Summary
Aquatic plants, comprising different divisions of embryophytes, derive from terrestrial ancestors. They have evolved to live in water, both fresh and salty, an environment that presents unique challenges and opportunities for photosynthesis and growth. These include, compared to air, a low water stress, a greater density, and attenuation of light, and a more variable supply of inorganic carbon, both in concentration and chemical species, but overall a lower carbon availability, and the opportunity to take up nutrients from the water. The leaves of many aquatic plants are linear, dissected, whorled, or cylindrical with a large volume of air spaces. They tend to have a high specific leaf area, thin cuticles, and usually lack functional stomata. Exploiting the availability of chemicals in their environment, freshwater macrophytes may incorporate silica in their cell wall, while seagrasses contain sulphated polysaccharides, similar to those of marine macroalgae; both groups have low lignin content. This altered cell wall composition produces plants that are more flexible and therefore more resistant to hydraulic forces (mechanical stress arising from water movement). Aquatic plants may have enhanced light harvesting complexes conferring shade adaptation, but also have mechanisms to cope with high light. Aquatic plants have evolved numerous strategies to overcome potential carbon-limitation in water. These include growing in micro-environments where CO2 is high, producing leaves and roots that exploit CO2 from the air or sediment and operating concentrating mechanisms that increase CO2 (CCM) around the primary carboxylating enzyme, ribulose-1,5-bisphosphate carboxylase-oxygenase. These comprise C4 metabolism, crassulacean acid metabolism, and the ability to exploit the often high concentrations of HCO3 −, and ~50% of freshwater macrophytes and ~85% of seagrasses have one or more CCM. Many of these adaptations involve trade-offs between conflicting constraints and opportunities while others represent ‘synergies’ that help to maximize the productivity of this important group of plants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
I. Introduction
Aquatic embryophytes (bryophytes, lycophytes, monilophytes, and angiosperms) are a heterogeneous group of plants with many common features. They have arisen as a result of convergent evolution from terrestrial ancestors to adapt to life in marine and fresh waters. In the ocean, seagrasses cover an area of about 3.1011 m2 (Duarte et al. 2005) and have an average net productivity rate that ranges from 500 to 1200 g C m−2 year−1 (van der Heijden and Kamenos 2015). This corresponds to a net global productivity of about 0.4 Pg C year−1, which is about 1% of marine primary productivity. Nevertheless, seagrass beds help to support coastal fisheries and are important habitats for fish, birds, invertebrates, and marine mammals, play an important role in nutrient cycling (Waycott et al. 2009) and keep waterbone pathogens in check (Lamb et al. 2017). They are also globally important for organic carbon sequestration, contributing about 30–40 Tg C year−1, or nearly 20% of global oceanic carbon burial (Duarte et al. 2005).
In fresh waters, macrophytes are important primary producers, particularly in shallow lakes and rivers. They can also be regarded as ‘ecological engineers’ producing a structurally complex environment that is very different from the open water and an important habitat for numerous organisms including invertebrates and fish (Chambers et al. 2008; Kovalenko et al. 2012). They also help to prevent turbid, phytoplankton-dominated systems as part of the alternative stable states that can exist in shallow lakes (Scheffer et al. 1993). Their presence in shallow lakes increases food-chain length (Ziegler et al. 2015) probably through their contribution to productivity, environmental variability, and their provision of a refuge for higher trophic levels. In some shallow environments, such as the Amazonian floodplains, macrophytes can contribute a large portion of the net primary productivity of the system (Silva et al. 2013).
Macrophytes comprise a range of life forms in a gradient ranging from emergent plants with leaves that photosynthesize in air (not considered in this chapter), through species with leaves floating on the water surface but rooted in the sediment, free-floating plants with leaves on the water surface, to completely submerged plants with leaves photosynthesizing in water, either free-floating in the water column or, more commonly, rooted in the sediment. The latter group can be broadly divided into two types: (i) rosette plants (often called isoetids) with leaves that are short, stiff, and often cylindrical and (ii) tall caulescent shoots (often called elodeids) with leaves with a range of morphologies (Sect. 3). Seagrasses typically belong to the caulescent, elodeid group of submerged plants. The life form system of Wiegleb (1991) gives a more detailed breakdown of hydrophyte growth forms. While most aquatic plants are rhizophytes with roots penetrating the sediment, some, such as the bryophytes, are haptophytes, attached to, but not penetrating hard surfaces.
In this chapter, we compare air and water as environments for photosynthesis and growth and describe how the structure and function of aquatic leaves allows them to thrive in aquatic environments. We deal primarily with submerged embryophytes, freshwater macrophytes, and seagrasses, but make occasional reference to macrophyte and microphyte algae.
II. Adaptation of Aquatic Plants to the Environmental Challenges and Opportunities in Water
A. Evolution of Aquatic Embryophytes
All aquatic embryophytes evolved from terrestrial ancestors that in turn evolved from aquatic green algae, probably within the Zygnematophyceae (Wickett et al. 2014), and therefore contain evolutionary traces of adaptations to life in both air and water. For example, terrestrial bryophytes inherited features from their algal ancestors, such as desiccation tolerance and lignin-like cell wall polymers, that allowed them to colonize land (Graham et al. 2014) while present day macrophytes possess visible features of their terrestrial past such as cuticles and stomata (functional and non-functional), xylem (Sculthorpe 1967) and leaves themselves.
Aquatic angiosperms are found in 17% of angiosperm families, comprising over 6000 species (Cook 1990; den Hartog and Kuo 2006), but represent less than 2% of all angiosperms (Les et al. 1997). Freshwater angiosperms have arisen at least 100 times (Les and Tippery 2013) and are represented predominantly by monocotyledons (Liliopsida) rather than dicotyledons (Magnoliopsida) (Les and Schneider 1995). In fresh waters, the cline between terrestrial, wetland, and submerged environments, blurred by episodic and seasonally variable water levels, produces a gradient that probably facilitated movement of plants between land and water. Indeed, there is evidence for progressive evolution from emergent to floating-leaved then to submerged leaves in aquatic lineages (Du and Wang 2014). Fossil and phylogenetic data show that invasion of the aquatic habitat occured very early in the evolution of the angiosperms and that they existed in the Cretaceous 120 million years ago (Friis et al. 2001; Gomez et al. 2015; Les 2015).
In coastal and marine waters, there are 72 species of seagrasses within 13 genera and five families if euryhaline genera such as Ruppia are included. All belong to the monocotyledon, largely freshwater order Alismatales (Les et al. 1997; den Hartog and Kuo 2006); Short et al. 2007; Papenbrock 2012; Les and Tippery 2013) and so probably evolved from freshwater ancestors. Seagrasses are believed to have evolved relatively recently, around 64–72 million years ago (Olsen et al. 2016) or possibly only 16–41 million years ago (Chen et al. 2012). The apparent evolutionary barrier to colonization of marine systems by embryophytes, indicated by low species diversity, is not clear. It does not appear to be linked to salinity per se since seagrasses have a range of mechanisms to deal with this factor (Touchette 2007). It has been suggested to be caused by difficulties of efficient pollination and potentially to the lack of co-evolution with insects, a group that is also limited in its success in marine environments (Van der Hage 1996). It could also result from competition with large macroalgae that were already present in marine systems in contrast to fresh waters where macroalgae, essentially members of the family Characeae, are relatively small and occupy a different niche, or simply result from a physical barrier caused by the hydraulic forces.
B. Comparison of Air and Water as Environments for Photosynthesis and Growth
The fluids of air and water provide contrasting environments for the photosynthesis, growth, and survival of photoautotrophs. These properties (Maberly and Spence 1989) set contrasting ecological challenges and opportunities (Table 11.1) that have resulted in different evolutionary solutions to maximize fitness in aquatic and terrestrial plants (Maberly 2014).
The most obvious distinction between air and water is water availability itself. In fresh water, the water potential is, by definition, 0 MPa and in seawater about −2.5 MPa. In air, it can range from 0 at 100% humidity to as low as −200 MPa in very dry air, setting extreme challenges for leaf water balance. Despite the many structural, morphological, and physiological attributes of terrestrial plants, water availability is still a major factor controlling plant productivity on land (Hsu et al. 2012). A second environmental difference is the greater density of water, compared to air, that reduces the need for investment in structural material, but increases the drag on a plant in flowing water. For a given velocity, the drag and lift forces on an object in water are about 29-times greater than in air (Denny 1993). Thus, plants in rivers with a relatively low water velocity of 1 m s−1 experience the same forces as plants in air with a wind speed of 100 km h−1. Plants, in littoral regions where waves are breaking, or in fast flowing water such as in rapids, often inhabited in sub-tropical and tropical areas by freshwater macrophytes of the family Podostemaceae (Koi et al. 2015), will experience much greater forces.
A third difference is the generally low levels of underwater light. This results from reflection at the air-water interface, but mainly by the much greater, and spectrally-selective, attenuation of light in water compared to air (Table 11.1). This is caused by light absorption by water molecules themselves, plus the ability of water to dissolve substances, such as coloured dissolved organic matter ((Kirk 2011). Furthermore, because of the density of water, particles including phytoplankton, sediment, and non-living organic material can be kept in suspension, scattering light and increasing attenuation. Finally, growth of epiphytes on aquatic leaves because of the absence of desiccation and presence of dissolved nutrients, can reduce light availability even further (Sand-Jensen 1977). As a consequence, light controls macrophyte depth limits (Krause-Jensen and Sand-Jensen 1998), macrophyte productivity at the whole population level (Sand-Jensen et al. 2007), and can also control the overall productivity of some freshwater systems (Karlsson et al. 2009). Although aquatic environments are essentially shade environments, wave focussing at the water surface can produce bursts of high light with a duration of milliseconds (Schubert et al. 2014). These differ from sunflecks, well-known in terrestrial vegetation (Pearcy 1990), in producing levels of surface light that can be five-times greater than surface light and consequently have the potential to cause photoinhibition and photodamage.
A fourth difference is the heat capacity of water that is around 3500-times greater than that of air on a volume basis (Maberly and Spence 1989). This tends to reduce the magnitude of diel temperature variation in water compared to air. Also, because in fresh water, ice is slightly less dense than liquid water at 4 °C, it forms at the surface in winter, thereby protecting freshwater plants from freezing in all but the shallowest or extremely cold environments.
A fifth difference relates to the availability of inorganic carbon, a key requirement for photosynthesis (Table 11.1). The solubility of CO2 in water decreases with temperature, but at 15 °C, the molar concentrations in air and water are similar. In contrast, oxygen is about 30-times less soluble in water than in air and this might suggest that aquatic photosynthesis would be favored over that in air because of reduced photorespiration. Furthermore, CO2 is the only form of inorganic carbon in air, while in water it is supplemented by bicarbonate (HCO3 −) and carbonate (CO3 2−). Bicarbonate and carbonate are produced by dissolution of limestone and weathering of silicates (Pagani et al. 2009) and hence their concentration is catchment-dependent and highly variable among fresh waters (Talling 1985), but relatively constant in the oceans. The different forms of inorganic carbon are interconnected by equilibria controlled largely by pH. In fresh water, HCO3 − is the dominant form of inorganic carbon at pH values between the two carbonate dissociation constants at about 6.4 and 10.4. In seawater, the equivalent dissociation constants are about pH 6.0 and 9.1 and the concentration of HCO3 − is around 2 mmol L−1, which is about 140-times greater than the concentration of CO2 at air-equilibrium.
However, these apparent photosynthetic advantages of inorganic carbon in water are not always realized because of other characteristics that reduce inorganic carbon availability. First, although atmospheric concentrations of CO2 have varied substantially over geological time (Pearson and Palmer 2000), they are relatively stable over decades with current seasonal ranges of the order of 2% of the annual mean and annual growth of less than 1% (Thoning et al. 1989). In contrast, concentrations of CO2 are highly variable in productive aquatic systems where rates of biological transformation between organic and inorganic carbon can greatly exceed rates of re-supply from the atmosphere, input from rivers, and entrainment from depth. Consequently, inland waters can be both substantially under- or over-saturated with CO2 relative to the atmosphere at different times of year (Cole et al. 1994; Maberly 1996).
Another major constraint on the supply of CO2 to photosynthesizing leaves in water is the approximately 10,000 lower rate of diffusion of CO2 compared to that in air, caused by the greater density of water (Raven 1970). Consequently, a transport limitation is imposed on leaf photosynthesis (Black et al. 1981) causing the K½ for CO2 uptake in water to be between 100 to 200 μmol L−1, roughly six- to eleven-times air-equilibrium concentrations (Maberly and Madsen 1998).
The high solubility of many ions and compounds in water provides an opportunity for nutrients, such as nitrogen and phosphorus, to be taken up by shoots as well as roots. This also produces a challenge because nutrients in the water column are a resource for planktonic and attached algae that may compete with the plants for these and other resources (Table 11.1). High solubility also allows allelochemicals and chemical signalling to produce an aquatic “smellscape” that affects interactions between different types of organism, potentially altering the competitive balance between plants and algae directly or indirectly via food-web interactions (van Donk and van de Bund 2002; Gross 2003).
III. Response of Leaf Morphology, Structure, and Composition to Aquatic Environments
Section 2 above describes some of the environmental opportunities and challenges faced by aquatic plants (outlined in Table 11.1) and the consequent biological responses are outlined below in Sects. 3 and 4.
