Abstract
Free-living stages of parasites are consumed by a variety of predators, which might have important consequences for predators, parasites, and hosts. For example, zooplankton prey on the infectious stage of the amphibian chytrid fungus, Batrachochytrium dendrobatidis (Bd), a pathogen responsible for amphibian population declines and extinctions worldwide. Predation on parasites is predicted to influence community structure and function, and affect disease risk, but relatively few studies have explored its consequences empirically. We investigated interactions among Rana cascadae tadpoles, zooplankton, and Bd in a fully factorial experiment in outdoor mesocosms. We measured growth, development, survival, and infection of amphibians and took weekly measurements of the abundance of zooplankton, phytoplankton (suspended algae), and periphyton (attached algae). We hypothesized that zooplankton might have positive indirect effects on tadpoles by consuming Bd zoospores and by consuming phytoplankton, thus reducing the shading of a major tadpole resource, periphyton. We also hypothesized that zooplankton would have negative effects on tadpoles, mediated by competition for algal resources. Mixed-effects models, repeated-measures ANOVAs, and a structural equation model revealed that zooplankton significantly reduced phytoplankton but had no detectable effects on Bd or periphyton. Hence, the indirect positive effects of zooplankton on tadpoles were negligible when compared to the indirect negative effect mediated by competition for phytoplankton. We conclude that examination of host-pathogen dynamics within a community context may be necessary to elucidate complex community dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity can profoundly affect the emergence and transmission of infectious diseases (Keesing et al. 2010). For example, active or passive foraging on free-living stages of helminths, fungi, protists, and some ectoparasites by a variety of aquatic and terrestrial predators might have consequences for predators, parasites, and hosts (Johnson et al. 2010). Parasite life cycle stages in aquatic ecosystems have, on average, 4.2–14.2 predators (Thieltges et al. 2013), and one study estimated that predation on parasites represented 44 % of links in an estuarine food web (Lafferty et al. 2006). Determining the strength of these interactions is important because they could lead to changes in food web structure, ecosystem function, and disease risk. However, few studies have empirically investigated the influence of these interactions on community dynamics.
Chytridiomycosis, a disease caused by the fungus Batrachochytrium dendrobatidis (Bd), is a major driver of ongoing loss of global amphibian biodiversity (Mendelson et al. 2006; Skerratt et al. 2007; Wake and Vredenburg 2008; Rohr et al. 2008a; Fisher et al. 2009). The infective stage of the fungus, an aquatic flagellated zoospore, infects keratinized tissues of amphibian hosts. In larvae, keratinized jaw sheaths and tooth rows may become infected, causing lethal (e.g., Blaustein et al. 2005) or sublethal effects, including reduced foraging efficiency (Venesky et al. 2009), reduced rates of growth and development (e.g., Parris and Cornelius 2004), and behavioral changes (Parris et al. 2006; Han et al. 2008). Effects of reduced growth and development during the larval stage may persist into adult stages (Metcalfe and Monaghan 2001; Altwegg and Reyer 2003; Rohr et al. 2006; Rohr and Palmer 2013). Larvae may also function as important reservoirs of the pathogen (Briggs et al. 2010).
Among various strategies suggested for the control of Bd (Woodhams et al. 2011) is the potential for predators to consume Bd zoospores, which could prevent infection load from reaching a critical threshold (Vredenburg et al. 2010). Zoospores of Bd are 3–5 µm in diameter (Longcore et al. 1999), well within the preferred range of food particle size for zooplankton (Knisely and Geller 1986; Sanders and Porter 1990; Pace and Funke 1991; Kagami 2004; Gleason et al. 2008). Buck et al. (2011) confirmed the presence of Bd in the gut of Daphnia, and several laboratory studies have demonstrated that filter-feeding species such as microcrustaceans (Woodhams et al. 2011; Hamilton et al. 2012; Searle et al. 2013) and tadpoles (Venesky et al. 2014) can reduce Bd zoospore densities in water. Furthermore, Daphnia (Searle et al. 2013) and filter-feeding tadpoles (Venesky et al. 2014) can dilute Bd infection risk for tadpoles. Zoospores of a different chytrid fungus, Zygorhizidium planktonicum, are consumed by Daphnia spp., thus protecting the diatom Asterionella formosa from infection (Kagami et al. 2004) and providing nutritional benefits to predatory zooplankton (Kagami et al. 2007). However, it is not known whether zooplankton consume Bd zoospores in the presence of alternative food sources, or what effects this might have on host-pathogen dynamics under natural conditions.
