Abstract
Behaviors have evolved in response to various selection pressures over evolutionary time. However, not all behaviors are adaptive. Some presumably “ancient” behaviors, persistent for millions of years, may be detrimental in the face of novel selection pressures in modern times. These pressures include a multitude of emerging infectious diseases which may be stimulated by environmental changes. We examined how a globally emerging amphibian pathogen, Batrachochytrium dendrobatidis (BD), affected two key evolutionarily persistent behaviors displayed by amphibian larvae: aggregation and thermoregulation. Larval aggregation behavior is often essential for foraging, thermoregulation, and antipredator defense, but varies among species. Thermoregulatory behavior speeds larval development in ephemeral habitats. Specifically, we examined whether aggregation and thermoregulatory behaviors changed when exposed to the BD pathogen in two species (Bufo boreas and Rana cascadae) whose larvae aggregate in nature. In laboratory choice tests, larvae of neither species avoided infected conspecifics. BD-exposed B. boreas larvae aggregated, while unexposed R. cascadae larvae associated more frequently with BD-exposed conspecifics. There was no evidence of behavioral fever or altered thermoregulation in larvae of four species we examined (Pseudacris regilla, Rana aurora, B. boreas, R. cascadae). The absence of behavioral fever may suggest an inability of the larvae of some host species to mediate infection risk by this pathogen. Thermoregulatory behaviors may exhibit a high degree of evolutionary inertia in amphibian hosts because they are linked with host physiology and developmental rates, while altered aggregation behaviors could potentially elevate pathogen transmission rates, leading to increased infection risk in social amphibian species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogens (both micro- and macro-parasites) present an important biotic selection pressure and often affect the behavior of their hosts (Freeland 1976; Altizer et al. 2003b; Parris et al. 2004; Moore 2002). In light of potentially strong selective forces imposed by some infectious diseases, it is not surprising that many animals behave in ways that may reduce disease risk. For example, some amphibians, crustaceans, and fishes avoid associating with diseased conspecifics (e.g., Kiesecker et al. 1999; Behringer et al. 2006; Dugatkin et al. 1994); some primate species quarantine newcomers to ensure they are free from latent infection before engaging in normal social interactions (Freeland 1976; Altizer et al. 2003b); and various parasitized ectotherms seek temperatures above their thermal optima, generating behavioral fevers that control infection (e.g., Ouedraogo et al. 2003; Roy et al. 2006). These and other behavioral defenses may mediate the severity of infectious diseases in natural populations.
Some behaviors (e.g., aggregation, thermoregulation) are expressed across modern taxa that diverged millions of years ago and may therefore be considered ancient (Parrish and Edelstein-Keshet 1999; Huey 1982). However, not all behaviors are adaptive. When confronted with novel selection pressures such as emerging infectious pathogens, some historically adaptive behaviors, such as aggregation behavior, may be detrimental in novel situations (Blaustein and Bancroft 2007). These ‘evolutionary traps’ are commonly observed in wildlife populations experiencing habitat changes (Schlaepfer et al. 2002; Battin 2004). Previously adaptive behaviors continue to be expressed, resulting in maladaptive reactions to new conditions or environments (Schlaepfer et al. 2005; Robertson and Hutto 2006; Koko and Sutherland 2001). Thus, with regard to pathogens, behaviors may help mediate disease risk, or they may be maladaptive, increasing disease risk.
Behaviors can also be directly altered by a pathogen. Infectious pathogens may induce changes in host behavior leading to increased pathogen transmission (reviewed in Moore 2002). One example of this phenomenon is illustrated by ground-dwelling arctiid caterpillars that climb to the tops of branches when infected by an entomopathogenic fungus, enhancing pathogen dispersal by wind and rainfall (Yamazaki et al. 2004). Additionally, some parasites with complex life cycles (requiring more than one host species to reproduce) cause their intermediate hosts to lose certain antipredator behaviors, predisposing them to predation by definitive hosts wherein parasites reproduce (reviewed in Moore 2002). These and other examples of infection-induced behavioral changes increase the chances of successful pathogen dissemination and transmission, and confirm the importance of examining the role of behavior in disease ecology.
