Abstract
The turnover of plant biomass largely determines the amount of energy flowing through an ecosystem and understanding the processes that regulate turnover has been of interest to ecologists for decades. Leaf life span theory has proven useful in explaining patterns of leaf turnover in relation to resource availability, but the predictions of this theory have not been tested for macroalgae. We measured blade life span, size, thickness, nitrogen content, pigment content, and maximum photosynthetic rate (P max) in the giant kelp (Macrocystis pyrifera) along a strong resource (light) gradient to test whether the predictions of leaf life span theory applied to this alga. We found that shorter blade life spans and larger blade areas were associated with increased light availability. In addition, nitrogen and P max decreased with blade age, and their decrease was greater in shorter lived blades. These observations are generally consistent with patterns observed for higher plants and the prevailing theory of leaf life span. By contrast, variation observed in pigments of giant kelp was inconsistent with that predicted by leaf life span theory, as blades growing in the most heavily shaded portion of the forest had the lowest chlorophyll content. This result may reflect the dual role of macroalgal blades in carbon fixation and nutrient absorption and the ability of giant kelp to modify blade physiology to optimize the acquisition of light and nutrients. Thus, the marine environment may place demands on resource acquisition and allocation that have not been previously considered with respect to leaf life span optimization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The turnover of plant biomass is a key component determining the amount of energy flowing through an ecosystem. Biomass turnover in plants is intimately linked to the life span of their leaves and factors that influence leaf life span have been extensively studied in many terrestrial systems (Reich et al. 1997, 1999; Kikuzawa and Ackerly 1999; Wright et al. 2004; Kikuzawa and Lechowicz 2006). While stochastic processes, such as fire, herbivory, and severe weather, can severely damage or destroy leaves, the natural course of cellular degeneration and overgrowth by new leaves largely determines the life span of leaves (Kikuzawa 1991; Kikuzawa and Ackerly 1999).
A cost-benefit analysis approach is useful in predicting variation in leaf life span as a function of resource availability (Chabot and Hicks 1982; Kikuzawa 1991; Kikuzawa and Ackerly 1999). Predictions of the cost-benefit approach are consistent with differences in leaf traits observed within and among individuals of the same species and among species over wide gradients in resource availability and environmental conditions (Reich et al. 1999; Dennison and Alberte 1982; Vincent 2006). This approach considers leaf life span to be a result of maximizing lifetime leaf carbon gain given the leaf’s maintenance and construction costs as well as decreases in photosynthetic capacity (P max) as the leaf ages (Chabot and Hicks 1982; Kikuzawa 1991).
In general, increased structural investment in a leaf results in a longer life span, but reduces resources available for plant growth (Coley et al. 1985; Herms and Mattson 1992). For example, thicker leaves tend to have longer life spans, but lower levels of mass-standardized photosynthesis (Reich et al. 1997; Terashima et al. 2005). Thicker leaves are usually found in low resource conditions, as the plant must defend the investment made in its leaves, because they are not easily replaced (Coley et al. 1985). Similarly, patterns of pigment and nitrogen allocation within a canopy can depend on light gradients (Terashima et al. 2005). Studies of resource allocation within leaf canopies suggest that the optimal use of light and nutrient resources would be achieved from an inverse relationship between pigment and light, so that leaves at all levels of the canopy are simultaneously saturated with light (Terashima et al. 2005). In contrast, optimization models predict that more nitrogen will be found in sun leaves than shade leaves, to maximize leaf canopy photosynthesis (Dietz and Heber 1984; Raines 2003). Optimization models also predict higher nitrogen levels in younger leaves, and more rapid nitrogen reduction with age in shorter lived leaves (Escudero and Mediavilla 2003; Hikosaka 2005, 2010).
