tissue before measuring organic content on the right valve using the weight-loss-on-ignition procedure. This pre-treatment also removed other external organic material, including the hinge ligament and the proteinaceous periostracum that covers the external shell surface (Carriker 1996). Thus, we believe that our values for shell organic content represent the conchiolin organic matrix internal to the shell (Carriker 1996) and hence we expected them to be slightly lower than published values for total shell organic content (i.e., shells that had not been pretreated to remove external organic material and the hinge ligament) in C. virginica. As expected, Thompson and Chow (1955) reported slightly higher organic content values (2.2 and 2.3%) than we found for two individual specimens of C. virginica, and Price et al. (1976) found values of 3.04 ± 1.16% (SD, n = 50).
Goulletquer and Wolowicz (1989) cautioned that the weight-loss-on-ignition procedure might overestimate shell organic content by a factor of ∼2 to 5 compared with chemical extraction procedures. For the purposes of our comparative study of changes in shell organic content in response to infochemicals from crab predators, the absolute value for the shell organic content that we report here is less important than the relative changes in organic content both within a species and between oyster species.
It has been suggested that higher amounts of organic material in the matrix of mollusc shells may confer increased resistance to fracturing because this conchiolin contributes protective flexibility to the shell (Taylor and Layman 1972; Carter 1980; Zuschin and Stanton 2001; Prezant et al. 2006). Based on an extensive literature review, Carriker (1996) states that, although there is no obvious correlation between the organic content of molluscan shells and shell strength, the microhardness of molluscan shell is higher than would be expected based on inorganic calcite and aragonite alone. Carriker (1996) concludes that the combination of organic layers and inorganic minerals found in molluscan shell contributes to attributes of microhardness and pliability not present in nonbiogenic polymorphs of calcium carbonate. We found that the shell of C. virginica, despite the fact that it has a lower organic content than that of C. ariakensis, was twice as dense and could resist more than twice the compressive force as could C. ariakensis. This pattern suggests that, even though shell organic content may be useful in reducing fractures, investing energy in producing a more massive shell is perhaps a more certain strategy for increasing a species’ resistance to predation. Palmer (1981) hypothesized that, for bivalves in general, faster-growing individuals should have thinner shells than slower-growing individuals because calcium carbonate deposition in molluscs is a rate-limited rather than an energy-limited process. The fact that we observed that diploid C. ariakensis grow at a rate that is about twice that of C. virginica (see above) and possess a thinner and less dense shell than C. virginica is consistent with this hypothesis.
When we exposed C. virginica and C. ariakensis for 54 days to R. harrisii and C. sapidus fed on conspecific oysters, both species of oyster showed a marked but very different response. Importantly, the induction of these changes in shell characteristics was not dependent on physical contact between the crab predator and the oysters, as the only contact between them was through water-borne infochemicals, either enemy-avoidance kairomones from the presence of the crab or alarm pheromones from damaged and ingested conspecifics. Shell compression strength of C. virginica exposed to both species of crabs increased significantly (P < 0.05) to ∼25% greater than controls, although shell density did not change significantly (Tables 4, 5). Shell organic content for C. virginica exposed to crabs tended to increase, although the magnitude of this change was only significant (P = 0.004) in the R. harrisii treatment. Shell compression strength of C. ariakensis exposed to both species of crabs also tended to increase, although the magnitude of this change was only significant (P = 0.002) in the C. sapidus treatment. This latter treatment increased shell compression strength by almost 100% compared to controls. Interestingly, changes in C. ariakensis shell composition were the converse of those observed for C. virginica. C. ariakensis exposed to C. sapidus but not R. harrisii grew significantly denser (P = 0.039) shells. In C. ariakensis exposed to R. harrisii and C. sapidus, the shell organic content declined to 1.9% (significant at P = 0.027) and 2.01% (not significant), respectively, which was ∼33% lower than the 2.95% found in control oysters (Tables 4, 5).
These results indicate that, in the presence of crab predators, C. ariakensis responded by growing a denser shell which, as discussed above, is a widely occurring strategy in bivalve molluscs for reducing susceptibility to shell-crushing predators. The reduction in percent organic content we observed in C. ariakensis shells may simply be an incidental consequence of changes in the magnitude of mineral deposition; alternatively, it could be due to repartitioning of energy to cover costs associated with forming this denser shell. Conversely, C. virginica responded by increasing percent shell organic content with no concomitant reduction in shell density. This increase in shell organic content in a shell that was already quite robust seemed to confer greater resistance to shell crushing without building an even more massive shell. Remarkably, these two very different responses of each oyster species both served to enhance the ability of their right valves to resist a compression force to a similar degree. Nevertheless, even after being held for 54 days in the presence of C. sapidus the shell of C. ariakensis still remained ∼57% weaker than C. virginica.