A. Leaf Morphology
Aquatic leaves vary hugely in area (Pierce et al. 2012) from the very small leaves of free-floating lemnids with a leaf area of <1 mm2 as in Wolfia arrhiza, through leaves of typical area of around 300 mm2, to the very large floating leaves of water lilies of over 44,000 mm2 for Nuphar alba and exceptionally nearly 5 106 mm2 in the giant water lily Victoria amazonica. The morphology of aquatic leaves is less variable than those of terrestrial plants. A number of different leaf forms are widespread as discussed in classic texts such as those of Arber (1920) and Sculthorpe (1967). For example, in freshwater macrophytes, the ‘isoetid’ leaf shape is common, comprising short, stubby, cylindrical leaves with a large lacunal volume found in the eponymous lycophyte Isoetes (Fig. 11.1a) and also in dicotyledons such as Littorella, Lobelia, and Subularia and monocotyledons such as Eleocharis. Dissected whorled leaves are found in many genera of dicotyledon macrophytes, including Myriophyllum (Fig. 11.1b), Limnophila, and Ceratophyllum while dissected leaves are also found in Cabomba and Ranunculus (Batrachium). Whorled, but mainly entire, leaves are present in monocotyledon genera such as Egeria (Fig. 11.1c), Elodea, Hydrilla, and Lagarosiphon and also in dicotyledon genera such as Hippuris. Rarely, large entire leaves are produced as in the monocotyledon Ottelia (Fig. 11.1d) and the submerged leaves of the dicotyledon Nuphar lutea. Within the large monocotyledon genus Potamogeton, there is a diversity of form including larger entire leaves such as in P. lucens (Fig. 11.1e), small linear leaves as in P. maackianus (Fig. 11.1f), and filiform leaves as in P. pectinatus (now Stukenia pectinata). Linear strap-like leaves are common, including those of the monocotyledons Vallisneria (Fig. 11.1g) and Sparganium. For the eudicots at least, small variations in a common development program can produce large changes in morphology (Runions et al. 2017). In seagrasses, all of which are monocotyledons, there is an even lower diversity of leaf-form with linear strap-like leaves being widespread, e.g., in Posidonia, Thalassia (Fig. 11.1h), and Zostera, although some genera contain species with linear leaves, e.g., Amphibolis or ovate leaves as in species of Halophila (Fig. 11.1i; (Kuo and den Hartog 2006).
Leaf form in freshwater macrophytes. (a) Isoetes sinensis (leaves in air), (b) Myriophyllum verticillatum, (c) Egeria densa, (d) Ottelia acuminata, (e) Potamogeton lucens, (f) Potamogeton maackianus, (g) Vallisneria spiralis, (h) Syringodium filiforme and Thalassia testudinum, and (i) Halophila ovalis. Photograph (a) was taken by Qing-Feng Wang, (h) by Ole Pedersen, and (i) by Marion Cambridge; the remainder were taken by the authors at the Botanical Garden of the Chinese Academy of Sciences in Wuhan
Thus, there is a high degree of convergent evolution in the leaf form of aquatic embryophytes, implying common responses to environmental pressures. This is supported by the different leaf morphologies commonly seen in amphibious freshwater plants with terrestrial-like floating or emergent leaves produced in air and typical aquatic leaves produced underwater (Arber 1920; Sculthorpe 1967; Maberly and Spence 1989).
B. Leaf Structure
One of the necessary developments in the invasion of land by aquatic plants was the evolution of stomata, which occurred about 400 million years ago (Edwards et al. 1998; Ruszala et al. 2011). Since stomata control gas exchange including water loss, they are obsolete in aquatic environments. Indeed, most submerged leaves do not possess stomata, or, where they do exist, they are non-functional (Sculthorpe 1967). However, leaves of aquatic plants that float at the surface of the water, such as water lilies (e.g., Nymphaea violacea), have stomata at the upper surface (Rudall and Knowles 2013). Stomata are also only present on the upper surface of the floating leaves of the aquatic pteridophyte, Marsilea, while stomata are present on both adaxial and abaxial surfaces of its aerial leaves (Lin and Yang 1999). In the seagrass Zostera marina, the genome of which has been sequenced (Olsen et al. 2016), the stomatal genes appear to have been lost. However, in another seagrass, Thalassia testudinum, paracytic stomata were observed in plants grown under ‘stressed’ conditions (Benzecry 2013). These contrasting data indicate that the loss of stomata is not universal in the seagrasses and there could be phylogenetic differences since Zostera belongs to the Zosteraceae while Thalassia is a member of the Hydrocharitaceae.
The lack of, or very low, water stress allows the cuticle to be very thin in many freshwater plants and seagrasses, typically about 0.1 μm (Frost-Christensen et al. 2003; Kuo and den Hartog 2006). It also allows chloroplasts to occur in epidermal cells (Fig. 11.2). This structure is typical of most aquatic plants, some of which have thin leaves with laminae of only two or three cell layers (Fig. 11.2g, h). Both laminar (Fig. 11.2c, d) and cylindrical leaves (Fig. 11.2e, f) can have large lacunal volumes although, unlike the sub-stomatal cavities of terrestrial leaves, these are not directly connected to the external environment. Floating leaves, in contrast, are thick exceeding 0.5 mm with many cells and a dorsi-ventral mesophyll structure similar to terrestrial leaves (Fig. 11.2a) or a homogenous mesophyll structure (Ronzhina and P’Yankov 2001). Within the seagrasses, small species such as Halophila (Figs. 11.1 and 11.2) and Halodule tend to have thin leaves while larger plants such as Thalassia and Posidonia tend to have thicker leaves (Papenbrock 2012) Figs. 11.1 and 11.2) presumably because they have to withstand greater hydraulic forces.
Cross-sections of leaves of freshwater macrophytes (left-hand column) and seagrasses (right-hand column). (a) Nuphar lutea floating leaf, (b) Amphibolis antarctica, (c) N. lutea submerged leaf, (d) Posidonia australis, (e) Ranunculus trichophyllous subsp. eradicatus, (f) Syringodium isoetifolium, (g) Potamogeton compressus, and (h) Halophila ovalis. (Panels a, c, e and g reproduced with permission from Ronzhina and P’Yankov (2001). Panels b, d, f and h courtesy of Lukasz Kotula. Scale bar 100 μm)
Leaf thickness in freshwater macrophytes and seagrasses has been compared to terrestrial plants by Enriquez et al. (1996). The median thickness of these aquatic leaves was about 130 μm while the median thickness of terrestrial herbs was 240 μm. Linked to this, the thickness of leaves of submerged freshwater macrophytes and seagrasses is much less than that of leaves that photosynthesize in air (Fig. 11.3). Since there is a negative relationship between leaf thickness and specific leaf area (m2 of projected leaf area per kg dry mass; (Vile et al. 2005), specific leaf area tends to be greater in submerged freshwater macrophytes than in terrestrial leaves. An extensive meta-analysis of leaf mass per area, the converse of specific leaf area, was undertaken by Poorter et al. (Poorter et al. 2009). They showed that aquatic leaves, particularly those of freshwater macrophytes, had a much lower leaf mass per unit area than terrestrial leaves (Fig. 11.4). Similar differences can be seen in comparisons of aquatic, floating, and terrestrial leaves (Klančnik et al. 2014). Some of the ecological and physiological consequences of this are discussed in Sect. 4. Leaf construction costs, the amount of energy required to produce a unit weight or area of leaf (Williams et al. 1987), varies with the structure of aquatic leaves and their ecological habitat (Ronzhina and Ivanov 2014). Construction cost was lower in submerged compared to floating leaved rooted hydrophytes and lower still in free-floating leaves. Leaves with large cells had lower construction costs than leaves with more, but smaller, cells (Ronzhina and Ivanov 2014).
Leaf thickness for leaves from different habitats calculated following Vile et al. (2005) for data from Pierce et al. (2012). Box and whisker plots show the lower and upper quartiles shaded in grey and the median as the horizontal line. The length of the whiskers is 1.5 times the interquartile range. The triangles represent the extreme outliers. Data for freshwater submerged (n = 33), freshwater floating (n = 19), freshwater secondary (n = 9), and terrestrial leaves (n = 506) were derived from (Pierce et al. 2012), data for marine seagrasses (n = 9) were from (Borum et al. 2015), and data from freshwater emergent plants (n = 6) were from (Colmer and Pedersen 2008). FW freshwater
Leaf mass per unit area (LMA, the inverse of specific leaf area) for different types of aquatic and terrestrial leaves. The bottom and top part of the box indicate the 25th and 75th percentile, respectively, the two whiskers the 10th and the 90th percentile, and the horizontal line within the box the median value. The median value is also printed above the box plots. The total number of species present in each functional group or habitat is indicated at the top of the figure. Aq. Aquatic, Trop. Tropical, Dec Deciduous, For. Forest, Temp. Temperate. (Reproduced with permission from Poorter et al. 2009)
Riparian plants close to the air-water interface essentially photosynthesize in air but are frequently inundated and so have to survive, and preferably photosynthesize, during periods of flooding. Some species have hydrophobic leaves that trap an air film around them when submerged (Colmer and Pedersen 2008). This has been dubbed a ‘plant plastron’ by Raven (2008) by analogy with some aquatic insects. The plastron can increase the rate of CO2 uptake by leaves in water by up to sixfold and the rate of oxygen influx to the leaf, helping to support respiration in the dark (Verboven et al. 2014; Voesenek and Bailey-Serres 2015).
The low solubility of oxygen in water, coupled with a 10,000-fold lower rate of diffusion, leads to an oxygen supply rate that is 300,000 times lower in water than air (Verberk et al. 2011). This, along with high rates of organic carbon breakdown in some environments such as the sediment, can lead to oxygen concentrations that are at or close to zero. This can prevent aerobic respiration of roots and rhizomes causing toxic products of glycolysis and fermentation, such as ethanol, to form. It can also produce redox shifts in heavy metal ions causing them to become more toxic (Crawford 1992).
The extensive lacunae of aquatic leaves, which are often continuous between shoots and roots, facilitate the transport of oxygen into the sediment (Sand-Jensen et al. 1982; Soana and Bartoli 2013). In aquatic plants with leaves in the air, pressurized ventilation can occur (Dacey 1980, 1981; Grosse et al. 1991). This arises from thermo-osmosis driven by a temperature difference between the ambient environment and a leaf, which forces gas molecules to diffuse into the warmer compartment. The process requires pores that are small enough to generate Knudsen diffusion, around 1 μm, which in the case of Nuphar lutea occurs in young, newly emerging floating leaves within a monolayer of cells that separates the palisade and spongy parenchyma (Schroder et al. 1986). The flow, into the young leaves and out via the old leaves, at rates of up to five litres per hour, can supply substantial amounts of oxygen to the roots and rhizomes, helping them to reduce damage in flooded sediments. However, the oxic conditions in the sediment around the roots produced by diffusion or ventilation may decrease the availability of phosphorus through changes in redox state (Wigand et al. 1997) see Sect. 3.3.1).
C. Leaf Composition
Leaf composition is influenced by the growth environment of the plant. Unlike most terrestrial leaves where essential mineral resources derive largely from uptake from the soil, aquatic leaves can access nutrients from the sediment and directly from the water.
1. Nitrogen and Phosphorus
Many early studies focused on the extent to which water or the sediment supplied the nitrogen and phosphorus requirements of aquatic plants (Denny 1980; Barko et al. 1991). Differences in nutrient availability in the sediment porewater and overlying water have a major effect on the contribution of roots and leaves to nutrient uptake. Thus, in conditions typical of nutrient-rich Danish streams, Madsen and Cedergreen (2002) showed that the water could completely satisfy the nutrient requirement of macrophytes since experimental removal of the roots, or enriching the water with additional nutrients, had no effect on the relative growth rate of the four species of plant tested.
Early work established critical average yield-limiting elemental composition for freshwater macrophytes of 0.13 and 1.3 as a percentage of dry mass for phosphorus and nitrogen, respectively (Gerloff and Krombholz 1966). These values represent the lower limits of content (yield-limiting), while rate-limiting contents for phosphorus are about 1.6- and 3-times greater for photosynthesis and growth respectively (Colman et al. 1987) and presumably similar for nitrogen. A survey of nutrient contents of 344 plant samples from 65 rivers and lakes in NE Scotland was undertaken by Demars and Edwards (2007). They found that only a few samples were close to being yield-limited, but many were potentially rate-limited, especially by phosphorus availability. There was a large taxon-specific variation in nutrient content: aquatic bryophytes (lacking roots) had lower tissue contents of nitrogen and particularly phosphorus than vascular plants. This suggests that, in addition to anchoring plants in the sediment, roots may play an important role in supplying nutrients to the plant when concentrations in the water are low. Furthermore, macrophytes can store excess phosphorus and levels exceeding 1% of dry mass have been recorded (Thiébaut 2008). These reserves can then prevent or reduce phosphorus limitation when demand exceeds supply. Overall, the flexibility afforded by alternative nutrient supply, from sediment or water, may be one reason why nutrient limitation appears not to be a major factor controlling the growth of freshwater plants.
2. Cell Walls
Cellulose, hemi-cellulose, and pectin are major components of angiosperm cell walls, as is lignin in terrestrial plants. Lignin is a complex phenolic polymer that increases mechanical strength and reduces the permeability of the vessel walls that transport water from the roots to the leaves. The content of lignin varies greatly in angiosperms: grasses contain 5–10% on a dry mass basis and some tropical hardwoods contain more than 40%; an average lignin content of 20% has been estimated for modern land plants (Novaes et al. 2010). Some aquatic species have a similar lignin content to modern land plants, however in many it is lower, although highly variable among species, ranging between 0.3% and 20% for aquatic species and between 2% and 14% for wetland species (Schoelynck et al. 2010). For example, the lignin content in submerged Berula erecta is only 0.8–1.4% while in Nuphar lutea it is about 1.2% in submerged leaves and 2% in floating leaves (derived from Fig. 11.1 in (Grasset et al. 2015)).
The cell walls of seagrass leaves and shoots also have low lignin contents (Espineira et al. 2011) and contain sulphated polysaccharides (anionic polymers), like marine macroalgae, but unlike freshwater and terrestrial plants (Aquino et al. 2005; Silva et al. 2012). In seagrasses, sulphated polysaccharides are made up of galactose units (Papenbrock 2012). The carbohydrate sulfotransferases encoding genes appear to be an ancient eukaryotic feature (Collen et al. 2013) that was lost in land plants, possibly because sulphate concentrations in soils are generally much lower than in the ocean, but regained in seagrasses (Olsen et al. 2016). In marine macroalgae, sulphated polysaccharides confer rheological properties such as flexibility (Kloareg and Quatrano 1988) and presumably perform a similar function in seagrasses.
In fresh waters, leaves have direct access to minerals in water and have a higher mineral content than terrestrial leaves (Ronzhina et al. 2009). The silica concentration in fresh water can be high (on average 200 μM in rivers; (Meybeck 1979)) and some freshwater macrophytes use silica in their cell walls since this produces some rigidity at a 10- to 20-fold lower energy cost than the production of lignin or cellulose (Raven 1983). The biogenic silica content varies between 7 and 28 mg g−1 dry matter for true aquatic species and between 2 and 14 mg g−1 dry matter for wetland species (Schoelynck et al. 2010). Across aquatic and wetland species, there is an antagonist relationship between biogenic silica, lignin, and cellulose content. Interestingly, the freshwater macrophyte Egeria densa acclimated for 3 weeks to higher hydrodynamic stress (exposure to 0.5 m s−1 water flow compared to low water movement) showed ‘thigmomorphogenetic’ responses. The composition of silica and lignin in leaves and stems increased at the higher flow, causing a greater resistance to breaking and a greater tensile strength, although flexibility was unaltered (Schoelynck et al. 2015).