As infectious diseases emerge with increasing frequency and severity on a global scale, they present novel selection pressures to wildlife populations (Altizer et al. 2003a), and in some cases, may affect population viability (reviewed by de Castro and Bolker 2005). An emerging infectious disease, amphibian chytridiomycosis, has been associated with many amphibian population declines on a global scale (Daszak et al. 2003), and is the putative agent for the decline of numerous frog populations (e.g., Rachowicz et al. 2006; Lips et al. 2006; Schloegel et al. 2006; Pounds et al. 2006). The fungal pathogen causing this disease, Batrachochytrium dendrobatidis (BD), has a waterborne infectious stage. Flagellated zoospores emerge from intracellular zoosporangia encysted within keratinizing epidermal tissue of amphibian hosts (Longcore et al. 1999; Altig 2007), which may affect larval, juvenile, and adult stages in a species-specific manner (Blaustein et al. 2005; Garcia et al. 2006; Rachowicz et al. 2006). Laboratory studies show that BD zoospores swim an average of 2 cm before encysting (Piotrowski et al. 2004), and zoospores were more successful at colonizing host tissue when in groups rather than singly or in low numbers (Longcore et al. 1999). Additionally, infection is commonly observed as patches of clustered zoosporangia in the epidermis, indicative of emergent zoospores re-encysting nearby the original point of infection, often on the same host (Weldon and Du Preez 2006; Longcore et al. 1999). These observations corroborate experimental evidence that still or slowly moving water may influence BD infection severity by leading to an accumulation of infectious zoospores in larval host habitats (Tunstall unpublished data). Though flagellated, BD resembles other members of order Chytridiales that are most effectively transported by flowing water or moving hosts (Berger et al. 1999; Sparrow 1968; Kriger and Hero 2007).
Thus, close proximity and direct contact between aggregating amphibians may be one important mode of BD transmission in the field. Dense aggregations formed by some larval amphibians may increase pathogen loads for each individual within the group. This may be particularly important for species like western toads (Bufo boreas) that can experience acute mortality as tadpoles from BD exposure (Blaustein et al. 2005). Despite potential negative consequences, aggregating tadpoles likely experienced benefits promoting social behavior, including protection from predation (via predator swamping and collective vigilance), and enhanced filter feeding through group agitation of organic matter into the water column (Hoff et al. 1999; Watt et al. 1997; Beiswenger 1977). Aggregation behavior also promotes thermoregulation. Temperatures within aggregating groups of tadpoles are higher than peripheral temperatures (Brattstrom 1962, 1963; Hoff et al. 1999). Though not all amphibian species aggregate, general heat-seeking behaviors are shared by all amphibian larvae of temperate zones, as warm temperatures expedite larval development and facilitate metamorphosis before ephemeral habitats dry or freeze (Dupré and Petranka 1985). The BD pathogen shows a thermal optimum ranging between 10°C and 25°C, with growth ceasing at 28°C (Piotrowski et al. 2004). Woodhams et al.( 2003) showed that housing adult frogs (Litoria chloris) in water baths at 37°C cleared experimental infection in less than 16 h. Rana yavapaiensis adults from thermal springs in Arizona (>30°C) tested negative for BD compared to conspecifics found in nearby habitats (ca. 550 m at temperatures between 20°C and 24°C; Schlaepfer et al. 2007). Collectively, these studies raise the interesting possibility that some hosts may clear BD infection through behavioral fever in the wild.
Thermoregulation of individuals and groups may mediate the risk of infection in amphibian larvae (Lefcort and Blaustein 1995). In this study, we sought to determine if hosts behaviorally respond to BD exposure by examining aggregation and thermoregulatory behaviors in larvae of multiple amphibian host species. Specifically, we tested whether social, schooling tadpoles of B. boreas and Rana cascadae (Cascades frog) (1) avoid BD-exposed conspecifics and (2) alter aggregation behaviors as a result of exposure status. Because amphibian larvae often exhibit opportunistic cannibalism by preying on sick or injured tadpoles (Crump 1983), we also measured activity rates to discern whether changes in aggregation behavior by healthy conspecifics were an indirect result of morbidity in BD-exposed animals. To examine thermoregulatory behaviors, we used larvae of four species: Pseudacris regilla and Rana aurora breed in the winter in lower elevation sites, and their larvae are found in cooler temperatures during development compared with B. boreas and R. cascadae larvae which are found at high elevation sites that develop in spring in warmer temperatures (O’Hara 1981; Bancroft et al. 2008). In these four species we tested (1) whether tadpoles induced behavioral fever as a response to BD exposure, and (2) if thermal preferences were altered as a result of exposure status. Thermoregulation and aggregation behaviors are closely tied to tadpole physiology and development and may present evolutionary constraints on potential adaptation to novel conditions (Ricklefs and Wikelski 2002; Huey et al. 2003). Thus, alterations of either of these evolutionarily persistent behaviors through BD infection may help explain some of the wide variation in susceptibility between host species. More generally, host behavioral responses to a globally emerging pathogen may reveal the adaptive potential of some host species to behaviorally respond to a novel biotic selection pressure.