To address these questions, we conducted a 2 × 2 × 2 factorial experiment in which we manipulated the presence and absence of Rana cascadae tadpoles, zooplankton, and Bd. Based on our previous research demonstrating that zooplankton consume Bd zoospores in the laboratory (Buck et al. 2011), we predicted that zooplankton would reduce Bd infection prevalence and load of tadpoles. Zooplankton could also benefit tadpoles by increasing periphyton resources through reduced shading of periphyton by phytoplankton (Leibold and Wilbur 1992; Rohr and Crumrine 2005; Rohr et al. 2008b; Relyea and Diecks 2008). Alternatively, zooplankton could reduce growth, development, and survival of tadpoles through competition for algal resources.
Materials and methods
We manipulated the presence of three members of the aquatic community in artificial ponds (mesocosms): R. cascadae tadpoles, zooplankton, and Bd. The experiment occurred at Oregon State University’s Lewis-Brown Horticulture Research Farm near Corvallis, Oregon (68 m a.s.l.; Benton County, USA) and ran from 16 June 2010 to 25 August 2010. Forty-eight plastic tanks (94 cm long × 70 cm wide × 33 cm high) were filled with ~120 L of tap water (pH = 8.0) on 31 May and were covered with weighted screen lids. On 1 June we added 30 g of leaf litter to each tank, which provided habitat heterogeneity. On 3 June we inoculated all tanks with 600 mL of water containing phytoplankton and periphyton from laboratory stocks. The experiment employed a completely randomized 2 × 2 × 2 factorial design. We crossed larval R. cascadae (absent or present at a density of 20 tadpoles per mesocosm) with zooplankton (absent or present) and Bd (absent or present at an average dose of 1.16 × 104 zoospores L−1). Each of the eight treatments was replicated six times for a total of 48 experimental units.
Partial clutches of R. cascadae (four masses) were collected on 6 May within 48 h of oviposition from site 1, a natural pond in the Cascade Mountains (elevation = 1,140 m). Eggs hatched and tadpoles were reared in outdoor holding tanks near the experimental site. Tadpoles were fed rabbit chow ad libitum. On 10 June 20 tadpoles of Gosner (1960) stage 25–27 (beginning to develop hind limb buds) were added to half of the experimental tanks. The initial mass of the tadpoles (mean ± 1SE) was 122 ± 7 mg. On the same day, half of the experimental tanks were inoculated with zooplankton filtered from 600 mL of water from laboratory stocks, originally collected from natural ponds in the area. All tanks receiving this treatment were checked after 7 days to confirm the presence of zooplankton.
On day 1 of the experiment (16-Jun) and every week thereafter for a total of ten inoculations, Bd was added to tanks assigned to the Bd treatment. We grew the fungus in pure culture on plastic Petri plates (10-cm diameter) with standard tryptone–gelatin hydrolysate–lactose nutrient agar medium (Longcore et al. 1999). We inoculated plates with liquid culture of Bd isolate JEL 274, originally isolated from western toads (Anaxyrus boreas) from Colorado, and incubated them at 22 °C for 8 days prior to use. A broth containing Bd scraped from 50 flooded plates was diluted to 800 mL using dechlorinated water and 30 mL of this broth was added to each tank in the Bd treatment. We examined a small sample of this broth in the laboratory with the use of a hemocytometer to determine zoospore concentration. Average zoospore concentration in mesocosms following Bd inoculation was 11,600 zoospores L−1. A broth containing water from 50 flooded control plates was diluted to 800 and 30 mL of this broth was added to each tank in the Bd control treatment.
Amphibians
On day 19 of the experiment, three tadpoles from each tank containing amphibians were haphazardly chosen and tested for Bd infection. Their mouthparts were swabbed for Bd using a sterile fine-tip swab (Medical Wire and Equipment), and the individuals were immediately returned to their tank (Retallick et al. 2006). Swabs were placed in sterile vials and stored at −20 °C for later analysis via quantitative polymerase chain reaction (qPCR).
Amphibians were removed from tanks upon emergence at metamorphosis. The first newly metamorphosed amphibian (metamorph) was observed on day 34. Following this initial observation, tanks were checked daily for metamorphs until the end of the experiment on day 71. Individuals emerging on day 34 and every third day thereafter were transported to the laboratory where snout-vent length was measured. Following general methods of Searle et al. (2011), these individuals were kept in Petri plates (140 × 30 mm) with air holes in the lid for 2 weeks to allow Bd infection to develop. The laboratory was maintained at a temperature of 14–16 °C with a 14-h:10-h light:dark photoperiod. Twenty-five milliliters of dechlorinated water was added to each Petri plate to cover the bottom of the dish, which kept the animals in constant contact with the water. Three days after metamorphosis and every 3 days subsequently, individuals were fed three pinhead crickets. Each individual’s Petri plate was changed 7 days after metamorphosis. After 14 days, individuals were euthanized using an overdose of MS-222 and preserved in 90 % ethanol. All other emerging individuals were transported to the laboratory, measured, immediately euthanized using an overdose of MS-222, and preserved in 90 % ethanol. At the end of the experiment, all remaining individuals (~18 % of all individuals added to mesocosms) were preserved, regardless of Gosner stage.