Despite significant progress made with respect to terrestrial plants and the generality of the theory used to explain leaf life span, there have been few attempts to apply this theory to other phototrophs. In particular, the extent that the life span of laminae in marine macroalgae is correlated to other traits predicted by leaf life span theory has not been examined, even though macroalgae support some of the most productive ecosystems in the world (Mann 2000). Large brown algae known as kelps (Laminariales) have convergently evolved a plant-like body plan and a vascular optimization strategy for transporting internal resources (Drobnitch et al. 2015). Key similarities between terrestrial plants and kelps in the need to transport and allocate resources to optimize performance suggest that leaf life span theory may be applicable to kelps.
The giant kelp Macrocystis pyrifera is well suited for investigating the effects of resource availability on the life span of its laminae (hereafter referred to as blades), which are its primary photosynthetic organ. A strong light gradient with depth and location in the kelp forest (Gerard 1984) provides ideal conditions to test the differential allocation of resources to tissue growth versus blade longevity in M. pyrifera. About 20 % of surface irradiance reaches a depth of 8 m on southern California reefs in the absence of giant kelp, compared to 1 % or less in the presence of a developed kelp canopy (Gerard 1984). Fronds in the interior of the kelp forest are often light-limited and exhibit morphological effects of resource limitation (Stewart et al. 2009). Blades (analogous to leaves) are formed from an apical meristem on an individual frond (analogous to a branch) that grows upwards toward the surface causing younger blades on a given frond to be distributed higher in the water column where light is more available (North 1971; Lobban 1978a; Graham et al. 2007). The amount of light required to saturate photosynthesis in blades varies with age and position in the water column (Wheeler 1980a).
The objective of this study was to determine whether spatial variability in light within a kelp forest affects the life span, size, thickness, nitrogen, and chlorophyll content of M. pyrifera blades. If leaf life span theory applies to giant kelp, then blades growing in higher light conditions (e.g., near the surface and at the forest edge) should have larger surface areas, shorter life spans, less structural investment per unit area (i.e., thinner), and less pigmentation, but higher percent tissue nitrogen content than blades in lower light conditions (near the bottom and in the interior of the forest). We also expect that if blade life span is internally regulated, indicators of photosynthetic performance (P max, blade nitrogen content) will decrease with increasing blade age, and this decrease will be more rapid in shorter lived blades. If blades are more commonly lost via disturbance (such as wave motion and herbivory), then reduction in performance indicators related to blade life span should be less pronounced. We used the natural variability in light that occurs from the sea surface to the sea floor as well as the effect of intraspecific shading to evaluate the response of M. pyrifera blades to different light environments and to separate the effects of depth and age on blade life span from other traits.
Materials and methods
Overview
Frond growth in M. pyrifera (hereafter referred to as Macrocystis) occurs primarily from its apical meristem, and to a lesser extent through internodal elongation (North 1971; Lobban 1978a, b). The effect of internodal elongation on the position of a blade in the water column is typically on the order of 1 m or less (North 1971), and most blades stay near the depth in which they were formed, except when elongation moves a blade into the canopy from just below the sea surface. After separation from the apical meristem, a blade increases in surface area and upon reaching its maximum size, it begins to senesce from the distal end until it erodes completely (Rodriguez 2015). We investigated the role of spatial variation in light on life span and other traits of Macrocystis blades in the Isla Vista kelp forest near Goleta, CA (34°24′18″N, 119°52′05″W). The study site was located on a relatively flat sandstone platform at 7 m depth. Two primary sources of spatial variation in light exist in a kelp forest—light extinction through the water column, and shading by the kelp canopy. To separate the effects of depth and shading, we sampled blades from midwater and in the canopy at the edge of the forest (where canopy shading is much reduced), and in the interior of the forest (where canopy shading is substantial).