There is a varied response of aquatic plants to the mechanical stress experienced in their environment (Schutten et al. 2004; Bornette and Puijalon 2011; Miler et al. 2012). Two broad strategies have been identified to minimize the risk of shoot breakage, ‘avoidance’ that minimizes the forces experienced by a plant and ‘tolerance’ that maximizes resistance to breakage (Puijalon et al. 2011). In a survey of 30 species, Puijalon et al. (2011) found an inverse correlation between the two strategies, implying that they are alternatives such that each involves a cost as well as a benefit. For example, ‘avoidance’ strategies may incur a cost resulting from self-shading (see Sect. 5), while ‘tolerance’ strategies require a greater resource allocation to structural compounds.
3. Storage Compounds
Starch is a polymer of glucose and a primary product of photosynthesis that serves as a storage compound supporting metabolism and growth when carbon and energy demands outstrip supply (Fondy and Geiger 1982; Weise et al. 2011). Its metabolism is highly influenced by key environmental factors such as day length, temperature, and nutrient availability (Zeeman et al. 2004). In land plants, starch content is extremely variable and ranges between 1 (e.g., in the fern Polypodium punctatum) and 40% (e.g., in the lycophyte Selaginella rupestris; (Sharkey et al. 2004). The starch content of freshwater plants is generally between 0.3% and 2.5% (Steinbachova-Vojtıskova et al. 2006; Cao et al. 2011; Grasset et al. 2015; Yang and Liu 2015). In seagrasses, starch contents as high as 14% have been reported (Touchette and Burkholder 2000) representing around 65% of the total non-structural carbohydrate content. For a given species, starch content varies with nutrient content. For example, in B. erecta and N. lutea, starch content decreased as the habitat nutrient content increased, presumably as starch can accumulate when biomass is limited by availability of nutrients such as nitrogen and phosphorus (Grasset et al. 2015). Ronzhina et al. (2009) found a slightly, but significantly, greater average non-structural polysaccharide (starch and fructosans) content in aquatic compared to terrestrial leaves, possibly caused by the greater lignin and structural polysaccharide content of terrestrial leaves.
4. Regulation of Leaf Form and Structure
Amphibious aquatic plants that live in habitats with fluctuating water levels have to survive and preferably thrive in air and water. Some species produce leaves of similar overall morphology (homophyllous) while others produce leaves of very different appearance in the two environments (heterophyllous; (Maberly and Spence 1989). Leaves of low stature plants, such as the isoetid Littorella uniflora, only differ slightly in morphology when growing in air or water. In a study of this species growing on the shores of a reservoir with seasonal fluctuating water levels, Robe and Griffiths (1998) showed that leaves produced in air were nearly twofold longer and 1.6-fold thinner than leaves produced underwater. Submerged leaves had a few stomata that appeared to be non-functional and terrestrial leaves had a tenfold greater stomatal density and 3.7-fold lower lacunal volume.
In heterophyllous amphibious plants, leaves produced in air are often similar to terrestrial leaves with thick cuticles, functional stomata, and sub-stomatal cavities, while leaves produced in water are typically aquatic in morphology and structure. A range of different environmental factors are known to trigger the switch between aquatic and terrestrial leaves (Maberly and Spence 1989; Wells and Pigliucci 2000). Low versus high temperature (Johnson 1967), short versus long daylength (Kane and Albert 1982), high versus low concentration of CO2 (Bristow 1969), and high versus low water potential (Deschamp and Cooke 1983) favor the production of submerged versus aerial leaves and vice versa. Phytochrome, affected by the ratio of red to far-red light, is involved in the production of aerial leaves in Hippuris vulgaris. Aerial leaves are produced by a low red:far-red ratio in the field and the laboratory even when underwater (Bodkin et al. 1980). This mechanism of sensing the environment is not present in the seagrass Z. marina, which has lost the genes coding for phytochrome production (Olsen et al. 2016), but appears to be present in the brackish water seagrass Ruppia maritima that responds to different red:far-red ratios (Rose and Durako 1994).
Some or all of these responses to environmental cues are probably mediated by hormones. Gibberellic acid often promotes the formation of submerged leaves, while abscisic acid, frequently produced in response to water stress, often promotes the formation of aerial leaves (Maberly and Spence 1989; Wells and Pigliucci 2000). Another plant hormone, ethylene, is involved in shoot elongation, a flooding response in some species including Callitriche platycarpa, Ranunculus sceleratus, and Rumex palustris (Jackson 1985). Potamogeton pectinatus (Stukenia pectinata) lacks the ability to produce ethylene, but responds to exogenously supplied ethylene (Summers and Jackson 1998). The seagrass Z. marina, in contrast, appears to lack ethylene responsive genes (Golicz et al. 2015; Olsen et al. 2016) but, apart from tidal fluctuations, seagrasses do not experience the same seasonal and episodic changes in water level as freshwater and riparian macrophytes.
IV. Resource Acquisition and Responses to Aquatic Environments
A. Light Acquisition
The aquatic environment is basically a shade environment because of a reflection loss at the air-water interface and the absorption of light by water and dissolved and suspended material, which can be exacerbated further by growth of epiphytes on leaf surfaces (Sand-Jensen 1977; Sect. 2.2). The rooting depth limit of freshwater macrophytes is controlled by light and varies between 2.2% of surface light in bryophytes, 5% in charophytes and caulescent (elodeid) angiosperms, 12.9% in Isoetes, and 16.3% in rosette (isoetid) angiosperms (Middelboe and Markager 1997). The differences among groups are partly caused by the proportional extent of roots with a large respiratory burden in isoetids and the ability of some species, such as caulescent angiosperms, to elongate their shoots at low light and therefore grow into shallower water with higher light levels. Seagrasses only have a depth limit of 16 to 18% of surface light (Lee et al. 2007), although in the generally more transparent marine waters, it has been suggested that this could be caused by the effects of pressure, rather than low light, at depth (Beer and Waisel 1982).
Enriquez (2005) studied the light absorption efficiency in Thalassia testudinum and other seagrasses. As expected, leaf light absorption efficiency decreased with pigment content per unit area as a consequence of the package effect. The lower pigment light absorption efficiency of T. testudinum compared to the more typical terrestrial leaf of Mentha aquatica might be explained by the absence of palisade cells and spongy mesophyll cells in seagrasses. However, the thin, flat leaves of tropical seagrasses were more efficient than M. aquatica, perhaps because of greater scattering within the leaf that helps to offset the package effect.
Individual submerged freshwater and marine macrophytes are generally shade-adapted with median compensation points for net photosynthesis (Ic) at a photon irradiance of about 16 μmol m−2 s−1 and the onset of light-saturation (Ik) at about 130 μmol m−2 s−1 (Binzer et al. 2006). In a compilation of data from temperate and tropical seagrasses, the median values of Ic and Ik were 30 and 116 μmol m−2 s−1 respectively (Lee et al. 2007). Freshwater macrophytes from deeper, low-light environments are more shade-adapted than those from shallower water (Spence and Chrystal 1970a, b). The genome of the seagrass Z. marina contains ten genes for LHCB1, one of three proteins that form the trimers of the photosystem II light-harvesting complex. The number of genes for LHCB1 in Z. marina is greater than in Spirodela polyrhiza and Arabidopsis thaliana (Olsen et al. 2016), consistent with enhancing performance at low light. Although individual leaves may be saturated by low levels of light, an individual leaf within a dense stand of plants maybe shaded by other leaves and therefore receive very low light so that the stand may not be fully light-saturated even at maximum light levels (Binzer et al. 2006). Individual leaves or shoots, however, may experience high light and photoinhibition may therefore occur that, although photoprotective, (Adams et al. 2013), will reduce net photosynthesis. When H. verticillata, grown at moderate light, was exposed to full sunlight for 15 minutes, photosynthesis was photoinhibited by about 50% at an inorganic carbon concentration of 0.6 mmol L−1 but was not inhibited at 2 mmol L−1 because carbon fixation provided a greater sink for the light energy absorbed (White et al. 1996). When three species of freshwater macrophyte, grown at low light, were exposed to light from 1.4-fold to 14-fold higher, a photoprotective response was triggered that down-regulated their light harvesting machinery (decreased chlorophyll content) and the photosynthetic electron transport chain (Hussner et al. 2010).
Enzymes and molecules that scavenge reactive oxygen species (ROS) are also involved in photoprotection. The amphibious plant Lobelia cardinalis relies on the xanthophyll cycle for the pre-emptive dissipation of excitation energy as heat preventing ROS formation (Nielsen and Nielsen 2006). In contrast, another amphibious species, Nesaea crassicaulis, when grown in water appeared to lack the xanthophyll cycle but had very high levels of anthocyanin that is a powerful antioxidant and acts as sunscreens and quencher of free radicals a (Nielsen and Nielsen 2006).
Ultraviolet radiation at the water surface can be substantial but it is rapidly attenuated with depth, especially in water with high concentrations of coloured dissolved organic carbon (Morris et al. 1995). However, no significant differences were found in the content of UV-B screening pigments (flavonoids) in floating versus submerged leaves of the amphibious macrophytes Ranunculus trichophyllum and Potamogeton alpinus (Germ et al. 2002). The authors concluded that, in these species, levels of screening pigments were saturating and sufficient to prevent damage at current or elevated (17%) levels of UV-B. In contrast, differences in UV-absorbing compounds were found on a unit area basis between submerged and floating or emerged leaves of Sagittaria sagittifolia and Ranunculus lingua (Klančnik et al. 2014). In the seagrass Z. marina, in contrast to the LHCB1 genes, a gene involved in UV-B-sensing and triggering photo-protective responses, UVR8 (Rizzini et al. 2011), appears to have been lost (Olsen et al. 2016), although associated genes such as COP1 are present. Olsen et al. (2016) hypothesized that the loss of UVR8 is linked to the attenuation of UV radiation in aquatic environments since UVR8 is present in another Alismatales that floats on the water surface, Spirodela. More sequenced genomes are required from a range of different aquatic plants to test this and other hypotheses.
B. Carbon Acquisition
The potential problems of acquiring inorganic carbon in aquatic environments are outlined in Sect. 2.2. Aquatic embryophytes exhibit a large range of anatomical, morphological, biochemical, physiological, and ecological carbon acquisition strategies to minimize this constraint (Klavsen et al. 2011; Maberly and Gontero 2017). This diversity of strategies reflects the various costs and benefits involved in acquiring inorganic carbon in different aquatic environments and contrasts with terrestrial plants where there are only three carbon dioxide acquisition strategies, all based on carboxylation enzymes as described below.
1. C3 Metabolism
Terrestrial and aquatic leaves assimilate CO2 by a common pathway known as the reductive pentose phosphate pathway, the Calvin-Benson-Bassham cycle or the C3 cycle. This pathway uses the products of the light reactions of photosynthesis, ATP and NADPH, to produce carbon skeletons that lead to the production of sucrose and starch and involves 13 reactions catalyzed by 11 enzymes. In the first step, ribulose-1,5-bisphosphate carboxylase-oxygenase (Rubisco) carboxylates ribulose-1,5-bisphosphate (RuBP) with CO2 to produce two molecules of phosphoglyceric acid (PGA), each with three carbon atoms. Rubisco can also oxygenate RuBP producing one molecule of PGA and one molecule of phosphoglycolate leading to photorespiration when the concentration of O2 at its active site is high relative to CO2 (Bowes et al. 1971; Bowes and Ogren 1972). The terrestrial evolutionary history of aquatic embryophytes means that the properties of their Rubisco enzyme are likely to be more similar to that of their direct land plant ancestors than to the microalgal and macroalgal photoautotrophs (Tabita et al. 2008) with which they grow and compete. Similarly, carboxylation in plants is unlikely to involve other enzymes (e.g., use of carbon monoxide dehydrogenase/Acetyl-CoA synthase; (Tabita et al. 2008; Berg 2011; Raven et al. 2012; Hadj-Saïd et al. 2015; Kroth 2015), in contrast to archaea and eubacteria. In order to be fully active, Rubisco must be activated by a non-substrate CO2, or carbamylated at the ϵ-amino group of a specific lysine residue at position 201 (numbered from the plant spinach enzyme) with the divalent cation Mg2+ (Lorimer and Miziorko 1980). Although the regulatory properties of Rubisco activase have not been examined in aquatic organisms, the regulation of this activase is probably similar to that employed by terrestrial shade plants (Gontero and Salvucci 2014).
While the properties of Rubisco in aquatic plants are likely to be similar to their terrestrial forebears, the levels of activity tend to be lower reflecting the lower resource availability in water than in air. A survey of 21 freshwater plants found a median Rubisco activity of about 150 μmol h−1 mg−1 Chla (range of 12 to 464 μmol h−1 mg−1 Chla; (Beer et al. 1991). Rubisco activity was lower in submersed than in emergent freshwater leaves (Beer et al. 1991; Fig. 11.5), in agreement with previous observations (Farmer et al. 1986). Moreover, Rubisco activity from seagrasses was similar to that of submersed freshwater species (Beer et al. 1991). From compiled data for C3 land plants, the average activity of Rubisco from 11 species was higher with a median of about 220 μmol h−1 mg−1 Chla (ranging from 80 to 622 μmol h−1 mg−1 Chla; Fig. 11.5).
Activity of Rubisco per unit chlorophyll a in different plant types. Box and whisker plots show the lower and upper quartiles shaded in grey and the median as the horizontal line. The length of the whiskers is 1.5 times the interquartile range. The maximum outlier is shown by a closed triangle. (Data for aquatic plants from Beer et al. (1991) and terrestrial plants predominantly from Vu et al. (1984). FW = freshwater)
2. Avoidance Strategies
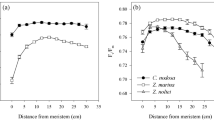
Avoidance strategies (sensu Klavsen et al. (2011) are employed by plants that live and grow in microhabitats with locally high CO2 concentrations, such as above the sediment surface close to sites where decomposition of organic matter produces CO2, and hence avoid problems caused by low CO2 availability. For example, the low-growing aquatic moss Fontinalis antipyretica is restricted to CO2 as a carbon source (Bain and Proctor 1980) yet grows in a lake, Esthwaite Water UK, where surface concentrations of CO2 are close to zero in the summer. It survives by growing just above the sediment surface where concentrations of CO2 are 2- to 10-times air-equilibrium (Maberly 1985a, b). About 20% of freshwater macrophytes lack a CO2-concentrating mechanism (CCM) but, based on plant stature, grow close enough to the sediment to have the possibility of benefitting from a high-CO2 microenvironment (Fig. 11.6).