Following methods of Boyle et al. (2004), we used real-time qPCR to quantify the infection status and load of the three tadpoles swabbed from each tank containing amphibians (72 individuals) and a randomly selected sample of 14 of the metamorphs that were kept in the laboratory from each treatment (56 individuals). qPCR analysis was conducted on an Applied Biosystems StepOne Plus real-time PCR machine (Applied Biosystems, CA). Each sample was run in triplicate against a Bd standard titration from 10−1 to 102 zoospores.
Community
To determine how treatments affected the pond community, we sampled zooplankton, phytoplankton, and periphyton on 17 and 18 June and once every week thereafter for 10 weeks. To measure the abundance of zooplankton, we plunged a 1.5-cm tube sampler holding approximately 30 mL of water vertically through the water column and sealed it near the bottom of the tank. Three samples were taken on opposite sides of each tank and in the center and combined. We repeated this procedure three times for each tank for a total of three samples of 90 mL each. Water samples were filtered through 150-μm mesh (Florida Aquatics), and zooplankton from each sample were combined and preserved in 30 % ethanol for later quantification.
To measure the concentration of phytoplankton, a 25-mL water sample from each of the three combined water samples described above was filtered through a Whatman GF/F filter. To minimize chlorophyll breakdown, filtering was conducted under full shade and filters were stored in 25-mL centrifuge tubes on ice. Samples were stored at −20 °C for 4 days before chlorophyll extraction. Following the Welschmeyer (1994) method, chlorophyll-a was extracted with 10 mL of 90 % acetone, agitated, and incubated for 24 h at −20 °C. Fluorescence measurements were taken using a Turner Designs fluorometer (model TD-700; Sunnyvale, CA), and chlorophyll-a concentration was calculated as the mean value of the three replicates from each mesocosm.
To measure the biomass of periphyton, ten standard glass microscope slides were mounted vertically using silicone I clear rubber sealant (General Electric) on one side of each tank 2 weeks before the tanks were filled. On each sampling occasion, one slide was removed and the periphyton on the outer side of the slide was scraped into a Petri plate using a straight-edge razor blade. Contents of the Petri plate were filtered through a 25-mm Whatman GF/F filter that had been previously dried for 24 h at 60 °C and weighed. Filters were dried again for 24 h at 60 °C and reweighed to determine periphyton biomass.
To ensure that the temperature in the tanks did not exceed the critical thermal maximum of Bd (Piotrowski et al. 2004) and did not differ among treatments, we deployed iButton temperature probes (Maxim, Sunnyvale, CA) in 12 tanks on 12 June. Each probe logged the temperature every hour during the experiment. Temperature measurements were averaged within tanks by week, and an ANOVA was used to test for differences between treatments.
Choice of venue
While field experiments are often recognized as the standard for ensuring the realism of experimental work in ecology, this option is not usually ethically acceptable when manipulating pathogens. We therefore resort to mesocosms as the next best option for community-level experimentation. We recognize the risk of releasing pathogens into the environment with this type of approach (Parris and Beaudoin 2004), and we took precautions to minimize the risk of pathogen escape following the methods of Buck et al. (2012).
Statistical analyses
To test for main and interactive effects of zooplankton and Bd on amphibians, we constructed linear mixed-effects models for the daily growth rate (snout-vent length) and larval period of amphibians. To meet parametric assumptions, these variables were log transformed. We also constructed a Cox mixed-effects model to test for main and interactive effects of zooplankton and Bd on survival of amphibians to metamorphosis. For all amphibian response variables, individuals were nested by tank to avoid pseudoreplication. Mixed-effects models were constructed using the nlme and the coxme packages in R version 3.0.2 (2013). To test for main and interactive effects of treatments on community response variables, including abundance of zooplankton, abundance of phytoplankton, and periphyton biomass, we used repeated-measures ANOVAs. All community response variables were log transformed to meet parametric assumptions. Repeated-measures ANOVAs were conducted using the ez package in R version 3.0.2 (2013). We also conducted qPCR analysis on three randomly chosen animals from each tank that were swabbed on day 19 of the experiment (n = 72), and 14 animals from each treatment that were held for 2 weeks past metamorphosis in the laboratory (n = 56). We used ANOVAs to test for effects of treatment on infection prevalence and infection load. Percent survival of animals held past metamorphosis was nearly 100 %, so we did not test for differences between treatments.