Blade area and life span
We tracked individual blades in situ from June to October 2012 to measure changes in their area and life span. Twenty mature plants consisting of 10–50 fronds were haphazardly chosen along a transect oriented along the offshore edge of the kelp forest (hereafter referred to as the forest edge). Likewise, another 20 mature plants were chosen from a parallel transect located 10 m inshore of the edge of the forest in an area with dense kelp cover (hereafter referred to as the forest interior). A single young frond measuring ~75 cm in total length was selected from each of the 40 plants. We measured weekly changes in area of the 2nd and 6th blades from the base of each frond (hereafter referred to as midwater blades) and the 40th blade (hereafter referred to as canopy blades) soon after they were formed and separated from the apical meristem. Measurements continued until the blades had senesced to less than 10 % of the maximum length or until the frond was lost from the holdfast. This sampling design accounted for internodal elongation and ensured that the blades spent their entire lifetime in either the midwater or surface canopy. It also allowed us to capture growth and senescence over the entire life span of blades occupying different positions in the water column.
We measured the blade length as the maximum distance along the primary axis of the blade and its width as the greatest distance perpendicular to the primary axis. Blade area was calculated from length and width, assuming the shape was elliptical. The life span of the blade was defined as the time from when the blade reached 80 % of its maximum area to when it senesced to less than 10 % of this maximum, which ensured that the entire process of senescence was observed. Thirty two of the 120 blades sampled did not meet this criterion (either because they had already reached 80 % of maximum size when we started sampling, or because the frond was lost before senescing to 10 % of its maximum) and were excluded from the analysis. No group (midwater or canopy, interior or edge) suffered severely disproportional sample loss.
Physical and chemical properties of blades
We collected a stratified random sample of blades of different age classes for laboratory analyses of thickness, pigment and nitrogen content, and photosynthetic performance. Blades used for this purpose were collected on the same date from different fronds on different plants to minimize the potential for results to be influenced by temporal differences in acclimation and resource allocation.
We analyzed pigment and dry mass density (a measure of blade thickness) from 46 haphazardly chosen mature canopy and midwater blades collected from the edge and interior of the Isla Vista kelp forest in April 2013. We also used these blades along with an additional 61 senescent canopy and midwater blades collected at the edge and in the interior of the kelp forest to examine the effect of age class on blade nitrogen content. Mature midwater blades (n = 35) were collected from young 3 to 4 m long fronds, senescent midwater blades (n = 47) and mature canopy blades (n = 11) were collected from fronds 5–7 m in length, and senescent canopy blades (n = 14) were selected from fronds >8 m in length that had stopped elongating.
Dry mass density, chlorophyll a (Chl a) mass per unit area of mature blades, and nitrogen as a percentage of dry mass for mature and senescent blades were estimated from six 1 cm diameter cores taken from the centerline of the blade, ~5 cm from the base of the blade. This standardization was done because the mass per unit area of blade decreases exponentially from the base of the blade to the tip (Clendenning 1971). Nitrogen content was estimated using a CE-440 CHN/O/S elemental analyzer (Exeter Analytical, Chelmsford, Massachusetts, USA). Chl a was extracted using a dimethyl sulfoxide/acetone solvent and analyzed using a Shimadzu UV 2401PC spectrometer (Shimadzu Scientific Instruments, Kyoto, Japan) following the methods of Seely et al. (1972).
Photosynthetic performance
Fourteen midwater blades of known ages were collected at the conclusion of the blade life span surveys to test the hypothesis that maximum photosynthesis (P max) decreases with blade age. Blades collected for P max measurements were obtained from 14 of the plants used to evaluate blade area and life span, but from different fronds than those monitored for blade life span. Eight blades came from plants at the forest edge and six blades from plants in the forest interior. Upon collection, blades were placed in dark sealed containers until P max was measured following the methods of Miller et al. (2012). Blades were incubated in nitrogen-purged, sealed aquaria and oxygen evolution was measured using a self-contained D-Opto dissolved oxygen logger (Zebra-Tech, Nelson, New Zealand). Blades were exposed to varying levels of photosynthetically active radiation ranging from complete darkness to 700 μEm−2 s−1, measured using spherical MkV/L Light Intensity Recorders manufactured by Alec Electronics Corporation (Kobe, Japan).