Carbon acquisition strategies in freshwater macrophytes (grey histograms) and seagrasses (black histograms); data from (Maberly and Madsen 2002; Koch et al. 2013; Borum et al. 2015). Local = leaves that have access to elevated levels of CO2 in their immediate vicinity; Atm = species with access to CO2 in the atmosphere; Sed = species that receive the bulk of CO2 from sediments via anatomical conduits and from their own respiratory CO2; CAM = species that utilize crassulacean acid metabolism to acquire CO2 during the night for subsequent use in light-driven chloroplast-localized photosynthesis during the day; C4 = plants that utilize C4 photosynthesis to acquire CO2 more efficiently during the day; and HCO3 − = plants that utilize bicarbonate as a source of carbon for photosynthesis
3. Exploitation Strategies
Exploitation strategies (sensu Klavsen et al. (2011)) involve morphological or anatomical features that give access to higher concentrations or higher availability of CO2. Examples include floating leaves that exploit the more constant and available concentrations of CO2 in the atmosphere. These can make a major contribution to carbon uptake of amphibious plants (Janauer and Englmaier 1986; Prins and Deguia 1986; Madsen and Breinholt 1995), but they may not be able to prevent overall carbon limitation, so restricting some species to sites with high concentrations of CO2 (Nielsen and Borum 2008). The high concentrations of CO2 in the sediment can be exploited by short isoetids, because extensive lacunae in roots and leaves form a continuous pathway for CO2 to diffuse from the sediment to the leaves. This was first shown by Wium-Andersen (1971) for Lobelia dortmanna and can account for a large, but variable proportion of carbon uptake in many isoetids (Raven et al. 1988; Madsen et al. 2002). The rate of diffusion restricts the length of leaf over which this process can occur. Accordingly, Bagger and Madsen (2004) found that leaves were longer in populations of L. uniflora growing in sediment with high concentrations of CO2. The thick cuticle of some isoetids, especially Lobelia dortmanna, reduces exchange of CO2 with the water, but acts to trap CO2 within the leaf. In Isoetes australis, achlorophyllous leaf bases in the sediment, comprising 34% of shoot surface area, are additional important points of CO2 entry to the photosynthesizing leaves (Pedersen et al. 2011b). Overall, about 30% of the tested species have access to the atmosphere via floating or emergent leaves but only about 3% have access via lacunae to CO2 within the sediment (Fig. 11.6).
4. Amelioration Strategies
C4 Metabolism
Amelioration strategies involve additional, energy-requiring processes. Some land plants, mainly those living in high light, hot, dry and/or saline environments, have evolved adaptations in which CO2 is first fixed by a supplementary pathway, namely crassulacean acid metabolism (CAM) or C4 photosynthesis. These CCMs elevate the CO2 concentration around Rubisco, thereby suppressing photorespiration (Giordano et al. 2005; Raven et al. 2011, 2012; Meyer and Griffiths 2013).
In both pathways, the first carboxylation step is achieved by the oxygen-insensitive enzyme phosphoenol pyruvate carboxylase (PEPC) that catalyzes carboxylation of phosphoenol pyruvate with HCO3 − to yield a four-carbon molecule, oxaloacetate, that is subsequently converted to malate (or aspartate). Malate (or aspartate) is then decarboxylated to produce CO2 near Rubisco by one of three enzymes: NADP malic enzyme (ME), NAD-ME, or PEP carboxykinase (PEPCK). In terrestrial plants, C4 metabolism has long been associated with a radial leaf anatomy (Kranz anatomy), involving two types of cells, to separate physically synthesis of the four carbon acids from decarboxylation in order to prevent futile cycling. Fixation of CO2 by PEPC occurs in mesophyll cells that surround bundle sheath cells where decarboxylation occurs and where Rubisco is present (Gutierre et al. 1974; Hatch 1987; Edwards et al. 2001). Detailed phylogenetic and carbon isotope studies have resolved C3, C4 and C3–C4 intermediate relationships within many taxonomic groups and showed that the C4 pathway of photosynthesis has evolved many times, suggesting that compartmentation is an intrinsic feature maximizing the efficiency of the process (Sage 2004; Sage et al. 2012). Interestingly, about a decade ago, variants of the C4 pathway within a single cell were discovered in terrestrial plants growing in salty, semi-desert regions, including Bienertia cycloptera and Borszczowia aralocaspica (family Chenopodiaceae) (Voznesenskaya et al. 2001; Voznesenskaya et al. 2002; Edwards et al. 2004) and more recently Bienertia sinuspersici (Offermann et al. 2011). These results show that Kranz anatomy is not essential for C4 metabolism.
In aquatic systems, a number of ‘amelioration strategies’ involving CCMs exist to counteract the limitations in inorganic carbon availability (Raven 1970; Maberly and Madsen 1998, 2002; Giordano et al. 2005; Klavsen et al. 2011). It is likely that C4 photosynthesis arose in aquatic systems, in response to limitations in dissolved CO2, before its advent in terrestrial ones, based on PEPCK in the green macroalga Udotea flabellum (Reiskind and Bowes 1991), and more controversially, and geologically later, in some marine diatoms putatively based on PEPC (Roberts et al. 2007; Reinfelder 2011; Clement et al. 2016).
Like the single-cell C4 metabolism in some terrestrial plants, aquatic C4 metabolism takes place in a single cell. The best characterized freshwater C4 plant is the submerged aquatic monocotyledon Hydrilla verticillata (Hydrocharitaceae); (Holaday and Bowes 1980; Salvucci and Bowes 1981; Bowes et al. 2002). C4 metabolism in H. verticillata is facultative, being induced by various environmental conditions that reduce the availability of CO2 and promote photorespiration (Reiskind et al. 1997). In H. verticillata, physical separation of PEP carboxylation and decarboxylation is achieved within a single cell since PEPC is confined to the cytosol and Rubisco to the chloroplast.
The regulatory patterns and properties of the enzymes involved in C4 metabolism have been studied in detail in H. verticillata. When C4 metabolism is induced in H. verticillata, the activities of PEPC, pyruvate phosphate dikinase (PPDK), NADP–ME, and asparagine and alanine aminotransferases are elevated, while Rubisco activity remains constant (Magnin et al. 1997). One out of the three PEPC isoforms was expressed uniquely in C4 leaves and transcript levels for this isoform increased (Rao et al. 2002). Genes for two isoforms of PPDK, as well as genes that encode a transporter, an aminotransferase, and two chaperonins, were also up-regulated (Rao et al. 2006). The activity of NADP-ME increased tenfold in C4 plants (Magnin et al. 1997) and a specific chloroplast isoform (HVME1) was induced. This isoform had specific and unusual regulatory properties that are likely to facilitate C4 metabolism in this species (Estavillo et al. 2007). Furthermore, the catalytic efficiency of recombinant HVME1 was twofold higher than that of rice but lower than the plastid forms of maize, and the Km for malate was higher than that for the maize enzyme but fourfold lower than that found in rice. Bowes and co-workers concluded that NADP-ME is the decarboxylating enzyme in H. verticillata (Holaday and Bowes 1980). Although both NAD- and NADP-ME increased under limiting CO2 conditions (Table 4 in Salvucci and Bowes 1981), 95% of the total NAD-ME activity was found in the mitochondrial fraction. Therefore, if NAD-ME was the decarboxylating enzyme, CO2 would be released in the cytosol causing a futile cycle since PEPC is also present within this compartment. In contrast, the NADP-ME isoform, HVME1, is specifically induced in the chloroplast, the site where CO2 is concentrated (Reiskind et al. 1997) and it was therefore concluded that it was the main decarboxylating enzyme in this species.
Egeria densa, another species from the Hydrocharitaceae, also exhibits a NADP-ME C4 syndrome, under high light and high temperature, that can also be triggered by exogenous application of the plant stress hormone abscisic acid (Casati et al. 2000). The kinetic properties of the purified NADP-ME that was induced were similar to those of terrestrial C3 plants. The partially purified PEPC synthesized during the induction of C4 photosynthesis had a Km value (ca 8 μM) for HCO3 − that is lower than those reported in the literature for other PEPC enzymes (Casati et al. 2000). PEPC isoforms are not only induced in leaves (Casati et al. 2000), but also in stems in E. densa under low CO2 conditions (Gu et al. 2015). Similar results were obtained for PPDK and NADP-ME, possibly indicating that the stem could act as a photosynthetic organ as has been described in some terrestrial plants such as tobacco and celery (Hibberd and Quick 2002) and several species of trees (Berveiller and Damesin 2008). As is often the case for an enzyme catalyzing the first step of a pathway, PEPC is finely regulated: phosphorylation and feedback inhibition by malate are well established in terrestrial plants (Chollet et al. 1996) and possibly also occur in aquatic systems (Casati et al. 2000; Lara et al. 2002; Gu et al. 2015). Recently, two further Hydrocharitaceae, Ottelia alismoides and O. acuminata, were also shown to exhibit the C4 syndrome in the absence of Kranz anatomy (Zhang et al. 2014; Shao et al. 2017). When grown at low CO2, the activity of PEPC and NAD-ME was higher than when grown at high CO2. If NAD-ME is the decarboxylating enzyme in these species it will be the first reported case for an aquatic plant. Four carbon acids accumulate in other freshwater macrophytes, such as Elodea canadensis (Degroote and Kennedy 1977), but there is currently no evidence for turnover and transfer of CO2 from these metabolites into the C3 pathway.
In seagrasses, the capacity to perform C4 photosynthesis seems limited to a few species. Evidence is strongest for Cymodocea nodosa (Beer et al. 1980), but Halophila stipulacea and Thalassia testudinum may have a facultative C4 metabolism induced by low CO2 concentrations (Koch et al. 2013; Larkum et al. 2017); further investigation is required to test this possibility. If confirmed, this would be another example of C4 photosynthesis in aquatic plants lacking Kranz anatomy since there is no indication of Kranz anatomy in any seagrasses (e.g., Fig. 11.2). Presently, about 4% of tested freshwater macrophytes and seagrasses show the capacity for C4 metabolism (Fig. 11.6).
CAM
The other supplementary pathway in terrestrial plants, CAM, is very similar to C4 photosynthesis, but PEPC and Rubisco are temporally regulated, the former being active at night and the latter during the day. At night, the four-carbon compound malic acid is stored in vacuoles and decarboxylated during the following day. The features of CAM are therefore: net uptake of CO2 and accumulation of malic acid during the night, diel change in acidity and starch content of the cells, opposite to one another, a high PEPC activity during the night, and a high PEPC:Rubisco activity ratio (Osmond 1984; Winter et al. 2015).
For decades, CAM was regarded as a water conserving measure in plants from environments with intermittent water availability. The intriguing discovery by J. Keeley that this metabolism was also present in an aquatic plant (Isoetes howellii; (Keeley 1981) was first rejected but later confirmed (Keeley 2014). In these plants, there is net CO2 uptake accompanied by malic acid accumulation during the night (Keeley et al. 1983; Keeley and Busch 1984). CAM has subsequently been found in all other species of Isoetis and other submerged macrophytes including L. uniflora (Madsen 1987a) and Crassula helmsii (Newman and Raven 1995; Klavsen and Maberly 2009). Other freshwater species show some evidence for diurnal acid fluctuations that may be related to CAM (Webb et al. 1988) including Vallisneria spiralis (Keeley 1998) and V. spinulosa, Nechamandra alternifolia, and E. densa (Yin et al. 2017). Recently, it was shown that O. alismoides (Zhang et al. 2014) and Deinostema violaceum (a member of the Plantaginaceae, like L. uniflora; (Yin et al. 2017) are able to operate CAM facultatively. CAM has been found in about 9% of tested aquatic species, which is comparable to the percentages estimated for terrestrial plants (Silvera et al. 2010).
Crassulacean acid metabolism is under environmental control in aquatic plants. Typically CAM is up-regulated when leaves are in water and down-regulated when leaves are in air in Isoetes howellii and L. uniflora (Keeley and Busch 1984; Aulio 1986; Robe and Griffiths 2000); the opposite to expectations for a water-conserving measure and to what occurs in some terrestrial CAM plants (Cushman and Borland 2002). After about 3 days emersion, CAM activity in L. uniflora aquatic leaves decreased by 70% and new terrestrial leaves had no CAM activity and exhibited high Rubisco activity (Robe and Griffiths 2000). Recently, using a range of approaches, including titratable acidity measurements, mRNA levels, and activity measurements of C3 and C4 enzymes, it was shown that Isoetes sinensis possesses a stronger CAM activity under submerged than under terrestrial conditions (Yang and Liu 2015), although, unlike other species of Isoetes, it appeared not to be completely down-regulated in leaves grown in air (Yin et al. 2017). CAM is also down-regulated when L. uniflora is grown at low light (43 μmol photon m−2 s−1 of photosynthetically active radiation) versus high light (450 μmol photon m−2 s−1) and CAM activity is reduced when this species is grown at high concentrations of CO2 (Madsen 1987b). Activity can also change seasonally (Klavsen et al. 2011; Klavsen and Madsen 2012).
CAM in aquatic ecosystems is correctly considered to be a carbon-conserving strategy that increases net inorganic carbon acquisition. For instance, many species with CAM live in soft-water lakes or in shallow seasonal pools of moderate alkalinity where the availability of CO2 is low (Sondergaard and Sand-Jensen 1979; Keeley et al. 1983; Madsen 1985). Aquatic CAM is beneficial in this habitat because, unlike the situation for many terrestrial CAM plants, uptake of external CO2 is not suppressed in the light allowing CO2 to take place over 24 hours and to exploit nocturnal concentrations of CO2 that are often elevated relative to those during day. A major carbon-conserving advantage of aquatic CAM is its ability to reduce respiratory loss of carbon by converting CO2 to malate. For example, in C. helmsii, CAM activity was equivalent to 74% of night-time respiration in spring and over 200% in summer (Klavsen and Maberly 2009). CAM also has a beneficial effect by increasing rates of photosynthesis and suppressing photorespiration in Isoetes australis (Pedersen et al. 2011a).