It is possible that the oysters used in our shell strength study had been exposed to crab infochemicals while being reared. All oysters had been held for 1 year in flowing ambient water pumped from the Choptank River while they were grown to a suitable size. This flowing seawater obviously contained some low level of crab infochemicals associated with the natural abundances of mud and blue crabs in the Choptank River. All our studies included control oysters not exposed experimentally directly to crabs nor any damaged conspecifics. These control oysters could only have been responding to possible background levels of crab infochemicals in the flowing seawater. All changes in shell strength or composition we discuss as being induced by the presence of crabs were statistically compared to those for control oysters (Tables 4, 5).
It is likely that the oysters used in the study of predator induction of defense responses were exposed to elevated concentrations of infochemicals in the experiments compared to what might be present in nature. Our experiments with diploid non-native oysters had to conducted under laboratory quarantine conditions, so oysters were not exposed to natural abundances of crab predators under normal field conditions of water flow and hence infochemical dilution. This same caveat applies to similar previous laboratory studies of induction of changes in mollusc shell composition in response to predators (e.g., Leonard et al. 1999; Prezant et al. 2006). However, the laboratory results obtained by Leonard et al. (1999) matched the pattern they observed in the field of increased shell thickness in blue mussels subject to greater crab predation. This confirmation between field observation and laboratory results suggests that the pattern of change in shell composition and strength we observed in our laboratory studies, even if not the absolute magnitude of the response, will likely apply to naturalized stocks of C. ariakensis.
We did find that the response of each species of oysters to the crabs from two different genera we tested was similar, although the type of response elicited differed appreciably between oyster species. Our experimental protocol did not include treatments to determine if the responsible infochemical was an enemy-avoidance kairomone emitted directly by the two species of crab or was an alarm pheromone released from the damaged conspecific oysters used to feed the crabs. Leonard et al. (1999) reported a greater increase in shell thickening of blue mussels in response to the presence of damaged conspecifics (14–42% shell thickening) than to crab predators (10–16%). However, this response pattern is not universal in bivalves as Cheung et al. (2004) found changes in shell growth in the green mussel Perna viridis only in response to predators and not to damaged conspecifics. The response we observed in both species of oyster, induced within a period of just 54 days, to some infochemical signifying an immediate threat of predatory attack, seems highly adaptive and perhaps capable of reducing that individual’s vulnerability to predation.
These adaptive changes in shell composition were expressed in the non-native C. ariakensis despite the fact that it shares no evolutionary history with these two species of Atlantic coast crabs. The oyster larvae we used came from the “Oregon” stock maintained entirely on the Pacific coast of the USA by Taylor Shellfish Company in Washington. Although the exact details concerning the introduction of C. ariakensis to the USA from Asia are not fully known, they were first found in the late 1960s among C. gigas oysters being cultured in Yaquina Bay, OR (Breese and Malouf 1977; Malouf, Oregon Sea Grant, personal communication). Because C. ariakensis responded rapidly to novel species of crabs feeding on congeners, it suggests that oysters have the ability to recognize some enemy-avoidance kairomones that are generic cues of potential crab predation or to alarm pheromones from damaged and ingested conspecifics. We suggest that such generic recognition of the threat of crab predation may be highly adaptive because in the native habitat of C. ariakensis in China, crabs, including portunid crabs (to which family the blue crab we tested belongs) are reported to be major predators on oysters (Zhou and Allen 2003). Further research will be required to isolate and characterize the exact nature of this infochemical cue.
The two oyster species exhibited markedly different changes in shell organic content in response to infochemicals from crab predators and damaged conspecifics. For C. virginica, both the organic content of the shell and the shell strength increased; conversely, C. ariakensis reduced shell organic content while shell strength increased. Prezant et al. (2006) found that when adult viviparous freshwater gastropods Bellamya chinensis were held in the presence of a predatory crayfish, there was an increase in numbers of juveniles released; these juveniles were smaller but possessed a significantly higher shell organic content. Prezant et al. (2006) suggested that possible explanations for this response might be that higher shell organic content either confers increased resistance to crustacean attack or maintains shell integrity after an incomplete attack. Obviously, changes in shell organic content in response to predatory attack are not necessarily the same among species and further research on this topic is required before generalizations can be made.