Light measurements
To characterize the reduction in light due to canopy shading and extinction with depth, we measured photosynthetic photon flux density using spherical PAR sensors placed just above the canopy (surface measurement) and at 4 m depth (midwater) and 6 m (bottom) depths near the start of each transect. Preliminary measurements showed no distinguishable difference in light at 4 and 6 m depths. Consequently, light sensors were deployed at the surface and midwater for two consecutive days during collection of the blade tissue samples. They recorded PAR every two minutes from about 10:30 to 13:30 h and were positioned to intercept the light reaching representative midwater and canopy blades.
Statistical analysis
The effects of location within the forest (interior vs. edge) and depth (canopy vs. midwater) on blade life span, maximum area, mass density, nitrogen, and Chl a were analyzed in separate two-way fixed factor ANOVAs with corrections for unequal sample sizes when appropriate (e.g., when fronds were lost prior to blade senescence). Because there was little difference in irradiance between the 4 m and 6 m depths, the blades sampled at these two depths were averaged into a single midwater group for analyses of blade life span and maximum area. The effect of age class and depth on nitrogen content was also tested with a similar two-way fixed factor ANOVA. Graphical analysis of residuals showed all response variables met the assumption of homoscedasticity.
A non-linear least-squares method was used to fit a hyperbolic tangent function to the oxygen evolution rates at each light intensity to estimate P max (Miller et al. 2012). Linear regression was used to assess the explanatory power of age (measured in days) on P max. Conformity to the homoscedasticity assumption of the linear regression was verified by graphical examination of the residuals.
Results
Characterization of the light environment
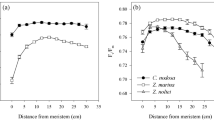
As expected, there were strong gradients in light associated with depth and location in the kelp forest (Fig. 1a). Light penetrating to the midwater in the interior of the forest during midday was very low, averaging 1.9 (±0.2 SE)μmol m−2 s−1, which was ~0.5 % of that in the midwater at the edge of the forest. By comparison, light reaching the canopy at the sea surface was nearly 800 times brighter than in the midwater of the interior of the forest and about four-fold brighter than in the midwater at the edge of the forest (Fig. 1a).
Characteristics of mature blades from different light environments
The life span of kelp blades varied with depth and location in the forest in a manner that was generally consistent with the predictions of leaf life span theory. Blades growing in the dim midwater lived on average 76 % longer than those growing in the bright environment of the surface canopy (81 vs. 46 days; Fig. 1b). The effects of location in the kelp forest on blade life span were less pronounced and varied with depth (F 1,57 = 7.62, P = 0.008 for location*depth). Blades in the midwater lived on average eight days longer in the interior of the forest compared to the edge, whereas the life span of blades in the canopy was on average 10 days shorter in the interior of the forest compared to the edge.
The size of blades at maturity increased with increasing light availability (Fig. 1a vs. c). The maximum area of blades growing in the canopy averaged about four times that of blades growing in the midwater (F 1,57 = 121.21, P < 0.001), while the maximum area of blades at the edge of the forest averaged twice that of blades in the interior of the forest (F 1,57 = 15.44, P < 0.001, Fig. 1c). The effects of depth and location on maximum blade area were additive (F 1,57 = 0.18, P = 0.67 for depth*location).
The mass density (Fig. 1d) and nitrogen content (Fig. 1e) of mature blades did not differ significantly with depth (F 1,42 = 3.22, P = 0.08, F 1,42 = 0.68, P < 0.41, for mass density and nitrogen, respectively) or location in the forest (F 1,42 = 0.12, P < 0.73, F 1,42 = 0.44, P < 0.51, for mass density and nitrogen, respectively). Blade mass density averaged 5.60 mg cm−2 (± 0.21 SE) across depths and locations in the forest (Fig. 1d), while blade nitrogen averaged 2.80 % ± 0.11 (Fig. 1e).