Materials and methods
Animal collection and rearing
Red-legged frog (R. aurora) and Pacific treefrog (P. regilla) eggs were collected from the Willamette Valley of Oregon (January–February, elevation 71.6 m; Benton County). R. cascadae and B. boreas eggs were collected from the Three Creeks recreational area (March–April, elevation 2,000 m; Deschutes County). All animals were reared in 38 L tanks at temperatures ranging between 11°C and 14°C on a 14:10-h photoperiod. Post-hatching, all tadpoles were fed a 2:1 mixture of ground fish flakes and alfalfa pellets ad libitum and transferred to lower densities. At developmental stages 25–26 (Gosner 1960), tadpoles were inoculated with either BD or a control treatment (see below). Complete water changes were conducted weekly. For brevity, we refer to animals inoculated with the BD pathogen as “BD+” and animals exposed to the control agar wash without BD as “BD−”. Importantly, tissue from animals used in these experiments were not retrospectively sampled to quantify pathogen concentration, thus BD+ and BD− designations refer only to exposure status. To confirm that our inoculation methods are sufficient to produce infection, we quantified BD pathogen loads in additional, non-experimental (new) larvae of these species. We inoculated six tadpoles held together in opaque plastic containers filled with 1 L dechlorinated water. We quantified BD loads for each tadpole using real-time polymerase chain reaction (PCR). DNA was extracted from tissue of excised oral disks using a DNAeasy 96 well kit (Qiagen, Valencia, CA, USA) and quantified using a spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). The real-time PCR was based upon established methodology (Boyle et al. 2004) using an ABI 7300 Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Twenty five microliter reactions contained: 5 μL of 20 ng/L template DNA and 20 μL of master mix (containing 900 nM forward and reverse primers, 125 nM MGB probe, and Taqman Master Mix). We obtained BD genome equivalent standards from D. Boyle (as used in Boyle et al. 2004) and included triplicates of each standard serially diluted on each plate (10−1, 100, 101, 102) and a duplicate of the high standard (103). Unknown samples were run in triplicate and values that differed by a coefficient of variation greater than 0.2 were rerun for greater accuracy. Values obtained from the quantitative PCR reaction are the mean BD zoospore genome equivalents per nanogram of excised mouthpart tissue. This measure accounts for differences between species based on size alone (i.e., more mouthpart tissue containing more zoospores).
Aggregation experiment
Inoculation regime
Ninety tadpoles of each species were held in 39 L aquaria. For BD+ treatments, aquaria were each inoculated with four culture dishes of isolate JEL 274 grown in pure culture for 10 days on 1% tryptone agar culture dishes. Five milliliters of water was added to each culture dish, which was scraped five times with a disposable pipette to dislodge BD colonies. Liquid containing zoospores and sporangia was added directly to aquaria. This process was repeated for control culture dishes (1% tryptone agar without BD), and the inoculate was added directly to aquaria holding BD− tadpoles. Experiments were conducted 14 days after inoculation. Animals were inoculated and held in multiple aquaria to avoid pseudoreplication (testing inoculated animals from a single tank).
Test chambers and data collection
Opaque commercial plastic containers (capacity 39.7 L, dimensions 0.16 × 0.86 × 0.42 m) were filled with 7.6 L dechlorinated water. Chambers were partitioned on either end by mesh screening, creating 0.10 m × 0.16 m enclosures to hold groups of five tadpoles of two treatments (BD+ or BD−) from two species (R. cascadae and B. boreas). A line drawn on the bottom of each chamber divided the remaining area in half (0.42 × 0.30 m). Chambers were rotated 180° between trials to remove possible effects of chamber orientation. The treatment sides were also randomized for each chamber in all trials. A focal tadpole (either BD+ or BD−) was placed in the center of each container. For brevity, we refer to the side of each test chamber nearest the BD+ group of conspecifics as the “BD+ side”, and the side of each test chamber nearest the unexposed group of conspecifics as the “BD− side”. All animals were allowed to acclimate to the test chambers for 20 min. As a proxy for aggregation, we recorded the side of the chamber occupied by the focal tadpole every 10 min for 240 min (N BD+ = 16, N BD− = 16, for each species). Observations were made over a 240-min period to capture an accurate mean for aggregation activity, as tadpole movements and behaviors can show high variability. All focal and stimulus tadpoles were used only once during the experiment. Test trials conducted before the experiment and observations made throughout the experiment showed that focal and stimulus animals were not repelled by the mesh used to create partitions, as they were observed touching, clinging, or very close to the mesh filters at both ends of the test chambers.
Using a binomial test, we tested the null hypothesis that stimulus tadpoles would not spend a significant proportion of time on either side of the test chamber. Therefore, we compared the number of focal animals that spent the majority of time on the side of the chamber containing BD+ stimulus animals to a null proportion of 0.5.