Many species of crabs, including blue crabs, will resort to peeling away the valve margins to expose the tissue, or prying open the valves with their chelae if the prey is large or the shell is too strong to crush directly (Vermeij 1987; Seed and Hughes 1995; White and Wilson 1996). We do not know if the changes we observed in shell compression strength and composition serve to protect larger oysters from crustacean predators using these peeling or prying techniques rather than direct crushing and shell compression techniques.
Our observation that all species of crab we tested selected C. ariakensis more often than C. virginica in choice experiments is consistent with the concurrent work of Bishop and Peterson (2006). These authors studied C. sapidus predation on single cultchless triploid C. ariakensis (>25 mm shell height) under controlled laboratory conditions and found that these oysters were consumed to a greater extent compared to similarly-sized cultchless diploid C. virginica. Bishop and Peterson (2006) also reported that the compression strength of the disarticulated right (upper) valve dried at 60°C was significantly greater in diploid C. virginica than in triploid C. ariakensis. Such comparisons between oysters of different ploidy must be interpreted with some caution, however, because triploid individuals partition less energy to reproductive processes and thus grow faster than diploids (Allen and Downing 1986). Such differences in growth rate may affect shell strength because faster-growing individuals tend to have thinner shells than slower-growing individuals (Palmer 1981). Moreover, if C. ariakensis were to be deliberately released in nature, or if some triploids reverted to the diploid state, then predators would be feeding on feral diploid individuals with their inherent growth characteristics and not on triploid individuals.
Flatworm predation study
Numerous studies have shown that flatworms are important predators of C. virginica (Webster and Medford 1961; Landers and Rhodes 1970; Christensen 1973; Newell et al. 2000). In our controlled laboratory studies we found that microscopic and macroscopic flatworms fed with the same high intensity on C. ariakensis as they did on C. virginica. This similarity in vulnerability between oyster species, despite their differences in shell strength, stems from the fact that flatworms gain access to oyster tissue by entering between the shell valves when the oysters are feeding and the valves are gaping (Loosanoff 1956).
Summary
We found that rates of predation by common invertebrate predators on <25 mm C. ariakensis were either similar to or higher than on C. virginica. We conclude, therefore, that the abundance of feral C. ariakensis in Chesapeake Bay will likely be controlled by the natural suite of predators. Furthermore, even if the faster-growing C. ariakensis reaches a size refuge from the smaller predators sooner than the slower-growing C. virginica, C. ariakensis will still be exposed to high rates of predation from the time of metamorphosis until its first winter. It must be recognized that these conclusions are based on studies performed under highly controlled conditions. In the natural environment along the Atlantic or Gulf coasts of North America, the situation may be sufficiently different in some way that allows naturalized populations of C. ariakensis to compensate for the species’ significantly greater vulnerability to crab predation. For example, if C. ariakensis were to have higher fecundity and larval survival, leading to greater recruitment success than C. virginica, then the species may still escape predator control. Further, in ongoing studies we have found that C. virginica is more susceptible to predation by oyster drills and starfish than is C. ariakensis. This could allow C. ariakensis to gain a competitive advantage in marine and polyhaline locations where such predators are abundant. Moreover, in some locations, predation by benthic predators may be low, thus allowing C. ariakensis to have such rapid population growth that they become ecologically and economically disruptive pests (e.g., Carlton 1999).