The effects of location in the forest on the Chl a content of blades varied inconsistently with depth (F 1,42 = 19.56, P < 0.001, depth*location). Contrary to expectations, the average Chl a content of blades from the darkest portion of the forest (interior midwater) was 25–44 % lower than that of blades from other areas of the forest (Fig. 1f).
Changes in blade characteristics with age
P max tended to decrease with the age of midwater blades, as predicted by leaf life span theory, but only marginally so (r 2 = 0.231, P = 0.08, Fig. 2). Closer examination revealed that the P max of shorter lived blades at the forest edge showed a rapid decrease with age (r 2 = 0.653, P = 0.015), whereas that of longer lived blades in the forest interior was unrelated to age (r 2 = 0.023, P = 0.78). In addition, consistent with optimization theory was our observation that the nitrogen content of blades was significantly lower in older senescent blades compared to younger mature blades (F 1,103 = 16.47, P < 0.001), and significantly lower in shorter lived canopy blades compared to longer lived midwater blades (F 1,103 = 8.98, P = 0.003; Fig. 3). The difference in nitrogen content between senescent and mature blades was similar in the canopy and midwater (F 1,103 = 1.72, P = 0.19 for age*depth).
Discussion
Leaf life span theory predicts that kelp blades growing in higher light (e.g., near the surface and at the forest edge) should be larger, thinner, shorter lived, and have less pigmentation, but higher nitrogen content than blades in areas that receive less light (near the bottom and in the interior of the forest). We found that the largest and shortest lived blades occurred in the surface canopy, which is consistent with the predictions of leaf life span theory. We did not detect a significant difference in the life span of canopy blades at the edge vs. interior of the forest, perhaps because the light environment is similarly high at these two locations. We also found that blade life span was not related to blade thickness. Increased wave disturbance has been shown to induce the kelp Ecklonia radiata to form thicker blades to reduce breakage in wave exposed areas (Fowler-Walker et al. 2006). However, differences in wave disturbance probably did not contribute significantly to differences in blade thickness or life span in our study, which was conducted during benign summer conditions (i.e., waves never exceeded 1.5 m in height, unpublished data). Instead, blades eroded at the distal ends at a relatively consistent rate, which is more consistent with loss due to senescence than episodic disturbance.
Spatial variation in nutrients and the movement of water that alters their delivery also have the potential to influence growth in Macrocystis (Wheeler 1980b; Wheeler and North 1981; Dean and Jacobsen 1984), and could have accounted for some of the differences that we observed. Although we were not able to isolate the potential effects of these factors, we attempted to minimize them by sampling plants in the interior of the forest that were only 10 m from the forest edge. This short distance was sufficient to greatly alter the availability of light, but likely had a little effect on the availability of nutrients. This assumption is supported by results obtained from a nearby kelp forest where nutrient concentrations were found to be similar between the interior and the edge of the forest (Stewart et al. 2009). Moreover, Stewart et al. (2009) used data on levels of carbon and nitrogen accumulation in kelp tissues to argue that the differences they observed in kelp growth between the interior and edge of the forest were due to differences in light rather than nutrients.
Epiphytes are common on the blades of giant kelp and have the potential to alter blade performance and life span. The most abundant and intensively studied of these epiphytes is the encrusting bryozoan Membranipora spp. which shows tremendous seasonal and spatial variation in its occurrence (Lobban 1978b; Bernstein and Jung 1979). Although the presence of dense colonies of Membranipora on Macrocystis blades has been shown not to inhibit photosynthesis (Wing and Clendenning 1971), it is purported to lead to accelerated blade loss via heightened senescence, breakage from water motion, and grazing by fishes (Lobban 1978b; Bernstein and Jung 1979; Dixon et al. 1981; Yoshioka 1982). Epiphytes in general, and Membranipora, in particular, were not abundant during our study as the vast majority of blades that we measured in the field and collected for analysis in the laboratory were virtually free of epiphytes. Thus, the effects of epiphytes on the life span and physical and chemical properties of Macrocystis blades did not influence our results. It is worth noting, however, that Membranipora tends to be most abundant on blades near the surface at the outer edges of kelp forests, whereas other epiphytes are more abundant in the interior of the forest on older, lower blades (Bernstein and Jung 1979). Thus, the extent to which the life span of Macrocystis blades is determined by resource allocation vs. increased rates of senescence, breakage, and grazing due to epiphytes is an interesting topic for future investigations.