Activity rate
Tadpoles were inoculated as described above, resulting in a BD− and a BD+ treatment. Single tadpoles were randomly chosen and placed in clear plastic chambers containing 1 L dechlorinated water. A grid was placed underneath each chamber so that gridlines were visible through the bottom of each chamber (grids = 25 mm2). Tadpoles were left to acclimate to new conditions for 1.5 h. We quantified tadpole activity by counting the number of lines crossed by each tadpole in 30 s continuously over a 2-h period. All observations were made from behind black curtains with small viewing windows to ensure that tadpoles would not respond to the presence of observers. We counted a tadpole as having crossed a gridline if the tadpole swam horizontally across a gridline. Vertical movement through the water column was not quantified. We replicated each treatment 15 times for each species. Treatments were randomly assigned and observers were blind to treatments during data collection.
Thermoregulation experiment
Inoculation regime
We tested four species: R. cascadae, B. boreas, P. regilla, and R. aurora. At stage 25 (Gosner 1960), R. cascadae and B. boreas were inoculated with BD isolate JEL 274 (B. boreas isolate) and R. aurora and P. regilla tadpoles were inoculated with BD isolate JEL 215 (ranid isolate). Infectious zoospores were harvested from 1% tryptone agar culture dishes by adding 20 mL filtered water to each dish and pouring the inoculum from each plate directly into holding aquaria after a 20-min period to allow for zoospore discharge. We used two culture dishes per tank of 20 tadpoles for each species. Animals were inoculated and held in multiple aquaria to avoid pseudoreplication (testing inoculated animals from a single tank). Animals were exposed to respective treatments for 40 days at the start of trials. We replicated each treatment 16 times for each species.
Test chambers and data collection
Thermal gradients were 1.2 m × 0.1 m lanes filled to a depth of 4.5 cm with 4 L dechlorinated water. Six digital temperature loggers (Thermacron i-Buttons, Dallas Semiconductor) placed along the length of each lane (25 cm apart) recorded temperatures every 10 min. A hot plate underneath one end of each lane heated the water and dry ice contained in a separated well (7 × 7 × 6 cm) at the opposite end of each lane cooled the water. Each lane was aerated at two locations (30 cm from either end) to minimize vertical and horizontal temperature stratification. Thermal gradients were allowed to establish for a minimum of 30 min (Fig. 1).
A single tadpole was added to the center of each of the eight lanes and allowed to acclimate for 15 min. We recorded the location of each tadpole every 10 min for 180 min. To approximate the water temperature at the tadpole as closely as possible, the distance between each of the six temperature loggers was further divided into 3 cm increments. The nearest temperature at each tadpole was extrapolated using the following formula:
where T x is the temperature of the logger nearer the cold end of the thermal gradient, T y is the temperature of the logger nearer the warm end, g is the grid mark where the tadpole was located, and g y,x is the total number of grid marks between the two loggers. Some ectotherms produce behavioral fever by periodically occupying high temperature environments over time, which may cause less damage from heat stress than continuous occupation of feverish temperatures (Kluger et al. 1998). Thus, as an additional measure of behavioral fever, we also counted the number of times tadpoles were found in temperatures ≥26°C, the temperature above which BD growth is presumably inhibited in laboratory cultures (Piotrowski et al. 2004).
We also quantified activity rates of tadpoles within thermal gradients by counting the number of grid marks (spaced approximately 3 cm apart) crossed by the tadpole during a 30-s period every 10 min over 180 min. Test chambers for all experiments were sterilized with 10% bleach solution after every trial.
Results
Inoculation
Tadpoles of all four species tested positive for BD infection, confirming that our inoculation methods are sufficient to produce infection. All control (BD−) tadpoles tested from the four species were negative for BD. There were species-specific variations in BD infection severity. The mean log BD zoospore genome equivalents per nanogram of excised mouthpart tissue are as follows: B. boreas, 0.53 (N = 8); P. regilla, 0.11 (N = 6); R. cascadae, 0.01 (N = 8); R. aurora, 0.35 (N = 5). Since it is not possible to match BD infection severity in tadpoles a priori without sacrificing them, our hypotheses and results examine the effects of BD exposure (not infection).