About 98% of the malic acid that is decarboxylated during the day to produce endogenous CO2 is re-assimilated in Littorella and Isoetes. This high efficiency is likely to be related to internal storage in the extensive lacunal system of the leaves, their relatively thick cuticles, and the resistance of the surrounding liquid medium. In these species, the maximum CO2 concentration in the lacunal air ranged from 0.8 to 1.5%, which is comparable to the values for terrestrial plants (Madsen 1987a). Last, it has been suggested that CAM could function as a nitrogen conserving mechanism because an increased concentration of CO2 at the active site of Rubisco makes the enzyme more efficient (Sage and Kubien 2003). However, for L. uniflora at least, this hypothesis could not be confirmed (Baattrup-Pedersen and Madsen 1999).
There is no suggestion so far that any seagrasses operate a CAM system (Koch et al. 2013). Some accumulation of 14C label in four carbon acids has been detected in Thalassia hemprichii, Thalassodendron ciliatum, and Halophila stipulacea that did not, however, appear to be linked to CAM metabolism (Beer et al. 1980). Nonetheless, more research is warranted to test the possibility that a form of CAM is in operation in some species.
Bicarbonate Use
Bicarbonate is the most abundant form of inorganic carbon in seawater and many fresh waters (Sect. 2.2) (Maberly and Gontero 2017). However, some form of active transport is required to acquire HCO3 − from the surrounding aqueous medium because the plasmalemma is impermeable to HCO3 − and the aquatic leaf has a negative internal membrane potential (Denny and Weeks 1970) creating a large electrochemical gradient opposing passive HCO3 − entry. A widespread mechanism of HCO3 − acquisition in freshwater macrophytes is the generation of low and high pH at the abaxial and adaxial leaf surfaces respectively (the so-called polar leaf). This mechanism has similarities to that associated with the well-studied banding on the giant cells of some freshwater green macroalgae (charophytes) Chara and Nitella (Lucas and Smith 1973). The formation of marl (calcite) on the upper surface of aquatic leaves of species within certain genera, e.g., Elodea, Egeria, Hydrilla and Potamogeton (Fig. 11.7), has been known for decades and studied as a mechanism of carbon uptake by scientists such as Arens, Ruttner, and Steemann-Nielsen in the middle of the twentieth century (see review by Prins (1989). Extensive studies by Prins and co-workers (1980, 1982; Prins and Elzenga 1989) have elucidated the various mechanisms involved. The cells of the lower epidermis often, but not always, have extensively folded plasma membranes with the appearance of transfer cells. The proposed mechanism involves active extrusion of protons at the lower epidermis, aided by the larger plasma membrane area, generating a local reduction of pH (down to pH 4). This converts HCO3 − into CO2 within the boundary layer that can then diffuse into the leaf. In order to maintain electrochemical balance, cations are transported through the apoplast from the lower to the upper side of the leaf and a net export of OH− occurs at the upper epidermis generating high pH values (pH 10 to 11) causing HCO3 − to be converted to carbonate and calcite to be precipitated the marl seen in (Fig. 11.7). The precipitation of carbonate also leads to the generation of CO2 close to the cell surface in Chara corallina that may be taken up (McConnaughey 1991) and a similar process may occur at the upper leaf surface. This is not the only mechanism involved in HCO3 − utilization since some species within genera such as Ranunculus or Myriophyllum are effective users of HCO3 − (Maberly and Spence 1983) but do not possess the physical leaf features described above, as is also true for Vallisneria spiralis (Prins et al. 1980). However, there might still be spatial horizontal patterns of high and low pH that act in a similar way, analogous to the banding in some species of Chara. Alternatively, or in addition, HCO3 −-H+ co-transport might be involved in HCO3 − uptake, but more work is needed to elucidate the mechanisms involved that are likely to vary among species.
Of the species of freshwater macrophyte tested, about 45% have the ability to use HCO3 − (Maberly and Madsen 2002; Fig. 11.6). Less extensive work has been performed on HCO3 − use in seagrasses compared to freshwater macrophytes. Compiled data (Carr and Axelsson 2008; Koch et al. 2013; Borum et al. 2015) suggest that about 85% of the 27 species that have been studied are able to use HCO3 − (Fig. 11.6). This is greater than the 57% found in the freshwater macrophytes tested, but the marine habitat has a higher HCO3 − concentration than many fresh waters, so the ecological advantage may be greater in the oceans. Detailed studies of the mechanism of HCO3 − use in seagrasses have been performed using inhibitors by a number of workers. For example, in Z. marina and Ruppia cirrhosa, HCO3 − use mainly involves HCO3 − dehydration in external acid zones, catalyzed by periplasmic carbonic anhydrase, followed by diffusion into the leaf (Hellblom et al. 2001; Hellblom and Axelsson 2003). In addition, non-catalyzed HCO3 − dehydration or direct uptake of HCO3 − has also been suggested to be involved. In a study of nine seagrass species (Borum et al. 2015), seven were definitely able to use HCO3 − and of these, five were suggested to operate a mechanism involving external acidification. In the two additional species, internal conversion of HCO3 − to CO2 was inferred. External carbonic anhydrase played a role in four of the species with the ability to use HCO3 − (see Table 3 in Borum et al. 2015). Fewer studies of this nature have been undertaken on freshwater macrophytes.
The more constant concentration of CO2 in the oceans and the closer coupling of the aqueous CO2 concentration with the atmospheric CO2 content makes it possible to assess whether seagrass photosynthesis is currently saturated by inorganic carbon and likely to be increased by changing atmospheric CO2 content. Borum et al. (2015) found that the rate of net photosynthesis in eight of the nine species they tested, the exception being Zostera polychlamys, was limited at pre-industrial concentrations of CO2 (280 ppm). This suggests that relief of carbon-limitation in future elevated CO2 environments might alter the competitive interactions among the species in non-light-limited situations. Experiments to determine carbon limitation in stream macrophytes showed that in situ rates of photosynthesis were 35% of CO2-saturated rates for a species unable to use HCO3 − and 60% for a species able to use HCO3 − despite the stream having a high CO2 concentration of about 220 μmol L−1 (Madsen and Maberly 1991). A similar type of assessment, but based on growth carried out in two contrasting Danish lakes with lower CO2 concentrations than the stream, showed that a species restricted to using CO2 was unable to grow while a species able to use HCO3 − grew slowly but the rate was stimulated by about 50% when supplied with higher CO2 concentrations (Vadstrup and Madsen 1995). These experiments, and others like them, show that freshwater macrophytes without the ability to use HCO3 − can be severely limited by carbon availability at typical environmental concentrations of CO2 and that although HCO3 − helps to reduce this it may not remove carbon-limitation completely.
V. Trade-Offs, Synergies, and Future Prospects
The contrasting environmental challenges and opportunities of air and water as environments for photosynthesis and growth have been described in the text and summarised in Table 11.1. These represent responses to a single environmental factor while in reality a leaf has to respond simultaneously to multiple environmental conditions. These may require a trade-off, where one response is optimised for one environmental factor but is less beneficial for another or a synergy where a particular feature has more than one benefit. Consequently, different characteristics are beneficial in different environments, although this is complicated by environmental variability that may alter the cost-benefit balance of a trade-off, and by acclimation at a range of time-scales that will help to optimize fitness by altering morphology and physiology. It is this interaction among environmental conditions, characteristics, trade-offs, and synergies that control fitness and the ecological distribution of species. In this penultimate section, three examples of different possible types of trade-offs, and three examples of synergies, are discussed.
A. Trade-Offs
Structural or physiological characteristics typically have energy or opportunity costs as well as fitness benefits. As a result, they confer an advantage under some, but not all, environments. As a first example, the flexible nature of many aquatic plants reduces the mechanical stress they experience in flowing water and thus reduces the risk of shoots breaking or being uprooted. However, the reconfiguration of shoots can have negative consequences by increasing self-shading and therefore reducing light availability to the plant (Sand-Jensen 1998).
A second example concerns the morphology of many aquatic leaves. Madsen (1991) showed that entire, as opposed to dissected, leaves were more common in oligotrophic compared to eutrophic lakes and more common in streams and marine systems than in lakes. This was attributed to trade-offs between minimizing boundary-layer thickness, and hence maximising rate of material exchange, and preventing mechanical damage at high flow. In seagrasses, the general pattern of thin leaves in some small species and thicker leaves in larger species is probably linked, at least in part, to a similar trade-off.
A third example relates to the use of HCO3 − in aquatic plants. This has costs and benefits that find different balances in different environments. The most obvious cost relates to the extra energy required to produce and operate the machinery required for HCO3 − use (Raven and Lucas 1985; Raven et al. 2008). The relevance of this cost in the ‘real world’ is supported by the lack of HCO3 − use in red macroalgae growing in low light environments in the ocean (Maberly 1990) and in bryophytes (Bain and Proctor 1980) that are frequently found at the depth-limit in lakes (Middelboe and Markager 1997). If energy was the only cost, one would expect HCO3 − to be ubiquitous in optically shallow water, but this is not the case as was observed (Maberly et al. 2015) in a transect down a fairly shallow river fed by a spring with very high HCO3 − concentrations (>4 mmol L−1). Concentrations of CO2 were very high close to the source, and the species found there were restricted to those that use CO2. A few kilometers downstream, where concentrations of CO2 were much lower but the concentration of HCO3 − was virtually the same, all the species tested were able to use HCO3 − (Maberly et al. 2015). A similar response also occurs within a species, Elodea canadensis, grown at high light and very high concentrations of CO2 (2200 μM), down-regulated its ability to use HCO3 − (Sand-Jensen and Gordon 1986). An additional cost of using HCO3 − relates to the affinity of using CO2 (Maberly and Madsen 1998). At a given limiting concentration of CO2, the CO2-dependent rate of photosynthesis is around twofold higher in species restricted to CO2 than in ones also able to use HCO3 −. This difference appears to be related to a lower internal permeability in species able to use HCO3 − (Madsen and Maberly 2003). This is teleologically consistent with, although not yet proved to be linked to, the prevention of futile cycling in species able to use HCO3 −: a low permeability reduces the efflux of inorganic carbon actively taken up by the leaf. It is also possible that species limited to the use of CO2 have a lower leaf construction cost than those species that also use HCO3 −. There are virtually no data on this, but in Table 11.1 in Ronzhina and Ivanov (2014), the single submerged species tested that lacks the ability to use HCO3 −, Utricularia vulgaris, had the lowest area-based construction cost. Clearly, this is worthy of further study.
B. Synergies
Some structural and physiological features have a synergistic benefit for more than one environmental challenge. For example, the lack of water stress allows chloroplasts to be present in the epidermis of the leaves of many submerged plants (e.g., Fig. 11.2). This is likely to be beneficial both for the supply of light and carbon although it has been noted (Black et al. 1981) that the large external transport resistance around the leaves of freshwater macrophytes means that internal diffusion resistances are relatively small in comparison.
In a second example, flexible shoots reduce the risk of breakage but also reduce water flow rate within the plant stand and so promote sedimentation. Sediment within a patch has been found to be 4.1 cm above the river bed on average (Sand-Jensen and Pedersen 2008) and since this comprises fine organic sediment, it has a high carbon, nitrogen, and phosphorus content. This may make an important contribution to nutrient availability to plants in streams and rivers that are not already eutrophic (Sand-Jensen 1998). However, in rivers with sufficient nutrients in the water, high organic carbon content in the sediment can cause a negative trade-off by limiting oxygen supply to the roots (Moller and Sand-Jensen 2011) and, in some species at least, reducing the strength of anchorage provided by the roots, increasing the risk that the whole plant might be washed out of the system (Sand-Jensen and Moller 2014).
In a third example, floating or emergent leaves give access to the much more reliable supply of CO2 in the atmosphere. This is a common feature in many emergent amphibious plants and makes them among the most productive plants in the world (Westlake 1975). Moreover, access to the atmosphere also helps to minimize the common problem of restricted oxygen supply to roots and rhizomes by providing a pathway for atmospheric oxygen to reach roots and rhizomes, especially in species with ‘forced ventilation’(Dacey 1980). The transpiration stream may also promote the transport of nutrients from root to shoot at a rate greater than that possible by acropetal transport (Pedersen 1993).
C. Future Prospects
As is hopefully apparent from this chapter, there is a large and growing body of information about how aquatic leaves respond to the challenges and opportunities presented by their environment and hence maximize their fitness. There are also some clear knowledge gaps, some of which are highlighted below. Unlike most terrestrial plants (but the C4/CAM Portulaca oleracea is an exception; (Koch and Kennedy 1980)), some aquatic plants can operate more than one CCM or carbon acquisition strategy. There are some apparent, but presently unexplained, associations between the different CCMs in freshwater macrophytes and seagrasses. All species known to have the ability to perform C4 photosynthesis are also able to use HCO3 −. While it is true that HCO3 − is the substrate for PEPC, the link between these two facts is not necessarily straightforward and direct. One can speculate that the association could be linked to the low concentrations of CO2 and high concentrations of O2 that can occur in productive beds where C4 plants may be found. While both processes act as a CCM, the C4 pre-fixation by PEPC may help to reduce oxygen sensitivity but HCO3 − provides the continued supply of carbon. In contrast, species known to have the ability to perform CAM are largely restricted to CO2. It is possible to speculate that species with the ability to undergo CAM tend to rely on a relatively thick cuticle to trap CO2 produced by decarboxylation of malate within the plant. This might be an effective strategy in species such as the isoetids that can obtain carbon from the sediment where it will also serve to increase internal concentrations of CO2, but will be detrimental to plants that rely on HCO3 − in the water. One interesting exception is O. alismoides that appears to operate three CCMs, HCO3 − use, C4 photosynthesis, and, facultatively, CAM (Zhang et al. 2014; Shao et al. 2017). If these mechanisms are all confirmed to be present, it will raise interesting questions about how these different processes are regulated and interact.
Unlike terrestrial plants, including A. thaliana, few aquatic plants have been sequenced, the recently published sequence of Z. marina (Olsen et al. 2016) being an exception as are the genomes of two species of two floating freshwater plants, Lemna minor (Van Hoeck et al. 2015) and Spirodela polyrhiza (Wang et al. 2014). Genomic sequencing and analysis of more aquatic species and their comparison with terrestrial species will lead to novel phylogenetic and functional insights. Along with analyses of proteomes, transcriptomes and metabolomes, it will dramatically increase our understanding of how aquatic plants respond to their environment at the level of their morphology, physiology, and biochemistry.