The decreases in tissue nitrogen and P max with age suggest that internal processes, rather than external factors (e.g., disturbance and herbivory), control the life span of Macrocystis blades much like they do the life span of Macrocystis fronds (Rodriguez et al. 2013). The decrease in photosynthetic performance of a leaf with age is an essential component of leaf life span optimization models: without such a decrease, leaves are predicted to have an infinite life span (Kikuzawa 1991). In addition, greater decreases in leaf nitrogen with age are associated with shorter leaf life spans (Hikosaka 2003, 2005, 2010). Our observation that the reduction in nitrogen content from mature blades to senescent blades was greater in the canopy (29 % decrease) than in the midwater (13 % decrease) where blades are shorter lived is consistent with predictions of leaf life span theory, as was our observation that the decline in nitrogen with age was greater in shorter lived blades at the edge of the forest compared to long-lived blades in the forest interior.
An important assumption of leaf life span theory is that leaves are abscised once they stop contributing to net carbon gain. Giant kelp blades found under a dense canopy may not contribute directly to net carbon gain, and this might explain why patterns of pigment allocation were not consistent with theory derived for leaves of higher plants. Light under a dense canopy is frequently at or below 10 μE m−2 s−1 (Gerard 1984; Deysher and Dean 1986), which is the compensation irradiance for Macrocystis (Gerard 1976). This suggests that blades occurring under a dense canopy are net carbon sinks. The lower concentration of Chl a that we observed in interior midwater blades where light was lowest is not consistent with strategic resource allocation to maximize photosynthesis (Terashima et al. 2005). Optimization of carbon gain cannot explain the longer life span observed for interior midwater blades if these blades consistently run a carbon deficit.
Bottom and midwater blades in the interior of the kelp forest may be important in nutrient uptake, because growth in Macrocystis can be sustained by nutrients supplied at depth (Zimmerman and Robertson 1985). Colombo‐Pallotta et al. (2006) showed that basal blades of Macrocystis had higher respiration than canopy blades. Since respiration is expected to be lower in the light-limited conditions, Colombo‐Pallotta et al. (2006) attributed this result to the enhancement of nutrient uptake systems. Thus, bottom and midwater blades may be retained by Macrocystis after they stop contributing to net positive carbon gains if they serve to enhance nutrient uptake.
This is the first study to explicitly test predictions of leaf life span theory in macroalgae. Our findings that Macrocystis blades were larger and shorter lived in areas of higher light had decreased photosynthesis with age and displayed a greater decrease in nitrogen in shorter lived blades were consistent with leaf life span theory. However, other observations (such as low pigment and high nitrogen concentrations in heavily shaded blades in the midwater) are inconsistent with theoretical predictions. Our finding that kelp blades in the interior of the kelp forest had longer life spans, despite having much lower pigment levels than predicted and living in a severely light-limited environment suggest that carbon fixation may not be the primary function of these blades. Data gathered during this study and others (Zimmerman and Robertson 1985; Colombo-Pallotta et al. 2006) indicate that the primary role of mature blades in the midwater of the interior of a kelp forest may actually be nutrient absorption, rather than carbon gain. This is consistent with the observation of an inverse relationship between the amount of photosynthetic pigment and nitrogen uptake by Macrocystis blades along a light gradient (Kopczak 1994).