Aggregation behavior
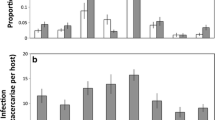
Neither BD+ nor BD− focal tadpoles of R. cascadae and B. boreas avoided BD+ conspecifics (Fig. 2). However, we observed other changes in aggregation behavior as a result of BD exposure. The majority of BD+ B. boreas focal tadpoles were found more frequently on the BD+ side of test chambers (p < 0.01, binomial test). BD− B. boreas focal tadpoles did not depart from random in their allocation of time between the two sides of the chamber (p = 0.19). BD+ R. cascadae focal tadpoles were associated randomly with both sides of test chambers (p = 0.18), but a majority of BD− R. cascadae focal tadpoles were observed more frequently on the BD+ side of test chambers (p < 0.03; Fig. 2). Activity rates between BD+ and BD− tadpoles did not differ in R. cascadae (p = 0.9; t 15 = 1.32), but BD+ B. boreas were more active than BD− tadpoles (p < 0.02; t 15 = −2.29; Fig. 3).
Thermoregulatory behavior
There were no differences in mean temperatures selected by BD+ tadpoles compared to BD− tadpoles for any of the four tested species: B. boreas (p = 0.37), R. cascadae (p = 0.23), P. regilla (p = 0.2), and R. aurora (p = 0.89; Mann–Whitney–Wilcoxon (MWW) test; Fig. 4 and Table 1). P. regilla and R. aurora generally chose colder temperatures compared to R. cascadae and B. boreas (Table 1 and Fig. 4). There was no significant difference in the mean number of observations at temperatures ≥26°C for any species in either treatment (BD+ or BD− tadpoles; Table 1, Fig. 5). Generally, activity rates quantified within thermal gradients corroborated the activity rate results observed for R. cascadae and B. boreas. There was no difference in activity rates within thermal gradients for R. cascadae between BD treatments. BD+ B. boreas and R. aurora tadpoles were more active compared with BD− conspecifics, although these differences were not statistically significant (Table 1). There was no difference in activity rates between treatments in P. regilla. There were no differences in body or tail length between treatments in any of the four species tested (MWW tests, Table 1).
Discussion
The behavioral repertoire of an animal has been molded over evolutionary time in response to numerous abiotic and biotic selection pressures. Yet extant behaviors that have persisted for millions of years may be maladaptive in a continuously changing environment that includes an onslaught of emerging infectious diseases (Morens et al. 2004). Our study examined two evolutionarily persistent ancestral behaviors in amphibian hosts in relation to a newly emerging infectious agent. Thermoregulatory behaviors, in particular, the ability to produce behavioral fever, may be one way that ectothermic hosts mediate or clear infection (Kluger et al. 1998; Lefcort and Blaustein 1995; Woodhams et al. 2003; Schlaepfer et al. 2007). Aggregation behaviors in many host species may increase disease risk (Kermack and McKendrick 1927; Rowley and Alford 2007).
Tadpole aggregations are likely formed and mediated on the basis of various cues (Wassersug 1973; O’Hara 1981; Hoff et al. 1999). Some of these cues may be altered as a result of infection and altered cues may lead to changes in individual host behaviors (e.g., Kavaliers and Colwell 1992; 1995), and the behavioral interactions between hosts in groups, although the latter possibility has not been well-studied experimentally. Among the two more social species we examined, neither B. boreas nor R. cascadae avoided BD+ groups of conspecifics. BD− B. boreas tadpoles associated randomly with conspecifics regardless of exposure status while BD+ tadpoles associated with other BD+ conspecifics. BD+ individuals were also more active than BD− tadpoles. BD+ B. boreas tadpoles may aggregate with other BD+ individuals more frequently because they are responding to cues from conspecifics sharing similar, high activity rates. Sharing similar activity patterns with conspecifics may further decrease individual conspicuousness to predators. It may therefore be advantageous for “hyperactive” BD-infected B. boreas to associate with conspecifics that share similar activity levels in the field.
Contrary to expectation, unexposed R. cascadae tadpoles associated more frequently with BD+ conspecifics, whereas BD+ tadpoles showed random associations with conspecifics regardless of exposure status. There was no difference in activity rate between BD+ and BD− R. cascadae. The larvae of many anuran species, including those examined in this study, exhibit opportunistic cannibalism of conspecifics in the field (Crump 1983) and in the laboratory (pers. obs.). Therefore, one possible explanation for the attraction of BD− tadpoles to BD+ conspecifics could be that BD− tadpoles were seeking opportunities to consume moribund conspecifics. However, since activity rates of BD+ R. cascadae tadpoles did not differ from BD− tadpoles, we could not attribute this trend to perceived opportunities for cannibalism from lowered activity rates. It remains to be tested whether altered chemical cues emitted from infected or moribund conspecifics attract uninfected conspecifics, or whether the BD pathogen itself may present cues that are attractive to potential hosts (Berger et al. 2005). Also, our observations of host activity rates were made on individual tadpoles. Groups of tadpoles may exhibit different activity rates than solitary tadpoles, or single tadpoles in the presence of conspecifics (e.g., Griffiths and Foster 1998).