To test our hypotheses regarding indirect effects, we employed structural equation modeling (SEM), a statistical technique based on the analysis of variance–covariance matrices (Grace 2006). SEM is well-suited to testing web-like hypotheses, because variables may serve as both independent and dependent variables. Measured variables used in our SEM included daily growth rate (snout-vent length), larval period, and percent survival of amphibians, abundance of zooplankton, abundance of phytoplankton, and periphyton biomass. To meet parametric assumptions, all variables were log transformed except for percent survival of amphibians, which was arcsine-square root transformed. For the community variables, we used the average value over the last four sampling occasions. We first constructed a full model (Fig. 3a), which included effects of tadpoles on phytoplankton (through possible predation, path a), covariance between zooplankton and phytoplankton (through predation and population feedbacks, path b), effects of tadpoles on periphyton (through predation, path c), effects of phytoplankton on periphyton (though shading, path d), effects of Bd on tadpoles (through infection, path e), effects of tadpoles on zooplankton [through possible predation, e.g., Hamilton et al. (2012), path f], and effects of zooplankton on Bd (through possible predation, path g). Tadpole growth rate, duration of the larval period, and tadpole survival all contributed to the overall predation and competition pressure that the tadpole populations could exert and all were strongly correlated. Consequently, we conducted a confirmatory factor analysis on these three variables and the primary axis served as the latent variable tadpole predation and competition pressure. Subsequently, we tested among eight a priori hypothesized path models that removed various sets of paths from the starting full model (Table 4). These eight models were ranked using Akaike information criterion (AIC). Treatments in which zooplankton were absent were excluded from the SEM. SEM was conducted using the lavaan package in R version 3.0.1 (2013).
One advantage of SEM is that multiplication of standardized path coefficients allows for the comparison of the strengths of indirect pathways (Grace 2006). We calculated and compared the strengths of the three hypothesized indirect effects of zooplankton on tadpoles: (1) via infection dilution (hereafter “dilution pathway,” paths e and g), (2) via consumption of phytoplankton and thus reduced shading of periphyton, an important resource to many tadpoles (hereafter “shading pathway,” paths b–d); and (3) via competition for algal resources (hereafter “competition pathway,” paths a and b). To calculate the strengths of these indirect effects, we used path coefficients from the winning model for all significant paths and path coefficients from the full model for all non-significant paths.
Results
Amphibians
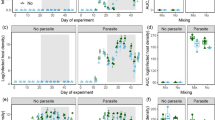
Linear mixed-effects models revealed that growth (snout-vent length) and larval period of amphibians were not significantly affected by zooplankton (P = 0.08 and P = 0.16, respectively), Bd (P = 0.12 and P = 0.24, respectively), or their interaction (P = 0.09 and P = 0.25, respectively) (Table 1; Fig. 1a, b). A Cox mixed-effects model showed non-significant effects of zooplankton (P = 0.78), Bd (P = 0.78), and their interaction (P = 0.14) on survival to metamorphosis (Table 2; Fig. 1c). qPCR analysis indicated that 26 of 36 Bd-exposed larvae that were swabbed on day 19 were infected; however, infection load was low (0.6–1.7 Bd genome equivalents) compared to exposed animals in many laboratory studies. Two weeks past metamorphosis, 14 of 28 exposed individuals harbored infections, and infection load was similarly low (0.9–2.0 Bd genome equivalents). All unexposed larvae and metamorphs that were tested (n = 36 and n = 28 respectively) were uninfected. Zooplankton presence during the larval period was not a significant predictor of infection prevalence or load for larvae or metamorphs.
a Daily growth rate (snout-vent length; n = 361 amphibians nested in 24 tanks), b larval period (n = 361 amphibians nested in 24 tanks), and c percent of larval Rana cascadae (n = 480 amphibians nested in 24 tanks) that reached metamorphosis in the absence (open circles) and presence (filled circles) of zooplankton and not exposed (left) and exposed (right) to Batrachochytrium dendrobatidis (Bd). Values plotted are means ± 1SE
Community
In experimental mesocosms, zooplankton and phytoplankton abundance, and periphyton biomass were comparable to densities found in natural systems and in other mesocosm studies (Fig. 2; McIntire 1996; Oregon Department of Environmental Quality 1998; Rohr and Crumrine 2005; Relyea 2005; Larson et al. 2007; Relyea and Diecks 2008; Buck et al. 2012). The abundance of zooplankton fluctuated over time (P < 0.001) and was reduced by an average of 46 % in the presence of amphibians (P < 0.001), but was not significantly affect by Bd (P = 0.95) (Table 3; Fig. 2a). Phytoplankton abundance fluctuated over time (P < 0.001) and was reduced by an average of 22 % in the presence of zooplankton (P < 0.001), but was not significantly affected by the presence of amphibians (P = 0.23) or Bd (P = 0.15) (Table 3; Fig. 2b). Periphyton biomass fluctuated over time (P < 0.001) and was reduced by an average of 48 % in the presence of amphibians (P < 0.001), but was not significantly affected by the presence of zooplankton (P = 0.13) or Bd (P = 0.97) (Table 3; Fig. 2c).