Our results indicate that it could be very fruitful to apply leaf life span theory to other species of marine macrophytes, particularly brown algae (Phaeophyceae) that have evolved plant-like body plans and are similar to terrestrial plants with respect to the need to optimize the transport of internal resources via phloem-like networks (e.g., Drobnitch et al. 2015). Our testing of the predictions of the theory for giant kelp revealed mechanisms that help explain tissue turnover that cannot be attributed to physical disturbance or other external influences (Rodriguez et al. 2013). Consistent with the predictions of leaf life span theory, the turnover of blades and fronds of Macrocystis ultimately serves to improve the efficiency of the organism, as older, less efficient tissue is replaced by more productive tissue. This study provides further evidence of the broad applicability of optimization models by applying a model developed for terrestrial plants to predict traits in M. pyrifera. However, while leaf life span theory and other optimization models provide a robust theoretical framework from which to base investigation of foliar turnover in marine algae, special consideration will need to be given to the unique demands of the fluid environment, in which macroalgae live, and the opportunities and constraints that result.
References
Bernstein BB, Jung N (1979) Selective pressures and coevolution in a kelp canopy community in southern California. Ecol Monogr 49:335–355
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Ann Rev Ecol Syst 13:229–259
Clendenning KA (1971) Photosynthesis and general development in Macrocystis. Nova Hedwig 32:169–190
Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Colombo-Pallotta MF, García-Mendoza E, Ladah LB (2006) Photosynthetic performance, light absorption, and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) blades from different depths. J Phycol 42:1225–1234
Dean TA, Jacobsen FR (1984) Growth of juvenile Macrocystis pyrifera (Laminariales) in relation to environmental factors. Mar Biol 83:301–311
Dennison WC, Alberte RS (1982) Photosynthetic responses of Zostera marina L. (Eelgrass) to in situ manipulations of light intensity. Oecologia 55:137–144
Deysher LE, Dean TA (1986) In situ recruitment of sporophytes of the giant kelp Macrocystis pyrifera (L.) C. A. Agardh: effects of physical factors. J Exp Mar Biol Ecol 103:41–63
Dietz KJ, Heber U (1984) Rate-limiting factors in leaf photosynthesis. I. Carbon fluxes in the calvin cycle. Biochim Biophys Acta 767:432–443
Dixon J, Schroeter SC, Kastendiek J (1981) Effects of the encrusting bryozoan, Membranipora membranacea, on the loss of blades and fronds by the giant kelp, Macrocystis pyrifera (Laminariales). J Phycol 17:341–345
Drobnitch ST, Jansen KH, Prentice P, Pittermann J (2015) Convergent evolution of vascular optimization in kelp (Laminariales). Proc R Soc B (1816) 282:20151667
Escudero A, Mediavilla S (2003) Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J Ecol 91:880–889
Fowler-Walker MJ, Wernberg T, Connell SD (2006) Differences in kelp morphology between wave sheltered and exposed localities: morphologically plastic or fixed traits? Mar Biol 148:755–767
Gerard VA (1976) Some aspects of material dynamics and energy flow in a kelp forest in Monterey Bay, California. PhD dissertation, Department of Biology, University of California. Santa Cruz, California, USA
Gerard VA (1984) The light environment in a giant kelp forest: influence of Macrocystis pyrifera on spatial and temporal variability. Mar Biol 84:189–195
Graham MH, Vasquez JA, Buschmann AH (2007) Global ecology of the giant kelp Macrocystis: from ecotypes to ecosystems. Oceanogr Mar Biol Ann Rev 45:39–88
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Hikosaka K (2003) A model of dynamics of leaves and nitrogen in a plant canopy: an integration of canopy photosynthesis, leaf life span, and nitrogen use efficiency. Am Nat 162:149–164
Hikosaka K (2005) Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Ann Bot 95:521–533
Hikosaka K (2010) Mechanisms underlying interspecific variation in photosynthetic capacity across wild plant species. Plant Biotechnol 27:223–229