References
Abbe GR, Breitburg DL (1992) The influence of oyster toadfish (Opsanus tau) and crabs (Callinectes sapidus and Xanthidae) on survival of oyster (Crassostrea virginica) spat in Chesapeake Bay: does spat protection always work? Aquaculture 107:21–31
Allen SK (2005) Stalemate over the new oyster. Va Mar Res Bull 37:2–16
Allen SK, Downing SL (1986) Performance of triploid Pacific oysters, Crassostrea gigas (Thunberg). 1. Survival, growth, glycogen-content, and sexual maturation in yearlings. J Exp Mar Biol Ecol 102:197–208
Bishop MJ, Peterson CH (2006) When r-selection may not predict introduced-species proliferation: predation of a non-native oyster. Ecol Appl 16:718–730
Bisker R, Castagna M (1987) Predation on single spat oysters Crassostrea virginica (Gmelin) by blue crabs Callinectes sapidus Rathbun and mud crabs Panopeus herbstii Milne-Edwards. J Shellfish Res 6:37–40
Breese WP, Malouf RE (1977) Hatchery rearing techniques for the oyster Crassostrea rivularis Gould. Aquaculture 12:123–126
Carlton JT (1999) Molluscan invasions in marine and estuarine communities. Malacologia 41:439–454
Carlton JT, Thompson JK, Schemel LE, Nichols FH (1990) Remarkable invasion of San Francisco Bay (California, USA) by the Asian clam Potamacorbula amurensis. I. Introduction and dispersal. Mar Ecol Prog Ser 66:81–94
Carriker MR (1996) The shell and ligament. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster, Crassostrea virginica. Maryland Sea Grant Publication, College Park, pp 75–168
Carter JG (1980) Environmental and biological controls of bivalve shell mineralogy and microstructure. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms: biological records of environmental change. Plenum Press, New York, pp 69–113
Cheung SG, Lam S, Gao QF, Mak KK, Shin PKS (2004) Induced anti-predator responses of the green mussel, Perna viridis (L.), on exposure to the predatory gastropod, Thais clavigera Küster, and the swimming crab, Thalamita danae Stimpson. Mar Biol 144:675–684
Christensen DJ (1973) Prey preference of Stylochus ellipticus in Chesapeake Bay. Proc Natl Shellfish Assoc 63:35–38
Cloern JE (1982) Does the benthos control phytoplankton biomass in South San Francisco Bay? Mar Ecol Prog Ser 9:191–202
Eggleston DB (1990) Foraging behavior of the blue crab, Callinectes sapidus, on juvenile oysters, Crassostrea virginica: effects of prey density and size. Bull Mar Sci 46:62–82
Ford SE, Tripp MR (1996) Diseases and defense mechanisms. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster, Crassostrea virginica. Maryland Sea Grant Publication, College Park, pp 581–660
Goulletquer P, Wolowicz M (1989) The shell of Cardium edule, Cardium glaucum and Ruditapes philippinarum: organic content, composition and energy value, as determined by different methods. J Mar Biol Assoc UK 69:563–572
Hare MP, Palumbi SR, Butman CA (2000) Single-step species identification of bivalve larvae using multiplex polymerase chain reaction. Mar Biol 137:953–961
Kennedy VS (1996) Biology of larvae and spat. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster, Crassostrea virginica. Maryland Sea Grant Publication, College Park, pp 371–421
Kennedy VS, Breisch LL (1983) Sixteen decades of political management of the oyster fishery in Maryland’s Chesapeake Bay. J Environ Manage 16:153–171
Kimmerer WJ, Gartside E, Orsi JJ (1994) Predation by an introduced clam as the likely cause of substantial declines in zooplankton in San Francisco Bay. Mar Ecol Prog Ser 113:81–93
Krantz GE, Chamberlin JV (1978) Blue crab predation on cultchless oyster spat. Proc Natl Shellfish Assoc 68:38–41
Landers WS, Rhodes EW (1970) Some factors influencing predation by the flatworm, Stylochus ellipticus (Girard), on oysters. Chesapeake Sci 11:55–60
Lee T, Siripattrawan S, Ituarte CF, Ó Foighil D (2005) Invasion of the clonal clams: Corbicula lineages in the New World. Am Malacol Bull 20:113–122
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14
Loosanoff VL (1956) Two obscure oyster enemies in New England waters. Science 123:1119–1120
McDermott JJ, Flower FB (1953) Preliminary studies of the common mud crabs on oyster beds of Delaware Bay. Proc Natl Shellfish Assoc 1952:47–50
Milke LM, Kennedy VS (2001) Mud crabs (Xanthidae) in Chesapeake Bay: claw characteristics and predation on epifaunal bivalves. Invert Biol 120:67–77
Nalepa TF, Schloesser DW (1993) Zebra mussels. Biology, impacts, and control. Lewis Publishers, Boca Raton
Newell RIE, Alspach GS, Kennedy VS, Jacobs D (2000) Mortality of newly metamorphosed eastern oysters (Crassostrea virginica Gmelin) in mesohaline Chesapeake Bay. Mar Biol 136:665–676