Responses of community variables zooplankton abundance (a; n = 24), phytoplankton concentration (b; n = 48), and periphyton biomass (c; n = 48) in the presence (solid line) and absence (dashed line) of tadpoles and the presence (filled circle) and absence (empty circle) of zooplankton. Note log scale. Values plotted are means ± 1SE
Average weekly temperatures in the experimental tanks ranged from 14.05 to 20.03 °C, which is suitable for growth of Bd (Piotrowski et al. 2004). An ANOVA indicated that temperature was not affected by the presence of tadpoles, zooplankton, or Bd.
Structural equation model
Mixed-effects models for the amphibian response variables and repeated-measures ANOVAs for the community response variables detected no two- or three-way interactions between treatments, so they were not included in the SEM. Based on AIC, the SEM with the greatest support (Fig. 3b) included negative effects of tadpoles on periphyton (P < 0.001), phytoplankton (P = 0.006) and zooplankton (P < 0.001). Additionally, zooplankton reduced phytoplankton abundance (P < 0.001; Fig. 3b), and competition between zooplankton and tadpoles for this shared resource may explain the negative association between these grazers. According to this model, phytoplankton did not reduce periphyton biomass and the addition of Bd to this system did not affect community dynamics. This model accounted for 72 % of the model weights; however, we could not discriminate between it and the second best model (ΔAIC < 2), which, in addition to the effects described above, also included negative effects of phytoplankton on periphyton (Table 4). Multiplication of standardized path coefficients revealed that the competition pathway (coefficient = 0.181) was roughly 1,000 times stronger than the dilution pathway (coefficient = 0.000228) and roughly five times stronger than the shading pathway (coefficient = −0.0385).
a Full model depicting the latent variable tadpole predation and competition pressure and paths a–g to be tested. b Best model (based on Akaike information criterion) showing a negative association between zooplankton and tadpoles and demonstrating that Bd did not affect members of the aquatic community. P-values and standardized coefficients are shown next to each path. R 2-values indicate total variance explained by predictor variables
Discussion
We hypothesized that zooplankton might benefit tadpoles by consuming Bd zoospores, thus diluting infection risk. Mixed-effects models revealed that growth, development, and survival of amphibians differed in the presence and absence of zooplankton and Bd (Fig. 1; Tables 1, 2), but these patterns were not statistically significant. Repeated-measures ANOVAs revealed non-significant effects of Bd on community response variables (Table 3). Using our SEM, we found no evidence for associations between Bd and tadpoles (path e) or Bd and zooplankton (path g). Multiplication of these standardized path coefficients revealed a negligible effect of zooplankton on tadpoles through this dilution pathway (coefficient = 0.000228). Previous studies suggesting that zooplankton might dilute Bd risk for tadpoles (Woodhams et al. 2011; Buck et al. 2011; Searle et al. 2013) occurred in the laboratory and have stressed that this effect is likely to vary depending on ecological context. For example, algae might interact negatively with Bd zoospores through physical interference or allelopathy (Searle et al. 2013). In our experiment, consumption of Bd zoospores by zooplankton might have been reduced by the presence of alternative and possibly preferred food sources [i.e., phytoplankton (Searle et al. 2013)].
Although zooplankton densities in this experiment were comparable to other mesocosm studies (Rohr and Crumrine 2005; Relyea 2005; Relyea and Diecks 2008; Hamilton et al. 2012; Buck et al. 2012), our qPCR analysis indicated that zooplankton presence did not significantly affect infection prevalence or load of tadpoles. Absence of an effect of zooplankton on infection prevalence corroborates the findings of Kagami et al. (2004), who showed that consumption of chytrid zoospores by zooplankton did not change infection prevalence of diatoms infected with a chytrid fungus (Zygorhizidium planktonicum). However, Kagami et al. (2004) found that consumption of chytrid zoospores by zooplankton reduced the mean infection intensity of diatoms. In our study, the majority of Bd-exposed tadpoles were successfully infected, but infection load was low, which might have prevented detection of an effect of zooplankton. In mesocosm studies, Bd transmission among tadpoles can be low (Buck et al. 2012; Hamilton et al. 2012) and abundant algal resources and weak competition among tadpoles might minimize effects of the pathogen on its hosts (Buck et al. 2012). In spite of these caveats, infected tadpoles may serve as important reservoirs of the pathogen in natural populations (Briggs et al. 2010), placing conspecifics and heterospecifics of all developmental stages at risk.