In the four species we tested, there was no evidence that thermoregulatory behaviors were altered in BD+ tadpoles compared to controls. Instead, all species occupied mean temperatures that paralleled their native habitats. P. regilla and R. aurora collected from similar cooler habitats occupied colder temperatures expected of winter breeding compared to R. cascadae and B. boreas, which inhabit warmer temperatures during larval development in summer months (Brattstrom 1963; Putnam and Bennett 1981; Bancroft et al. 2008). Furthermore, we did not detect any evidence of behavioral fever in these species. Our results suggest that during larval stages these anuran species do not engage in behavioral fever as a result BD exposure, even though temperatures ≥26°C were consistently available in all thermal gradients.
We observed thermoregulatory and aggregation behaviors of individual hosts separately in a controlled laboratory environment. Temperatures selected by amphibian larvae in laboratory settings may differ widely from those selected in natural settings (Brattstrom 1963). This may be due partly to conspecific aggregations contributing to thermoregulation (reviewed in Hoff et al. 1999). In at least two of the four species tested (R. cascadae, B. boreas), conspecific aggregations aid thermoregulation by raising water temperatures within larval aggregations. If schooling tadpoles can routinely reach sufficiently high temperatures in the field to regulate BD infection levels, there may not exist a strong force to select for behavioral fever in response to BD infection. Field studies that track both body temperatures of aggregating individuals and infection status over time would provide valuable insight to this system.
From an evolutionary standpoint, our data also lead us to speculate about the potential interactions between altered aggregation and conserved thermoregulation behaviors in response to BD infection in the field. For example, contrary to the trend we observed, if some infected B. boreas in a given host population avoid infected conspecifics, these individuals may avoid superinfection (increased pathogen load through infected neighbors or through reinfection of self), thus standing a better chance of surviving infection by avoiding the accumulation of a lethal BD load. However, the hyperactivity of these hosts may render them more conspicuous to predators, increasing their chances of being removed from the population through predation. Similarly, if some infected B. boreas tadpoles in the population exhibit tendencies towards behavioral fever unlike the majority of the infected population, these individuals may also become more conspicuous, increasing their chances of becoming prey. Both scenarios would slow the evolution of adaptive behaviors in response to BD infection in the host population. Although speculative, these and similar conjectures highlight the importance of considering multiple behaviors in a natural context, as well as in context with one another. Behaviors are considered among the most labile characteristics of organisms and may form the basis of trait evolution (Wcislo 1989; West-Eberhard 1989). However, there are costs to behavioral plasticity that may render some behaviors relatively slow to change (Huey et al. 2003). As hosts encounter rising numbers of emerging diseases, it will be important to consider the relative roles of both behavioral plasticity and behavioral inertia in interpreting host responses to these novel selection pressures.
The lack of avoidance behavior in susceptible tadpoles suggests that the risk of successful BD transmission to susceptible tadpoles may increase, especially in social species. Moreover, if susceptible tadpoles cannot or do not avoid infected conspecifics (such as R. cascadae), they could experience increased infection risk through close proximity, direct contact, or cannibalizing infected conspecifics. Indeed, heightened risk of infection resulting from consuming infected conspecifics is one hypothesis that may explain the general infrequency of opportunistic cannibalism in nature (e.g., Pfennig et al. 1998) despite the developmental advantages gained through consuming conspecifics (Heinen and Abdella 2005).
The alteration of ecologically important host behaviors in response to non-lethal infection has not been examined in the amphibian–chytridiomycosis system. The transmission dynamics that may arise from interactions between susceptible and infected hosts remains a challenging area for future study. In particular, the relationship between ancient host behaviors and transmission of non-lethal infections warrants further examination. It is likely that social behaviors such as aggregation and pathogen-mediated behavioral changes in social species are making important and understudied contributions to the distribution and prevalence of horizontally transmitted pathogens and their hosts in nature.