Kikuzawa K (1991) A cost-benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am Nat 138:1250–1263
Kikuzawa K, Ackerly D (1999) Significance of leaf longevity in plants. Plant Species Biol 14:39–45
Kikuzawa K, Lechowicz MJ (2006) Toward synthesis of relationships among leaf longevity, instantaneous photosynthetic rate, lifetime leaf carbon gain, and the gross primary production of forests. Am Nat 168:373–383
Kopczak CD (1994) Variability of nitrate uptake capacity in Macrocystis pyrifera (Laminariales, Phaeophyta) with nitrate and light availability. J Phycol 30:573–580
Lobban CS (1978a) The growth and death of the Macrocystis sporophyte (Phaeophyceae, Laminariales). Phycologia 17:196–212
Lobban CS (1978b) Growth of Macrocystis integrifolia in Barkley Sound, Vancouver Island, BC. Can J Bot 56:2607–2711
Mann KH (2000) Ecology of coastal waters: with implications for management. Blackwell Science, Malden
Miller RJ, Harrer S, Reed DC (2012) Addition of species abundance and performance predicts community primary production of macroalgae. Oecologia 168:797–806
North WJ (1971) The biology of giant kelp beds (Macrocystis) in California. Nova Hedwig 32:1–600
Raines CA (2003) The Calvin cycle revisited. Photosynth Res 75:1–10
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Rodriguez GE (2015) Turnover dynamics of the giant kelp, Macrocystis pyrifera. PhD dissertation, Department of Ecology, Evolution and Marine Biology, University of California Santa Barbara, Santa Barbara, California, USA
Rodriguez GE, Rassweiler A, Reed DC, Holbrook SJ (2013) The importance of progressive senescence in the biomass dynamics of giant kelp (Macrocystis pyrifera). Ecology 94:1848–1858
Seely GR, Duncan MJ, Vidaver WE (1972) Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Mar Biol 12:184–188
Stewart HL, Fram JP, Reed DC, Williams SL, Brzezinski MA, MacIntyre S, Gaylord B (2009) Differences in growth, morphology and tissue carbon and nitrogen of Macrocystis pyrifera within and at the outer edge of a giant kelp forest in California, USA. Mar Ecol Prog Ser 375:101–112
Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Ann Bot 95:507–519
Vincent G (2006) Leaf life span plasticity in tropical seedlings grown under contrasting light regimes. Ann Bot 97:245–255
Wheeler WN (1980a) Pigment content and photosynthetic rate of the fronds of Macrocystis pyrifera. Mar Biol 56:103–110
Wheeler WN (1980b) Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol 56:103–110
Wheeler PA, North WJ (1981) Nitrogen supply, tissue composition and frond growth rates for Macrocystis pyrifera off the coast of southern California. Mar Biol 64:59–69
Wing BL, Clendenning KA (1971) Kelp surfaces and associated invertebrates. Nova Hedwig 32:319–341
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villa R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Yoshioka PM (1982) Role of planktonic and benthic factors in the population dynamics of the bryozoan Membranipora membranacea. Ecology 63:457–468
Zimmerman RC, Robertson DL (1985) Effects of El Niño on local hydrography and growth of the giant kelp, Macrocystis pyrifera, at Santa Catalina Island, California. Limnol Oceanogr 30:1298–1302
Acknowledgments
We thank E. Barba, J. Mandoske, and P. Salinas-Ruiz for help in data collection and S. Harrer and C. Nelson for technical and logistical assistance. Financial support was provided by the US National Science Foundation’s Long Term Ecological Research program (OCE-0620276, OCE-1232779).
Author contribution statement
GER, DCR, and SJH conceived and designed the experiments. GER performed the experiments. GER and DCR analyzed the data. GER wrote the manuscript and DCR and SJH provided editorial advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joel Trexler.
Rights and permissions
About this article
Cite this article
Rodriguez, G.E., Reed, D.C. & Holbrook, S.J. Blade life span, structural investment, and nutrient allocation in giant kelp. Oecologia 182, 397–404 (2016). https://doi.org/10.1007/s00442-016-3674-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3674-6