Previous studies have shown that zooplankton can benefit tadpoles by consuming phytoplankton, thus increasing water clarity, allowing sunlight to reach periphyton, a primary food resource of scraping tadpoles (Leibold and Wilbur 1992; Rohr and Crumrine 2005; Rohr et al. 2008b; Relyea and Diecks 2008). Although we detected negative effects of zooplankton on phytoplankton abundance (Fig. 2b; Table 3), our best model did not support a negative association between phytoplankton and periphyton (path d). However, we could not discriminate between the best and second best model, the latter of which did include a negative effect of phytoplankton on periphyton. Multiplication of standardized path coefficients from our SEM revealed that positive effects of zooplankton on tadpoles through this shading pathway were small under the conditions tested here (coefficient = −0.0385).
Instead of these positive indirect effects, the path model with the greatest support included a negative association between tadpoles and zooplankton (P < 0.001). In support of this association, a repeated-measures ANOVA revealed negative effects of tadpoles on zooplankton (Fig. 2a; Table 3). Furthermore, mixed-effects models supported a negative association between tadpoles and zooplankton (Fig. 1), though these effects were not statistically significant (Tables 1, 2). This negative association was probably mediated through competition for phytoplankton, as both zooplankton and tadpoles were associated with reduced phytoplankton abundance in the structural equation model (P < 0.001 and P = 0.006 respectively; Fig. 3b). Multiplication of standardized path coefficients from our SEM revealed that negative effects of zooplankton on tadpoles through this competition pathway (coefficient = 0.181) were roughly 1,000 times stronger than positive effects via the dilution pathway and roughly five times stronger than positive effects via the shading pathway. It is often assumed that tadpoles consume periphyton, and thus do not compete with filter-feeding zooplankton. However, negative associations between tadpoles and zooplankton have been reported from mesocosm studies (e.g., Boone and Semlitsch 2001, 2002, 2003) and from a study in a permanent pond (Seale 1980). Rasping tadpoles such as R. cascadae, are facultative suspension feeders (Seale 1982), and phytoplankton (including diatoms, dinoflagellates, and cryptophytes) may be an important food source for them (Altig 2007; Whiles et al. 2010; Buck et al. 2012), introducing the potential for competition with filter-feeding zooplankton. Interphyletic competition (competition between vertebrates and invertebrates) is more common than previously recognized, and may be important in shaping community dynamics (Brown and Davidson 1977; Schoener and Spiller 1987; Morin et al. 1988; Mokany and Shine 2003; Jennings et al. 2010).
Conclusion
Although zooplankton may benefit tadpoles through infection dilution (Searle et al. 2013) and by increasing periphyton resources (Leibold and Wilbur 1992; Rohr and Crumrine 2005; Rohr et al. 2008b; Relyea and Diecks 2008), we found that interactions between zooplankton and tadpoles were dominated by competition for phytoplankton resources. However, because infection loads were low in our study, we had little power to detect effects of zooplankton on Bd dynamics. Our results show that complex community dynamics may be revealed when hosts and disease agents are incorporated into realistic ecological communities.
References
Altig R, Whiles MR, Taylor CL (2007) What do tadpoles really eat? Assessing the trophic status of an understudied and imperiled group of consumers in freshwater habitats. Freshwater Biol 52:386–395
Altwegg R, Reyer HU (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Blaustein AR, Romansic JM, Scheessele EA, Han BA, Pessier AP, Longcore JE (2005) Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol 19:1460–1468
Boone MD, Semlitsch RD (2001) Interactions of an insecticide with larval density and predation in experimental amphibian communities. Conserv Biol 15:228–238
Boone MD, Semlitsch RD (2002) Interactions of an insecticide with competition and pond drying in amphibian communities. Ecol Appl 12:307–316
Boone MD, Semlitsch RD (2003) Interaction of bullfrog tadpole predators and an insecticide: Predation release and facilitation. Oecologia 137:610–616
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. PNAS 107:9695–9700
Brown JH, Davidson DW (1977) Competition between seed-eating rodents and ants in desert ecosystems. Science 196:880–882