References
Altig R (2007) Comments on the descriptions and evaluations of tadpole mouthpart anomalies. Herpetol Conserv Biol 2:1–4
Altizer S, Harvell D, Friedle E (2003a) Rapid evolutionary dynamics and disease threats to biodiversity. TREE 18:589–596
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, Dobson AP, Ezenwa V, Jones KE, Pedersen AB, Poss M, Pulliam JRC (2003b) Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu Rev Ecol Evol Syst 34:517–547 doi:10.1146/annurev.ecolsys.34.030102.151725
Bancroft BA, Baker NJ, Searle CL, Garcia TS, Blaustein AR (2008) Larval amphibians seek warm temperatures and do not avoid harmful UVB radiation. Behav Ecol 19(4):879–886
Battin J (2004) When good animals love bad habits: ecological traps and the conservation of animal populations. Conserv Biol 16:1482–1491
Behringer DC, Butler MJ, Shields JD (2006) Avoidance of disease by social lobsters. Nature 441:421 doi:10.1038/441421
Beiswenger RE (1977) Diel patterns of aggregative behavior in tadpole of Bufo americanus, in relation to light and temperature. Ecology 58:98–108
Berger L, Speare R, Hyatt AD (1999) Chytrid fungi and amphibian declines: overview, implications and future directions. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 23–33
Berger L, Hyatt AD, Speare R, Longcore JE (2005) Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 68:51–63
Blaustein AR, Bancroft BA (2007) Amphibian population declines: evolutionary considerations. BioScience 57:437–444 doi:10.1641/B570517
Blaustein AR, Romansic JM, Scheessele EA, Han BA, Pessier AP, Longcore JE (2005) Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol 19:1460–1468
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Brattstrom BH (1962) Thermal control of aggregation behavior in tadpoles. Herpetologica 18:38–46
Brattstrom BH (1963) A preliminary review of the thermal requirements of amphibians. Ecology 44:238–255
Crump ML (1983) Opportunistic cannibalism by amphibian larvae of temporary aquatic environments. Am Nat 121:281–289
Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9:141–150
de Castro F, Bolker B (2005) Mechanisms of disease-induced extinction. Ecol Lett 8:117–126
Dugatkin LA, FitzGerald GJ, Lavoie J (1994) Juvenile three-spined sticklebacks avoid parasitized conspecifics. Environ Biol Fishes 39:215–218
Dupré RK, Petranka JW (1985) Ontogeny of temperature selection in larval amphibians. Copeia 1985:462–467
Freeland WJ (1976) Pathogens and the evolution of primate sociality. Biotropica 8:12–24
Garcia TS, Romansic JM, Blaustein AR (2006) Survival of three species of anuran metamorphs exposed to UV-B radiation and the pathogenic fungus Batrachochytrium dendrobatidis. Dis Aquat Org 72:163–169
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Griffiths RA, Foster JP (1998) The effect of social interactions on tadpole activity and growth in the British anuran amphibians (Bufo bufo, B. calamita, and Rana temporaria). J Zool Lond 245:431–437
Heinen JT, Abdella JA (2005) On the advantages of putative cannibalism in American toad tadpoles (Bufo a. americanus): is it active or passive and why? Am Midl Nat 153:338–347
Hoff KVS, Blaustein AR, McDiarmid RW, Altig R (1999) Behavior: interactions and their consequences. In: McDiarmid RW, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago London, pp 215–239
Huey RB (1982) Temperature, physiology, and the ecology of reptiles. In: Gans C, Pough FH (eds) Biology of the Reptilia. Academic, New York, pp 25–74
Huey RB, Hertz PE, Sinervo B (2003) Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat 161:357–366 doi:10.1086/346135
Kavaliers M, Colwell DD (1992) Aversive responses of female mice to the odors of parasitized males: neuromodulatory mechanisms and implications for mate choice. Ethology 95:202–212
Kavaliers M, Colwell DD (1995) Discrimination by female mice between the odours of parasitized and non-parasitized males. Proc R Soc Lond Ser B 261:31–35
Kermack WO, McKendrick AG (1927) A contribution to the mathematical theory of epidemics. Proc R Soc Lond Ser A 115:700–721
Kiesecker JM, Skelly DK, Beard KH, Preisser E (1999) Behavioral reduction of infection risk. Proc Natl Acad Sci U S A 96:9165–6198
Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D (1998) Role of fever in disease. Ann N Y Acad Sci 856:224–233
Koko H, Sutherland WJ (2001) Ecological traps in changing environments: ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol Ecol Res 3:537–551
Kriger KM, Hero J-M (2007) The chytrid fungus Batrachochytrium dendrobatidis in non-randomly distributed across amphibian breeding habitats. Divers Distrib 13:781–788
Lefcort H, Blaustein AR (1995) Disease, predator avoidance, and vulnerability to predation in tadpoles. Oikos 74:469–474
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A 103:3165–3170 doi:10.1073/pnas.0506889103
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
Moore J (2002) Parasites and the behavior of animals. Oxford University Press, Oxford