VI. Conclusions
The challenges and opportunities faced by aquatic plants photosynthesising in water are very different to those of their terrestrial ancestors photosynthesising in air. This has led to distinctive differences in the morphology, structure and resource acquisition processes of aquatic compared to terrestrial leaves. The contrasting properties of air and water, and the biological characteristics that they trigger, can be useful to highlight fundamental processes. For example, the interrelationships between CCMs discussed above are also relevant to discussions about the evolution of terrestrial C4 photosynthesis and CAM (Sage 2002). In another example, the discovery of CAM in aquatic plants (Keeley 1981) and the subsequent realization that its function was linked to carbon-conservation, rather than water-conservation, has also illuminated the carbon-conserving function of CAM in terrestrial plants. Within the field of food security, the possibility of introducing genes for C4 photosynthesis into C3 crops such as rice (Leegood 2013) has relied in part on the extensive work on the best-characterized plant with single-cell photosynthesis, Hydrilla verticillata (Bowes 2011). Aquatic leaves are also part of the global spectrum of leaf and shoot types. In a recent analysis, Díaz et al. (2016) showed that aquatic leaves lie on one of the major axes that they identified. They fell within the group of ephemeral, small plants with low investment in vegetative structures along with small terrestrial ruderals such as Arabidopsis thaliana. Recent work that related the size of terrestrial leaves (Wright et al. 2017) to leaf-energy budgets and water-loss could be confronted with data from aquatic leaves where other challenges are likely to be dominant as a result of the physical properties of water.
Abbreviations
- A:
-
air
- Atm:
-
atmospheric
- ATP:
-
adenosine-5′-triphosphate
- CAM:
-
crassulacean acid metabolism
- CCM:
-
CO2-concentrating mechanism
- Chl:
-
chlorophyll
- COP1:
-
E3 ubiquitin ligase constitutively photomorphogenic 1
- Env:
-
environment
- FW:
-
freshwater
- HVME1:
-
Hydrilla verticillata malic enzyme isoform1
- Ic :
-
incident light at which respiratory CO2 generation is balanced by photosynthetic CO2 fixation
- Ik :
-
incident light at which photosynthetic CO2 fixation approaches saturation
- LHCB1:
-
light-harvesting chlorophyll-binding complex 1 of photosystem II
- ME:
-
malic enzyme
- NAD:
-
nicotinamide adenine dinucleotide
- NADP:
-
nicotinamide adenine dinucleotide phosphate
- PEPC:
-
phosphoenolpyruvate carboxylase
- PEPCK:
-
PEP carboxykinase
- PGA:
-
phosphoglyceric acid
- PPDK:
-
pyruvate phosphate dikinase
- ROS:
-
reactive oxygen species
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase-oxygenase
- RuBP:
-
ribulose-1,5-bisphosphate
- Sed:
-
sedimentary
- UV:
-
ultraviolet radiation
- UVR8:
-
UV-B photoreceptor UV resistance locus 8
- W:
-
water
References
Adams WW III, Muller O, Cohu CM, Demmig-Adams B (2013) May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth Res 117:31–44
Aquino RS, Landeira-Fernandez AM, Valente AP, Andrade LR, Mourao PAS (2005) Occurrence of sulfated galactans in marine angiosperms: evolutionary implications. Glycobiology 15:11–20
Arber A (1920) Water plants: a study of Aquatic Angiosperms. Cambridge University Press, Cambridge
Aulio K (1986) CAM-like photosynthesis in Littorella uniflora (L.) Aschers – the role of humidity. Ann Bot 58:273–275
Baattrup-Pedersen A, Madsen TV (1999) Interdependence of CO2 and inorganic nitrogen on crassulacean acid metabolism and efficiency of nitrogen use by Littorella uniflora (L.) Aschers. Plant Cell Environ 22:535–542
Bagger J, Madsen TV (2004) Morphological acclimation of aquatic Littorella uniflora to sediment CO2 concentration and wave exposure. Funct Ecol 18:946–951
Bain JT, Proctor MCF (1980) The requirement of aquatic bryophytes for free CO2 as an inorganic carbon source, some experimental evidence. New Phytol 86:393–400
Barko JW, Gunnison D, Carpenter SR (1991) Sediment interactions with submersed macrophyte growth and community dynamics. Aquat Bot 41:41–65
Beer S, Shomerilan A, Waisel Y (1980) Carbon metabolism in seagrasses. 2. Patterns of photosynthetic CO2 incorporation. J Exp Bot 31:1019–1026
Beer S, Waisel Y (1982) Effects of light and pressure on photosynthesis in 2 seagrasses. Aquat Bot 13:331–337
Beer S, Sandjensen K, Madsen TV, Nielsen SL (1991) The carboxylase activity of rubisco and the photosynthetic performance in aquatic plants. Oecologia 87:429–434
Benzecry A (2013) Field notes on Thalassia testudinum growing under stress conditions. Eur J Environ 1:7–10
Berg IA (2011) Ecological aspects of the distribution of different autotrophic CO2 fixation pathways. Appl Environ Microbiol 77:1925–1936
Berveiller D, Damesin C (2008) Carbon assimilation by tree stems:potential involvement of phosphoenolpyruvate carboxylase. Trees- Struct Func 22:149–157
Binzer T, Sand-Jensen K, Middelboe A-L (2006) Community photosynthesis of aquatic macrophytes. Limnol Oceanogr 51:2722–2733
Black MA, Maberly SC, Spence DHN (1981) Resistance to carbon dioxide fixation in four submerged freshwater macrophytes. New Phytol 89:557–568
Bodkin PC, Spence DHN, Weeks DC (1980) Photoreversible control of heterophylly in Hippuris vulgaris L. New Phytol 84:533–542
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14
Borum J, Pedersen O, Kotula L, Fraser MW, Statton J, Colmer TD, Kendrick GA (2015) Photosynthetic response to globally increasing CO2 of co-occurring temperate seagrass species. Plant Cell Environ 39:1240–1250
Bowes G, Ogren WL, Hageman RH (1971) Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun 45:716–722
Bowes G, Ogren WL (1972) Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem 247:2171–2176
Bowes G, Rao SK, Estavillo GM, Reiskind JB (2002) C4 mechanisms in aquatic angiosperms: comparisons with terrestrial C4 systems. Funct Plant Biol 29:379–392
Bowes G (2011) Single-cell C4 photosynthesis in aquatic plants. In: Raghavendra AS, Sage RF (eds) C4 photosynthesis and related CO2 concentrating mechanisms, pp 63–80
Bristow JM (1969) The effects of carbon dioxide on the growth and development of amphibious plants. Can J Bot 47:1803–1807
Cao T, Ni L, Xie P, Xu J, Zhang M (2011) Effects of moderate ammonium enrichment on three submersed macrophytes under contrasting light availability. Freshw Biol 56:1620–1629
Carr H, Axelsson L (2008) Photosynthetic utilization of bicarbonate in Zostera marina is reduced by inhibitors of mitochondrial ATPase and electron transport. Plant Physiol 147:879–885
Casati P, Lara MV, Andreo CS (2000) Induction of a C-4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiol 123:1611–1621
Chambers PA, Lacoul P, Murphy KJ, Thomaz SM (2008) Global diversity of aquatic macrophytes in freshwater. Hydrobiologia 595:9–26
Chen LY, Chen JM, Gituru RW, Wang QF (2012) Generic phylogeny, historical biogeography and character evolution of the cosmopolitan aquatic plant family Hydrocharitaceae. BMC Evol Biol 12:30
Chollet R, Vidal J, Oleary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47:273–298
Clement R, Dimnet L, Maberly SC, Gontero B (2016) The nature of the CO2 -concentrating mechanisms in a marine diatom, Thalassiosira pseudonana. New Phytol 209:1417–1427
Cole JJ, Caraco NF, Kling GW, Kratz TK (1994) Carbon dioxide supersaturation in the surface waters of lakes. Science 265:1568–1570
Collen J, Porcel B, Carre W, Ball SG, Chaparro C, Tonon T,. .. Boyen C (2013) Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc Natl Acad Sci U S A 110:5247--5252.
Colman JA, Sorsa K, Hoffmann JP, Smith CS, Andrews JH (1987) Yield- and photosynthesis-derived critical concentrations of tissue phosphorus and their significance for growth of Eurasian water milfoil, Myriophyllum spicatum. Aquat Bot 29:111–122
Colmer TD, Pedersen O (2008) Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytol 177:918–926
Cook CDK (1990) Aquatic plant book. SPB Publishing, The Hague
Crawford RMM (1992) Oxygen availability as an ecological limit to plant distribution. Adv Ecol Res 23:93–185
Cushman JC, Borland AM (2002) Induction of Crassulacean acid metabolism by water limitation. Plant Cell Environ 25:295–310
Dacey JWH (1980) Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science 210:1017–1019
Dacey JWH (1981) Pressurized ventilation in the Yellow Waterlily. Ecology 62:1137–1147
Degroote D, Kennedy RA (1977) Photosynthesis In Elodea canadensis Michx: four-carbon acid synthesis. Plant Physiol 59:1133–1135
Demars BOL, Edwards AC (2007) Tissue nutrient concentrations in freshwater aquatic macrophytes: high inter-taxon differences and low phenotypic response to nutrient supply. Freshw Biol 52:2073–2086
den Hartog C, Kuo J (2006) Taxonomy and biogeography of seagrasses. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 1–23
Denny MW (1993) Air and water: the biology and physics of life’s media. Princeton University Press, Princeton
Denny P, Weeks DC (1970) Effects of light and bicarbonate on membrane potential in Potamogeton schweinfurthii (Benn). Ann Bot 34:483–496
Denny P (1980) Solute movement in submerged angiosperms. Biol Rev Camb Philos Soc 55:65–92
Deschamp PA, Cooke TJ (1983) Leaf dimorphism in aquatic angiosperms: significance of turgor pressure and cell expansion. Science 219:505–507
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S et al (2016) The global spectrum of plant form and function. Nature 529:167–171
Du Z-Y, Wang Q-F (2014) Correlations of life form, pollination mode and sexual system in aquatic angiosperms. Plos One 9:e115653
Duarte CM, Middelburg JJ, Caraco N (2005) Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2:1–8
Edwards D, Kerp H, Hass H (1998) Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot 49:255–278
Edwards GE, Franceschi VR, Ku MSB, Voznesenskaya EV, Pyankov VI, Andreo CS (2001) Compartmentation of photosynthesis in cells and tissues of C4 plants. J Exp Bot 52:577–590
Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55:173–196
Enriquez S, Duarte CM, SandJensen K, Nielsen SL (1996) Broad-scale comparison of photosynthetic rates across phototrophic organisms. Oecologia 108:197–206
Enriquez S (2005) Light absorption efficiency and the package effect in the leaves of the seagrass Thalassia testudinum. Mar Ecol Prog Ser 289:141–150
Espineira JM, Novo Uzal E, Gomez Ros LV, Carrion JS, Merino F, Ros Barcelo A, Pomar F (2011) Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol 13:59–68
Estavillo GM, Rao SK, Reiskind JB, Bowes G (2007) Characterization of the NADP malic enzyme gene family in the facultative, single-cell C4 monocot Hydrilla verticillata. Photosynth Res 94:43–57
Farmer AM, Maberly SC, Bowes G (1986) Activities of carboxylation enzymes in freshwater macrophytes. J Exp Bot 37:1568–1573
Fondy BR, Geiger DR (1982) Diurnal pattern of translocation and carbohydrate-metabolism in source leaves of Beta vulgaris L. Plant Physiol 70:671–676
Friis EM, Pedersen KR, Crane PR (2001) Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 410:357–360
Frost-Christensen H, Jogensen LB, Floto F (2003) Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant Cell Environ 26:561–569
Gerloff GC, Krombholz PH (1966) Tissue analysis as a measure of nutrient availability for the growth of angiosperm aquatic plants. Limnol Oceanogr 11:529–537
Germ M, Mazej Z, Gaberscik A, Hader DP (2002) The influence of enhanced UV-B radiation on Batrachium trichophyllum and Potamogeton alpinus - aquatic macrophytes with amphibious character. J Photochem Photobiol B Biol 66:37–46
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
Golicz AA, Schliep M, Lee HT, Larkum AWD, Dolferus R, Batley J et al (2015) Genome-wide survey of the seagrass Zostera muelleri suggests modification of the ethylene signalling network. J Exp Bot 66:1489–1498
Gomez B, Daviero-Gomez V, Coiffard C, Martin-Closas C, Dilcher DL (2015) Montsechia, an ancient aquatic angiosperm. Proc Natl Acad Sci U S A 112:10985–10,988
Gontero B, Salvucci ME (2014) Regulation of photosynthetic carbon metabolism in aquatic and terrestrial organisms by Rubisco activase, redox-modulation and CP12. Aquat Bot 118:14–23
Graham L, Lewis LA, Taylor W, Wellman C, Cook M (2014) Early terrestrialization: transition from algal to bryophyte grade. In: Hanson DT, Rice SK (eds) Photosynthesis in Bryophytes and Early Land Plants. Springer, Dordercht, pp 9–28
Grasset C, Delolme C, Arthaud F, Bornette G (2015) Carbon allocation in aquatic plants with contrasting strategies: the role of habitat nutrient content. J Veg Sci 26:946–955
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
Grosse W, Buchel HB, Tiebel H (1991) Pressurized ventilation in wetland plants. Aquat Bot 39:89–98
Gu S, Yin L, Wang Q-f (2015) Phosphoenolpyruvate carboxylase in the stem of the submersed species Egeria densa may be involved in an inducible C4-like mechanism. Aquat Bot 125:1–8
Gutierre M, Gracen VE, Edwards GE (1974) Biochemical and cytological relationships in C4 plants. Planta 119:279–300
Hadj-Saïd J, Pandelia M-E, Léger C, Fourmond V, Dementin S (2015) The carbon monoxide dehydrogenase from Desulfovibrio vulgaris. Biochim Biophys Acta 1847:1574–1583
Hatch MD (1987) C4 Photosynthesis - a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Hellblom F, Beer S, Bjork M, Axelsson L (2001) A buffer sensitive inorganic carbon utilisation system in Zostera marina. Aquat Bot 69:55–62
Hellblom F, Axelsson L (2003) External HCO3 − dehydration maintained by acid zones in the plasma membrane is an important component of the photosynthetic carbon uptake in Ruppia cirrhosa. Photosynth Res 77:173–181
Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415:451–454
Holaday AS, Bowes G (1980) C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiol 65:331–335
Hsu JS, Powell J, Adler PB (2012) Sensitivity of mean annual primary production to precipitation. Global Change Biol 18:2246–2255
Hussner A, Hoelken HP, Jahns P (2010) Low light acclimated submerged freshwater plants show a pronounced sensitivity to increasing irradiances. Aquat Bot 93:17–24