Buck JC, Truong L, Blaustein AR (2011) Predation by zooplankton on Batrachochytrium dendrobatidis: biological control of the deadly amphibian chytrid fungus? Biodivers Conserv 20:3549–3553
Buck JC, Scheessele EA, Relyea RA, Blaustein AR (2012) The effects of multiple stressors on wetland communities: pesticides, pathogens, and competing amphibians. Freshwater Biol 57:61–73
Fisher MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310
Gleason FH, Kagami M, Lefevre E, Sime-Ngando T (2008) The ecology of chytrids in aquatic ecosystems: roles in food web dynamics. Fungal Biol Rev 2:17–25
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, Cambridge
Hamilton PT, Richardson JML, Anholt BR (2012) Daphnia in tadpole mesocosms: trophic links and interactions with Batrachochytrium dendrobatidis. Freshwater Biol 57:676–683
Han BA, Bradley PW, Blaustein AR (2008) Ancient behaviors of larval amphibians in response to an emerging fungal pathogen, Batrachochytrium dendrobatidis. Behav Ecol Sociobiol 63:241–250
Jennings DE, Krupa JJ, Raffel TR, Rohr JR (2010) Evidence for competition between carnivorous plants and spiders. Proc R Soc B—Biol Sci 277:3001–3008
Johnson PTJ, Dobson A, Lafferty KD, Marcogliese DJ, Memmott J, Orlofske SA, Poulin R, Thietges DW (2010) When parasites become prey: ecological and epidemiological significance of eating parasites. Trends Ecol Evol 25:362–371
Kagami M, Van Donk E, de Bruin A, Rijkeboer M, Ibelings BW (2004) Daphnia can protect diatoms from fungal parasitism. Limnol Oceanogr 49:680–685
Kagami M, von Elert E, Ibelings BW, de Bruin A, Van Donk E (2007) The parasitic chytrid, Zygorhizidium, facilitates the growth of the cladoceran zooplankter, Daphnia, in cultures of the inedible alga, Asterionella. Proc R Soc B 274:1561–1566
Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652
Knisely K, Geller W (1986) Selective feeding of 4 zooplankton species on natural lake phytoplankton. Oecologia 69:86–94
Lafferty KD, Dobson AP, Kuris AM (2006) Parasites dominate food web links. PNAS 103:11211–11216
Larson GL, McIntire CD, Buktenica MW, Girdner SF, Truitt RE (2007) Distribution and abundance of zooplankton populations in Crater Lake, Oregon. Hydrobiologia 574:217–233
Leibold MA, Wilbur HM (1992) Interactions between food-web structure and nutrients on pond organisms. Nature 360:341–343
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp.nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
McIntire CD, Larson GL, Truitt RE, Debacon MK (1996) Taxonomic structure and productivity of phytoplankton assmblages in Crater Lake, Oregon. J Lake Reservior Manage 12:259–280
Mendelson JR, Lips KR, Gagliardo RW, Rabb GB, Collins JP, Diffendorfer JE, Daszak P, Ibanez R, Zippel KC, Lawson DP, Wright KM, Stuart SN, Gascon C, da Silva HR, Burrowes PA, Joglar RL, La Marca E, Lotters S, du Preez LH, Weldon C, Hyatt A, Rodriguez-Mahecha JV, Hunt S, Robertson H, Lock B, Raxworthy CJ, Frost DR, Lacy RC, Alford RA, Campbell JA, Parra-Olea G, Bolanos F, Domingo JJC, Halliday T, Murphy JB, Wake MH, Coloma LA, Kuzmin SL, Price MS, Howell KM, Lau M, Pethiyagoda R, Boone M, Lannoo MJ, Blaustein AR, Dobson A, Griffiths RA, Crump ML, Wake DB, Brodie ED (2006) Biodiversity. Confronting amphibian declines and extinctions. Science 313:48
Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16:254–260
Mokany A, Shine R (2003) Competition between tadpoles and mosquito larvae. Oecologia 135:615–620
Morin PJ, Lawler SP, Johnson EA (1988) Competition between aquatic insects and vertebrates: interaction strength and higher-order interactions. Ecology 69:1401–1409
Oregon Department of Environmental Quality (1998) Columbia Slough total maximum daily loads (TMDLs) for: chlorophyll a, dissolved oxygen, pH, phosphorus, bacteria, DDE/DDT, PCBs, Pb, dieldrin, and 2,3,7,8 TCDD. www.deq.state.or.us/wq/tmdls/docs/willamettebasin/columbiaslough/tmdl.pdf
Pace ML, Funke E (1991) Regulation of planktonic microbial communities by nutrients and herbivores. Ecology 72:904–914
Parris MJ, Beaudoin JG (2004) Chytridiomycosis impacts predator-prey interactions in larval amphibian communities. Oecologia 140:626–632
Parris MJ, Cornelius TO (2004) Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85:3385–3395
Parris MJ, Reese E, Storfer A (2006) Antipredator behavior of chytridiomycosis infected northern leopard frog (Rana pipiens) tadpoles. Can J Zool 84:58–65
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618–627
Relyea RA, Diecks N (2008) An unforeseen chain of events: lethal effects of pesticides on frogs at sublethal concentrations. Ecol Appl 18:1728–1742