Newell RIE, Fisher TR, Holyoke RR, Cornwell JC (2005) Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In: Dame R, Olenin S (eds) The comparative roles of suspension feeders in ecosystems, vol 47. NATO Science Series: IV—Earth and environmental sciences. Springer, Netherlands, pp 93–120
NRC (National Research Council) (2004) Nonnative oysters in the Chesapeake Bay. The National Academies Press, Washington, District of Columbia
Osman RW, Abbe GR (1994) Post-settlement factors affecting oyster recruitment in the Chesapeake Bay, USA. In: Dyer K, Orth RJ (eds) Changes in fluxes in estuaries: implications from science to management. Olsen and Olsen, Fredensborg, pp 335–340
Palmer AR (1981) Do carbonate skeletons limit the rate of body growth? Nature 292:150–152
Prezant RS, Chapman EJ, McDougall A (2006) In utero predator-induced responses in the viviparid snail Bellamya chinensis. Can J Zool 84:600–608
Price J, Thayer GW, LaCroix MW, Montgomery GP (1976) The organic content of shells and soft tissues of selected estuarine gastropods and pelecypods. Proc Natl Shellfish Assoc 65:26–31
Reimer O, Harms-Ringdahl S (2001) Predator-inducible changes in blue mussels from the predator-free Baltic Sea. Mar Biol 139:959–965
Ruther J, Meiners T, Steidle JLM (2002) Rich in phenomena—lacking in terms. A classification of kairomones. Chemoecology 12:161–167
Seed R, Hughes RN (1995) Criteria for prey size-selection in molluscivorous crabs with contrasting claw morphologies. J Exp Mar Biol Ecol 193:177–195
Seed R, Suchanek TH (1992) Population and community ecology of Mytilus. In: Gosling E (ed) The Mussel Mytilus: ecology, physiology, genetics and culture. Elsevier, Amsterdam, pp 87–169
Smith RJF (1992) Alarm signals in fishes. Rev Fish Biol Fish 2:33–63
Smith LD, Jennings JA (2000) Induced defensive responses by the bivalve Mytilus edulis to predators with different attack modes. Mar Biol 136:461–469
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. WH Freeman, New York
Stabell OB, Ogbebo F, Primicerio R (2003) Inducible defences in Daphnia depend on latent alarm signals from conspecific prey activated in predators. Chem Senses 28:141–153
Strayer DL, Caraco NF, Cole JJ, Findlay S, Pace ML (1999) Transformation of freshwater ecosystems by bivalves. A case study of zebra mussels in the Hudson River. Bioscience 49:19–27
Taylor JD, Layman M (1972) The mechanical properties of bivalve (Mollusca) shell structures. Paleontology 15:73–87
Thompson TG, Chow TJ (1955) The strontium-calcium atom ratio in carbonate-secreting marine organisms. Deep Sea Res 3(suppl):20–39
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton
Webster JR, Medford RZ (1961) Flatworm distribution and associated oyster mortality in Chesapeake Bay. Proc Natl Shellfish Assoc 50:89–95
White ME, Wilson EA (1996) Predators, pests, and competitors. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster, Crassostrea virginica. Maryland Sea Grant Publication, College Park, pp 559–579
Zhou M, Allen SK (2003) A review of published work on Crassostrea ariakensis. J Shellfish Res 22:1–20
Zuschin M, Stanton RJ (2001) Experimental measurement of shell strength and its taphonomic interpretation. Palaios 16:161–170
Acknowledgments
We are grateful to Ed Jones (Taylor Shellfish) for providing Suminoe oyster larvae; Mark Luckenbach (Virginia Institute for Marine Science) for providing eastern oyster larvae; Melissa Radcliffe (Horn Point Laboratory) for supplying cultured microalgae; John Thiravong (University of Delaware Center for Composite Materials) for providing technical assistance with the Instron instrument; Matt Hare (University of Maryland College Park) for performing genetic analysis for oyster species identification; Angela Freeman for initial technical assistance; George Abbe, Don Boesch, and Robert Prezant and two anonymous reviewers for constructive comments. This research was supported by award NA16RG2207/07-5-28068J from Maryland Sea Grant, National Oceanic and Atmospheric Administration and award NA04NMF4570425 from NMFS-NOAA non-native oyster research program. The experiments reported herein comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Thompson.
Rights and permissions
About this article
Cite this article
Newell, R.I.E., Kennedy, V.S. & Shaw, K.S. Comparative vulnerability to predators, and induced defense responses, of eastern oysters Crassostrea virginica and non-native Crassostrea ariakensis oysters in Chesapeake Bay. Mar Biol 152, 449–460 (2007). https://doi.org/10.1007/s00227-007-0706-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0706-0