Jackson MB (1985) Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Plant Physiol 36:145–174
Janauer GA, Englmaier P (1986) The effects of emersion on soluble carbohydrate accumulations in Hippuris vulgaris L. Aquat Bot 24:241–248
Johnson MP (1967) Temperature dependent leaf morphogenesis in Ranunculus flabellaris. Nature 214:1354–1355
Kane ME, Albert LS (1982) Growth regulators in aquatic plants: environmental and growth regulator effects on heterophylly and growth of Proserpinaca intermedia (Haloragaceae). Aquat Bot 13:73–85
Karlsson J, Bystrom P, Ask J, Ask P, Persson L, Jansson M (2009) Light limitation of nutrient-poor lake ecosystems. Nature 460:506–509
Keeley JE (1981) Isoetes howellii a submerged aquatic CAM plant. Am J Bot 68:420–424
Keeley JE, Mathews RP, Walker CM (1983) Diurnal acid metabolism in Isoetes howellii from a temporary pool and a permanent lake. Am J Bot 70:854–857
Keeley JE, Busch G (1984) Carbon assimilation characteristics of the aquatic CAM plant, Isoetes howellii. Plant Physiol 76:525–530
Keeley JE (1998) CAM photosynthesis in submerged aquatic plants. Bot Rev 64:121–175
Keeley JE (2014) Aquatic CAM photosynthesis: a brief history of its discovery. Aquat Bot 118:38–44
Kirk JTO (2011) Light and photosynthesis in aquatic environments. Cambridge University Press, Cambridge
Klančnik K, Pančić M, Gaberščik A (2014) Leaf optical properties in amphibious plant species are affected by multiple leaf traits. Hydrobiologia 737:121–130
Klavsen SK, Maberly SC (2009) Crassulacean acid metabolism contributes significantly to the in situ carbon budget in a population of the invasive aquatic macrophyte Crassula helmsii. Freshw Biol 54:105–118
Klavsen SK, Madsen TV, Maberly SC (2011) Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: a review. Photosynth Res 109:269–279
Klavsen SK, Madsen TV (2012) Seasonal variation in crassulacean acid metabolism by the aquatic isoetid Littorella uniflora. Photosynth Res 112:163–173
Kloareg B, Quatrano RS (1988) Structure of the cell walls of marine algae and ecophysiological function of the matrix polysaccharides. Oceanogr Mar Biol 26:259–315
Koch K, Kennedy RA (1980) Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiol 65:193–197
Koch M, Bowes G, Ross C, Zhang X-H (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Chang Biol 19:103–132
Koi S, Ikeda H, Rutishauser R, Kato M (2015) Historical biogeography of river-weeds (Podostemaceae). Aquat Bot 127:62–69
Kovalenko KE, Thomaz SM, Warfe DM (2012) Habitat complexity: approaches and future directions. Hydrobiologia 685:1–17
Krause-Jensen D, Sand-Jensen K (1998) Light attenuation and photosynthesis of aquatic plant communities. Limnol Oceanogr 43:396–407
Kroth PG (2015) The biodiversity of carbon assimilation. J Plant Physiol 172:76–81
Kuo J, den Hartog C (2006) Seagrass morphology, anatomy, and ultrastructure. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 57–87
Lara MV, Casati P, Andreo CS (2002) CO2 concentrating mechanisms in Egeria densa, a submersed aquatic plant. Physiol Plant 115:487–495
Larkum AWD, Davey PA, Kuo J, Ralph PJ, Raven JA (2017) Carbon-concentrating mechanisms in seagrasses. J Exp Bot 68:3773–3784
Lamb JB, van de Water JAJM, Bourne DG, Altier C, Hein MY, Fiorenza EA, Abu N, Jompa J, Harvell CD (2017) Seagrass ecosystems reduce exposure to bacterial pathogens of humans, fishes, and invertebrates. Science 355:731–733
Lee K-S, Park SR, Kim YK (2007) Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: a review. J Exp Mar Biol Ecol 350:144–175
Leegood RC (2013) Strategies for engineering C4 photosynthesis. J Plant Physiol 170:378–388
Les DH, Schneider EL (1995) The nymphaeales, alismatidae, and the theory of an aquatic monocotyledon origin. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ (eds) Monocotyledons: systematics and evolution. Royal Botanic Gardens, Kew, pp 23–42
Les DH, Cleland MA, Waycott M (1997) Phylogenetic studies in alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily. Syst Bot 22:443–463
Les DH, Tippery N (2013) In time and with water... the systematics of alismatid monocotyledons. In: Wilkin P, Mayo SJ (eds) Early events in monocot evolution. Cambridge University Press, Cambridge, pp 118–164
Les DH (2015) Water from the rock: ancient aquatic angiosperms flow from the fossil record. Proc Natl Acad Sci U S A 112:10825–10,826
Lin B-L, Yang W-J (1999) Blue light and abscisic acid independently induce heterophyllous switch in Marsilea quadrifolia. Plant Physiol 119:429–434
Lorimer GH, Miziorko HM (1980) Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulose bisphosphate carboxylase by CO2 and Mg2+. Biochemistry 19:5321–5328
Lucas WJ, Smith FA (1973) Formation of alkaline and acid regions at surface of Chara corallina cells. J Exp Bot 24:1–14
Maberly SC, Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. J Ecol 71:705–724
Maberly SC (1985a) Photosynthesis by Fontinalis antpyretica. 1. Interaction between photon irradiance, concentration of carbon dioxide and temperature. New Phytol 100:127–140
Maberly SC (1985b) Photosynthesis by Fontinalis antipyretica 2. Assessment of environmental factors limiting photosynthesis and production. New Phytol 100:141–155
Maberly SC, Spence DHN (1989) Photosynthesis and photorespiration in freshwater organisms- amphibious plants. Aquat Bot 34:267–286
Maberly SC (1990) Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae. J Phycol 26:439–449
Maberly SC (1996) Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshw Biol 35:579–598
Maberly SC, Madsen TV (1998) Affinity for CO2 in relation to the ability of freshwater macrophytes to use HCO3. Funct Ecol 12:99–106
Maberly SC, Madsen TV (2002) Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Funct Plant Biol 29:393–405
Maberly SC (2014) The fitness of the environments of air and water for photosynthesis, growth, reproduction and dispersal of photoautotrophs: an evolutionary and biogeochemical perspective. Aquat Bot 118:4–13
Maberly SC, Berthelot SA, Stott AW, Gontero B (2015) Adaptation by macrophytes to inorganic carbon down a river with naturally variable concentrations of CO2. J Plant Physiol 172:120–127
Maberly SC, Gontero B (2017) Ecological imperatives for aquatic CO2-concentrating mechanisms. J Exp Bot 68:3797–3814
Madsen JD (1991) Ecology of submersed aquatic macrophytes resource allocation at the individual plant level. Aquat Bot 41:67–86
Madsen TV (1985) A community of submerged aquatic CAM plants in lake Kalgaard, Denmark. Aquat Bot 23:97–108
Madsen TV (1987a) Interactions between internal and external CO2 pools in the photosynthesis of the aquatic CAM plants Littorella uniflora (L) Aschers and Isoetes lacustris L. New Phytol 106:35–50
Madsen TV (1987b) The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiol Plant 70:183–188
Madsen TV, Maberly SC (1991) Diurnal variation in light and carbon limitation of photosynthesis by two species of submerged freshwater macrophyte with a differential ability to use bicarbonate. Freshw Biol 26:175–187
Madsen TV, Breinholt M (1995) Effects of air contact on growth, inorganic carbon sources and nitrogen uptake by an amphibous freshwater macrophyte. Plant Physiol 107:149–154
Madsen TV, Cedergreen N (2002) Sources of nutrients to rooted submerged macrophytes growing in a nutrient-rich stream. Freshw Biol 47:283–291
Madsen TV, Olesen B, Bagger J (2002) Carbon acquisition and carbon dynamics by aquatic isoetids. Aquat Bot 73:351–371
Madsen TV, Maberly SC (2003) High internal resistance to CO2 uptake by submerged macrophytes that use HCO3 −: measurements in air, nitrogen and helium. Photosynth Res 77:183–190
Magnin NC, Cooley BA, Reiskind JB, Bowes G (1997) Regulation and localization of key enzymes during the induction of Kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol 115:1681–1689
McConnaughey T (1991) Calcification in Chara corallina: CO2 hydroxylation generates protons for bicarbonate assimilation. Limnol Oceanogr 36:619–628
Meybeck M (1979) Major elements contents of river waters and dissolved inputs to the oceans. Rev. Geol Dyn Geogr Phys 21:215–246
Meyer M, Griffiths H (2013) Origins and diversity of eukaryotic CO2 concentrating mechanisms: lessons for the future. J Exp Bot 64:769–786
Middelboe AL, Markager S (1997) Depth limits and minimum light requirements of freshwater macrophytes. Freshw Biol 37:553–568
Miler O, Albayrak I, Nikora V, O’Hare M (2012) Biomechanical properties of aquatic plants and their effects on plant-flow interactions in streams and rivers. Aquat Sci 74:31–44
Moller CL, Sand-Jensen K (2011) High sensitivity of Lobelia dortmanna to sediment oxygen depletion following organic enrichment. New Phytol 190:320–331
Morris DP, Zagarese H, Williamson CE, Balseiro EG, Hargreaves BR, Modenutti B et al (1995) The attentuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol Oceanogr 40:1381–1391
Newman JR, Raven JA (1995) Photosynthetic carbon assimilation by Crassula helmsii. Oecologia 101:494–499
Nielsen LT, Borum J (2008) Why the free floating macrophyte Stratiotes aloides mainly grows in highly CO2-supersaturated waters. Aquat Bot 89:379–384
Nielsen SL, Nielsen HD (2006) Pigments, photosynthesis and photoinhibition in two amphibious plants: consequences of varying carbon availability. New Phytol 170:311–319
Novaes E, Kirst M, Chiang V, Winter-Sederoff H, Sederoff R (2010) Lignin and biomass: a negative correlation for wood formation and lignin content in trees. Plant Physiol 154:555–561
Offermann S, Okita TW, Edwards GE (2011) Resolving the compartmentation and function of C4 photosynthesis in the single-cell C4 species Bienertia sinuspersici. Plant Physiol 155:1612–1628
Olsen JL, Rouzé P, Verhelst B, Lin Y-C, Bayer T, Collen J et al (2016) The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 530:331–335
Osmond CB (1984) CAM: regulated photosynthetic metabolism for all seasons. In: Sybesma C (ed) Advances in photosynthesis research. Junk, The Hague, pp 557–563
Pagani M, Caldeira K, Berner R, Beerling DJ (2009) The role of terrestrial plants in limiting atmospheric CO2 decline over the past 24 million years. Nature 460:85–88
Papenbrock J Highlights in seagrasses’ phylogeny, physiology, and metabolism: what makes them special? ISRN Bot 2012, 2012:103892
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41:421–453
Pearson PN, Palmer MR (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406:695–699
Pedersen O (1993) Long-distance water transport in aquatic plants. Plant Physiol 103:1369–1375
Pedersen O, Rich SM, Pulido C, Cawthray GR, Colmer TD (2011a) Crassulacean acid metabolism enhances underwater photosynthesis and diminishes photorespiration in the aquatic plant Isoetes australis. New Phytol 190:332–339
Pedersen O, Pulido C, Rich SM, Colmer TD (2011b) In situ O2 dynamics in submerged Isoetes australis: varied leaf gas permeability influences underwater photosynthesis and internal O2. J Exp Bot 62:4691–4700
Pierce S, Brusa G, Sartori M, Cerabolini BEL (2012) Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Ann Bot 109:1047–1053
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Prins HBA, Snel JFH, Helder RJ, Zanstra PE (1980) Photosynthetic HCO3 − utilization and OH− excretion in aquatic angiosperms: light induced pH changes at the leaf surface. Plant Physiol 66:818–822
Prins HBA, Snel JFH, Zanstra PE, Helder RJ (1982) The mechanisms of bicarbonate assimilation by the polar leaves of Potamogeton and Elodea: CO2 concentrations at the leaf surface. Plant Cell Environ 5:207–214
Prins HBA, Deguia MB (1986) Carbon source of the water soldier, Stratiotes aloides L. Aquat Bot 26:225–234
Prins HBA, Elzenga JTM (1989) Bicarbonate utilization: function and mechanism. Aquat Bot 34:59–83
Puijalon S, Bouma TJ, Douady CJ, van Groenendael J, Anten NP, Martel E, Bornette G (2011) Plant resistance to mechanical stress: evidence of an avoidance-tolerance trade-off. New Phytol 191:1141–1149
Rao SK, Magnin NC, Reiskind JB, Bowes G (2002) Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single-cell, facultative C4 system of Hydrilla verticillata. Plant Physiol 130:876–886
Rao SK, Fukayama H, Reiskind JB, Miyao M, Bowes G (2006) Identification of C4 responsive genes in the facultative C4 plant Hydrilla verticillata. Photosynth Res 88:173–183
Raven JA (1970) Exogenous inorganic carbon sources in plant photosynthesis. Biol Rev Camb Philos Soc 45:167–221
Raven JA (1983) The transport and function of silicon in plants. Biol Rev 58:179–207
Raven JA, Lucas WJ (1985) Energy cost of carbon acquisition. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. American Society of plant physiologists, Rockville, pp 305–324
Raven JA, Handley LL, Macfarlane JJ, McInroy S, McKenzie L, Richards JH, Samuelsson G (1988) The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form- A review with new data on Eriocaulon decangulare L. New Phytol 108:125–148
Raven JA (2008) Not drowning but photosynthesizing: probing plant plastrons. New Phytol 177:841–845
Raven JA, Cockell CS, De La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos Trans R Soc Lond Ser B Biol Sci 363:2641–2650
Raven JA, Giordano M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109:281–296
Raven JA, Giordano M, Beardall J, Maberly SC (2012) Algal evolution in relation to atmospheric CO2: carboxylases, carbon-concentrating mechanisms and carbon oxidation cycles. Philos Trans R Soc Lond Ser B Biol Sci 367:493–507
Reinfelder JR (2011) Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annu Rev Mar Sci 3:291–315
Reiskind JB, Bowes G (1991) The role of phosphoenolpyruvate carboxykinase in a marine macroalga with C4-like photosynthetic characteristics. Proc Natl Acad Sci USA 88:2883–2887
Reiskind JB, Madsen TV, VanGinkel LC, Bowes G (1997) Evidence that inducible C4 type photosynthesis is a chloroplastic CO2 concentrating mechanism in Hydrilla, a submersed monocot. Plant Cell Environ 20:211–220
Rizzini L, Favory J-J, Cloix C, Faggionato D, O’Hara A, Kaiserli E,... Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 Protein. Science 332:103--106.