Retallick RWR, Miera V, Richards KL, Field KJ, Collins JP (2006) A non-lethal technique for detecting the chytrid fungus Batrachochytrium dendrobatidis on tadpoles. Dis Aquat Org 72:77–85
Rohr JR, Crumrine PW (2005) Effects of an herbicide and an insecticide on pond community structure and processes. Ecol Appl 15:1135–1147
Rohr JR, Palmer BD (2013) Climate change, multiple stressors, and the decline of ectotherms. Conserv Biol 27:741–751
Rohr JR, Sager T, Sesterhenn TM, Palmer BD (2006) Exposure, postexposure, and density-mediated effects of atrazine on amphibians: breaking down net effects into their parts. Environ Health Perspect 114:46–50
Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ (2008a) Evaluating the links between climate, disease spread, and amphibian declines. PNAS 105:17436–17441
Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR (2008b) Agrochemicals increase trematode infection in a declining amphibian species. Nature 455:1235–1240
Sanders RW, Porter KG (1990) Bacterivorous flagellates as food resources for the freshwater crustacean zooplankter Daphnia ambigua. Limnol Oceanogr 35:188–191
Schoener TW, Spiller DA (1987) Effects of lizards on spider populations: manipulative reconstruction of a natural experiment. Science 236:949–952
Seale DB (1980) Influence of amphibian larvae on primary production, nutrient flux, and competition on a pond ecosystem. Ecology 61:1531–1550
Seale DB (1982) Obligate and facultative suspension feeding in anuran larvae—feeding regulation in Xenopus and Rana. Biol Bull 162:214–231
Searle CL, Gervasi SS, Hua J, Hammond JI, Relyea RA, Olson DH, Blaustein AR (2011) Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv Biol 25:965–974
Searle CL, Mendelson JR III, Green LE, Duffy MA (2013) Daphnia predation on the amphibian chytrid fungus and its impacts on disease risk in tadpoles. Ecol Evol 3:4129–4138
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N (2007) Spread of chytridiomycosis has cause the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Thieltges DW, Amundsen PA, Hechinger RF, Johnson PTJ, Lafferty KD, Mouritsen KN, Preston DL, Reise K, Zander CD, Poulin R (2013) Parasites as prey in aquatic food webs: implications for predator infection and parasite transmission. Oikos 122:1473–1482
Venesky MD, Parris MJ, Storfer A (2009) Impacts of Batrachochytrium dendrobatidis infection of tadpole foraging performance. EcoHealth 6:565–575
Venesky MD, Liu X, Sauer EL, Rohr JR (2014) Linking manipulative experiments to field data to test the dilution effect. J Anim Ecol 83:557–565. doi:10.1111/1365-2656.12159
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. PNAS 107:9689–9694
Wake DB, Vredenburg VT (2008) Are we in the midst of the sixth mass extinction? A view from the world of amphibians. PNAS 105:11466–11473
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll-a in the presence of chlorophyll-b and pheopigments. Limnol Oceanogr 39:1985–1992
Whiles M, Gladyshev MI, Sushchik NN, Makhutova ON, Kalachova GS, Peterson SD (2010) Fatty acid analyses reveal high degrees of omnivory and dietary plasticity in pond-dwelling tadpoles. Freshwater Biol 55:1533–1547
Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, Muths E, Puschendorf R, Schmidt BR, Sheafor B, Voyles J (2011) Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool 8:8
Acknowledgments
We thank M. James, S. Moyers, L. Biga, and P. Buck for field assistance. Members of the Blaustein laboratory, E. Borer, and M. Albins provided advice regarding experimental design and execution, data analysis, and comments on the manuscript. We also thank the R. Tanguay, J. Spatafora, B. Menge, and S. Hacker laboratories, and E. Scheessele for use of equipment and protocol, and S. Robbins and D. Hinds-Cook for assistance at the Horticulture Farm. We thank J. Trexler, R. Alford, and several anonymous reviewers for insightful comments and suggestions that improved the manuscript substantially. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under grant no. 0802268 and a Howard Hughes Medical Institute Summer Undergraduate Research Fellowship to K. I. S. Supplementary funding was provided by the Oregon State University Zoology Research Fund and the Society of Wetland Scientists.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Andrew Alford.
Rights and permissions
About this article
Cite this article
Buck, J.C., Scholz, K.I., Rohr, J.R. et al. Trophic dynamics in an aquatic community: interactions among primary producers, grazers, and a pathogenic fungus. Oecologia 178, 239–248 (2015). https://doi.org/10.1007/s00442-014-3165-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3165-6