Morens DM, Folkers GK, Fauci AS (2004) The challenges of emerging and re-emerging infectious diseases. Nature 430:242–249
O’Hara RK (1981) Habitat selection behavior in three species of anuran larvae: environmental cues, ontogeny, and adaptive significance. PhD dissertation, Oregon State University
Ouedraogo RM, Cusson M, Goettel MS, Brodeur J (2003) Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. J Invertebr Path 82:103–109 doi:10.1016/S0022-2011(02)00185-4
Parris MJ, Davis A, Collins JP (2004) Single-host pathogen effects on mortality and behavioral responses to predators in salamanders (Urodela: Ambystomatidae). Can J Zool 82:1477–1483
Parrish JK, Edelstein-Keshet L (1999) Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284:99–101
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15
Pfennig DW, Ho SG, Hoffman EA (1998) Pathogen transmission as a selective force against cannibalism. Anim Behav 55:1255–1261
Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, Ron SR, Sanchez-Azofeifa GA, Still CJ, Young BE (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167
Putnam RW, Bennett AF (1981) Thermal dependence of behavioural performance of anuran amphibians. Anim Behav 29:502–509
Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, Briggs CJ (2006) Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87:1671–1683
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. TREE 17:462–468
Robertson BA, Hutto RL (2006) A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87:1075–1085
Rowley JJL, Alford RA (2007) Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis Aquat Org 77:1–9 doi:10.3354/dao01830
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357 doi:10.1146/annurev.ento.51.110104.150941
Schlaepfer MA, Runge MC, Sherman PW (2002) Ecological and evolutionary traps. TREE 17:474–480
Schlaepfer MA, Sherman PW, Blossey B, Runge MC (2005) Introduced species as evolutionary traps. Ecol Lett 8:241–246
Schlaepfer MA, Sredl MJ, Rosen PC, Ryan MJ (2007) High prevalence of Batrachochytrium dendrobatidis in wild populations of lowland leopard frogs Rana yavapaiensis in Arizona. EcoHealth 4:421–427
Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P (2006) The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3:35–40 doi:10.1007/s10393-005-0012-6v
Sparrow FK (1968) Ecology of freshwater fungi. In: Gainsworth GC, Sussman AS (eds) The fungi. Academic, New York, pp 41–93
Wassersug RJ (1973) Aspects of social behavior in anuran larvae. In: Vial JL, Blair WF, Bogart JP, Duellman WE, Estes R, Guttman SI, Lynch JD, Merrell DJ, Rabb GB, Reig OA, Salthe SN, Savage JM, Schiøtz A, Starrett PH, Straughan IR, Trueb L, Wassersug RJ (eds) Evolutionary biology of the anurans: contemporary research on major problems. University of Missouri Press, Columbia, Missouri
Watt PJ, Nottingham SF, Young S (1997) Toad tadpole aggregation behaviour: evidence for a predator avoidance function. Anim Behav 54:865–872
Wcislo WT (1989) Behavioral environments and evolutionary change. Ann Rev Ecolog Syst 20:137–169
Weldon C, Du Preez LH (2006) Quantitative measurement of Batrachochytrium dendrobatidis in amphibian skin. Dis Aquat Org 72:153–161
West-Eberhard MJ (1989) Phenotypic plasticity and the origins of diversity. Ann Rev Ecolog Syst 20:249–279
Woodhams DC, Alford RA, Marantelli G (2003) Emerging disease of amphibians cured by elevated body temperature. Dis Aquat Org 55:65–67
Yamakazi K, Sugiura S, Fukasawa Y (2004) Epizootics and behavioral alteration in the arctiid caterpillar Chionarctia nivea (Lepidoptera: Arctiidae) caused by an entomopathogenic fungus, Entomophaga aulicae (Zygomycetes: Entomophthorales). Entomol Sci 7:219–223
Acknowledgments
We thank J Ng, H Lee, S Smith, R LeMaster, M Westphal, BW Patton, J Takishita, J Romansic, R Hill, T Young, J Hubbard, B Moore, S Yi, K Bryant and P House for assistance. B Bancroft and N Baker constructed thermal gradients. J Kerby ran supplemental PCR analyses and provided insightful discussion to improve this manuscript. Training by JE Longcore and LB Kats made this work possible. Authors were funded by the Budweiser Conservation Scholarship (BAH), the Howard Hughes Medical Institute grant for undergraduate research (PWB), and the National Science Foundation Integrated Research Challenges in Environmental Biology (NSF IRCEB) Program (DEB0213851 and IBN9977063). All aspects of this study comply with US laws and adhere to standards of the Institutional Animal Care and Use Committee at Oregon State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Christensen-Dalsgaard
Rights and permissions
About this article
Cite this article
Han, B.A., Bradley, P.W. & Blaustein, A.R. Ancient behaviors of larval amphibians in response to an emerging fungal pathogen, Batrachochytrium dendrobatidis . Behav Ecol Sociobiol 63, 241–250 (2008). https://doi.org/10.1007/s00265-008-0655-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0655-8