Robe WE, Griffiths H (1998) Adaptations for an amphibious life: changes in leaf morphology, growth rate, carbon and nitrogen investment, and reproduction during adjustment to emersion by the freshwater macrophyte Littorella uniflora. New Phytol 140:9–23
Robe WE, Griffiths H (2000) Physiological and photosynthetic plasticity in the amphibious, freshwater plant, Littorella uniflora, during the transition from aquatic to dry terrestrial environments. Plant Cell Environ 23:1041–1054
Roberts K, Granum E, Leegood RC, Raven JA (2007) Carbon acquisition by diatoms. Photosynth Res 93:79–88
Ronzhina DA, P’Yankov VI (2001) Structure of the photosynthetic apparatus in leaves of freshwater hydrophytes: 2. Quantitative characterisation of leaf mesophyll and the functional activity of leaves with different degress of submersion. Russ J Plant Physiol 48:567–575
Ronzhina DA, Ivanov LA, Lambers G, VI P’y (2009) Changes in chemical composition of hydrophyte leaves during adaptation to aquatic environment. Russ J Plant Physiol 56:355–362
Ronzhina DA, Ivanov LA (2014) Construction costs and mesostructure of leaves in hydrophytes. Russ J Plant Physiol 61:776–783
Rose CD, Durako MJ (1994) Induced photomorphogenesis by an altered R:FR light ratio in axenic Ruppia maritima L. Bot Mar 37:531–535
Rudall PJ, Knowles EVW (2013) Ultrastructure of stomatal development in early-divergent angiosperms reveals contrasting patterning and pre-patterning. Ann Bot 112:1031–1043
Runions A, Tsiantis M, Prusinkiewicz P (2017) A common developmental program can produce diverse leaf shapes. New Phytol 216:401–418
Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21:1030–1035
Sage RF (2002) Are crassulacean acid metabolism and C4 photosynthesis incompatible? Funct Plant Biol 29:775–785
Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth Res 77:209–225
Sage RF (2004) The evolution of C4 photosynthesis. New Phytol 161:341–370
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 Photosynthesis. In: Merchant SS (ed) Annual review of plant biology, vol 63, pp 19–47
Salvucci ME, Bowes G (1981) Induction of reduced photorespiratory activity in submersed and amphibious aquatic macrophytes. Plant Physiol 67:335–340
Sand-Jensen K (1977) Effect of epiphytes on eelgrass photosynthesis. Aquat Bot 3:55–63
Sand-Jensen K, Prahl C, Stokholm H (1982) Oxygen release from roots of submerged aquatic macrophytes. Oikos 38:349–354
Sand-Jensen K, Gordon DM (1986) Variable HCO3 − affinity of Elodea canadensis Michaux in response to different HCO3 − and CO2 concentrations during growth. Oecologia 70:426–432
Sand-Jensen K (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw Biol 39:663–679
Sand-Jensen K, Binzer T, Middelboe AL (2007) Scaling of photosynthetic production of aquatic macrophytes - a review. Oikos 116:280–294
Sand-Jensen K, Pedersen ML (2008) Streamlining of plant patches in streams. Freshw Biol 53:714–726
Sand-Jensen K, Moller CL (2014) Reduced root anchorage of freshwater plants in sandy sediments enriched with fine organic matter. Freshw Biol 59:427–437
Scheffer M, Hosper SH, Meijer ML, Moss B, Jeppesen E (1993) Alternative equilbria in shallow lakes. Trends Ecol Evol 8:275–279
Schoelynck J, Bal K, Backx H, Okruszko T, Meire P, Struyf E (2010) Silica uptake in aquatic and wetland macrophytes: a strategic choice between silica, lignin and cellulose. New Phytol 186:385–391
Schoelynck J, Puijalon S, Meire P, Struyf E (2015) Thigmomorphogenetic responses of an aquatic macrophyte to hydrodynamic stress. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00043
Schroder P, Grosse W, Woermann D (1986) Localization of thermo-osmotically active partitions in young leaves of Nuphar lutea. J Exp Bot 37:1450–1461
Schubert H, Sagert S, Forster RM (2014) Evaluation of the different levels of variability in the underwater light field of a shallow estuary. Helgol Mar Res 55:12–22
Schutten J, Dainty J, Davy AJ (2004) Wave-induced hydraulic forces on submerged aquatic plants in shallow lakes. Ann Bot 93:333–341
Sculthorpe CD (1967) The biology of aquatic vascular plants. Edward Arnold, London
Shao H, Gontero B, Maberly SC, Jiang HS, Cao Y, Li W, Huang WM (2017) Responses of Ottelia alismoides, an aquatic plant with three CCMs, to variable CO2 and light. J Exp Bot 68:3985–3995
Sharkey TD, Weise SE, Standish AJ, Terashima I (2004) Chloroplast to leaf. In: Smith WK, Vogelmann TC, Critchley C (eds) Photosynthetic adaptation, vol 178. Springer, New York, pp 171–206
Short F, Carruthers T, Dennison W, Waycott M (2007) Global seagrass distribution and diversity: a bioregional model. J Exp Mar Biol Ecol 350:3–20
Silva TH, Alves A, Popa EG, Reys LL, Gomes ME, Sousa RA et al (2012) Marine algae sulfated polysaccharides for tissue engineering and drug delivery approaches. Biomatter 2:278–289
Silva TSF, Melack JM, Novo EMLM (2013) Responses of aquatic macrophyte cover and productivity to flooding variability on the Amazon floodplain. Glob Chang Biol 19:3379–3389
Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC (2010) Evolution along the crassulacean acid metabolism continuum. Funct Plant Biol 37:995–1010
Soana E, Bartoli M (2013) Seasonal variation of radial oxygen loss in Vallisneria spiralis L.: an adaptive response to sediment redox? Aquat Bot 104:228–232
Sondergaard M, Sand-Jensen K (1979) Carbon uptake by leaves and roots of Littorella uniflora (L) Aschers. Aquat Bot 6:1–12
Spence DHN, Chrystal J (1970a) Photosynthesis and zonation of freshwater macrophytes. 1. Depth distribution and shade tolerance. New Phytol 69:205–215
Spence DHN, Chrystal J (1970b) Photosynthesis and zonation of fresh-water macrophytes. 2. Adaptability of species of deep and shallow water. New Phytol 69:217–217
Steinbachova-Vojtıskova L, Tylovaa E, Soukupa A, Novickaa H, Votrubovaa O, Lipavskaa H, Cızkovab H (2006) Influence of nutrient supply on growth, carbohydrate,and nitrogen metabolic relations in Typha angustifolia. Environ Exp Bot 57:246–257
Summers JE, Jackson MB (1998) Light- and dark-grown Potamogeton pectinatus, an aquatic macrophyte, make no ethylene (ethene) but retain responsiveness to the gas. Aust J Plant Physiol 25:599–608
Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS (2008) Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J Exp Bot 59:1515–1524
Talling JF (1985) Inorganic carbon reserves of natural waters and ecophysiological consequences of their photosynthetic depletion: microalgae. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, pp 403–435
Thiébaut G (2008) Phosphorus and aquatic plants. In: White PJ, Hammond JP (eds) The ecophysiology of plant-phosphorus interactions. Springer, Dordrecht, pp 31–49
Thoning KW, Tans PP, Komhyr WD (1989) Atmospheric carbon dioxide at Mauna Loa observatory. 2. Analysis of the NOAA GMCC data, 1974–1985. J Geophys Res Atmos 94:8549–8565
Touchette BW, Burkholder JM (2000) Overview of the physiological ecology of carbon metabolism in seagrasses. J Exp Mar Biol Ecol 250:169–205
Touchette BW (2007) Seagrass-salinity interactions: physiological mechanisms used by submersed marine angiosperms for a life at sea. J Exp Mar Biol Ecol 350:194–215
Vadstrup M, Madsen TV (1995) Growth limitation of submerged aquatic macrophytes by inorganic carbon. Freshw Biol 34:411–419
Van der Hage JCH (1996) Why are there no insects and so few higher plants, in the sea? New thoughts on an old problem. Funct Ecol 10:546–547
van der Heijden LH, Kamenos NA (2015) Reviews and syntheses: calculating the global contribution of coralline algae to total carbon burial. Biogeosciences 12:6429–6441
van Donk E, van de Bund WJ (2002) Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot 72:261–274
Van Hoeck A, Horemans N, Monsieurs P, Cao HX, Vandenhove H, Blust R (2015) The first draft genome of the aquatic model plant Lemna minor opens the route for future stress physiology research and biotechnological applications. Biotechnol Biofuels 8:188
Verberk W, Bilton DT, Calosi P, Spicer JI (2011) Oxygen supply in aquatic ectotherms: partial pressure and solubility together explain biodiversity and size patterns. Ecology 92:1565–1572
Verboven P, Pedersen O, Ho QT, Nicolai BM, Colmer TD (2014) The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant Cell Environ 37:2433–2452
Vile D, Garnier E, Shipley B, Laurent G, Navas ML, Roumet C et al (2005) Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann Bot 96:1129–1136
Voesenek L, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73
Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE (2001) Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414:543–546
Voznesenskaya EV, Franceschi VR, Kiirats O, Artyusheva EG, Freitag H, Edwards GE (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J 31:649–662
Vu JCV, Allen LH, Bowes G (1984) Dark/light modulation of ribulose bisphosphate carboxylase activity in plants from different photosynthetic categories. Plant Physiol 76:843–845
Wang W, Haberer G, Gundlach H, Glaesser C, Nussbaumer T, Luo MC et al (2014) The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nat Commun 5:3311
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S,. .. Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci U S A 106:12377--12,381.
Webb DR, Rattray MR, Brown JMA (1988) A preliminary survey for crassulacean acid metabolism (CAM) in submerged aquatic macrophytes in New Zealand. N Z J Mar Freshw Res 22:231–235
Weise SE, van Wijk KJ, Sharkey TD (2011) The role of transitory starch in C3, CAM, and C4 metabolism and opportunities for engineering leaf starch accumulation. J Exp Bot 62:3109–3118
Wells CL, Pigliucci M (2000) Adaptive phenotypic plasticity: the case of heterophylly in aquatic plants. Perspect Plant Ecol Evol Syst 3:1–18
Westlake DF (1975) Primary production of freshwater macrophytes. In: Cooper JP (ed) Photosynthesis and productivity in different environments. Cambridge University Press, Cambridge, pp 189–206
White A, Reiskind JB, Bowes G (1996) Dissolved inorganic carbon influences the photosynthetic responses of Hydrilla to photoinhibitory conditions. Aquat Bot 53:3–13
Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N et al (2014) Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc Natl Acad Sci U S A 111:E4859–E4868
Wiegleb G (1991) Die lebens und wuchsformen der makrophytischen wasserpflanzen und deren beziehungen zu ökologie, verbreitung und vergesellschaftung der arten. Tuexenia 11:135–148
Wigand C, Stevenson JC, Cornwell JC (1997) Effects of different submersed macrophytes on sediment biogeochemistry. Aquat Bot 56:233–244
Williams K, Percival F, Merino J, Mooney HA (1987) Estimation of tissue construction costs from heat of combustion and organic nitrogen content. Plant Cell Environ 10:725–734
Winter K, Holtum JAM, Smith JAC (2015) Crassulacean acid metabolism: a continuous or discrete trait. New Phytol 208:73–78
Wium-Andersen S (1971) Photosynthetic uptake of free CO2 by roots of Lobelia dortmanna. Physiol Plant 25:245–248
Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Díaz S,. .. Wilf P (2017) Global climatic drivers of leaf size. Science 357:917--921.
Yang T, Liu X (2015) Comparing photosynthetic characteristics of Isoetes sinensis Palmer under submerged and terrestrial conditions. Sci Rep 5:17783
Yin L, Li W, Madsen TV, Maberly SC, Bowes G (2017) Photosynthetic inorganic carbon acquisition in 30 freshwater macrophytes. Aquat Bot 140:48–54
Zeeman SC, Smith SM, Smith AM (2004) The breakdown of starch in leaves. New Phytol 163:247–261
Zhang Y, Yin L, Jiang H-S, Li W, Gontero B, Maberly SC (2014) Biochemical and biophysical CO2 concentrating mechanisms in two species of freshwater macrophyte within the genus Ottelia (Hydrocharitaceae). Photosynth Res 121:285–297
Ziegler JP, Solomon CT, Finney BP, Gregory-Eaves I (2015) Macrophyte biomass predicts food chain length in shallow lakes. Ecosphere 6:1–16
Acknowledgments
We are extremely grateful to Marion Cambridge, Lukasz Kotula, Ole Pedersen, and Quing-Feng Wang for contributing photographs to Figs. 11.1 and 11.2, to Dina Ronzhina for giving permission to reproduce her drawings of leaf sections reproduced in Fig. 11.2 and to Hendrik Poorter for permission to reproduce Fig. 11.4. The Chinese Academy of Sciences is thanked for providing Visiting Professorships for Senior International Scientists and the President’s International Fellowship Initiative to the authors (2015VBA023, 2016VBA006). Stephen Maberly’s work is supported by the UK Natural Environment Research Council. Brigitte Gontero’s group is supported by Centre National de la Recherche Scientifique, Aix-Marseille Université, A*Midex project (No. ANR-11-IDEX-0001-02), Agence National de la Recherche (Signaux-BioNRJ, ANR-15-CE05-0021-03).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Maberly, S.C., Gontero, B. (2018). Trade-offs and Synergies in the Structural and Functional Characteristics of Leaves Photosynthesizing in Aquatic Environments. In: Adams III, W., Terashima, I. (eds) The Leaf: A Platform for Performing Photosynthesis. Advances in Photosynthesis and Respiration, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-319-93594-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-93594-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93592-8
Online ISBN: 978-3-319-93594-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)