Abstract
Tadpoles and mosquito larvae often co-occur, and may compete for scarce resources. However, competition between such distantly related organisms has attracted less scientific attention than have interactions among closely related taxa. We examined ecological interactions in two tadpole-mosquito systems in southeastern Australia, one from freshwater ponds (Limnodynastes peronii and Culex quinquefasciatus) and one from brackish-water habitats (Crinia signifera and Ochlerotatus australis). Diets of these tadpoles and mosquito larvae overlap considerably, potentially leading to competition for food. Laboratory experiments show that, in both study systems, mosquitoes reduced the growth rates of tadpoles, and tadpoles reduced the growth rates and survival of mosquito larvae. These negative effects were seen even at high food levels. Thus, our study suggests that tadpoles and mosquito larvae affect each other strongly, and do so via pathways other than simple consumptive competition. Because mosquitoes are important vectors for human diseases, the global decline in amphibian populations may have more impact on human health than has generally been anticipated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Competitive interactions among organisms are widespread in natural ecosystems, and may often be important determinants of their species composition and persistence (Bronmark et al. 1991). Although the study of competition has thus been a major theme of ecological research for many decades (e.g. Lotka 1932), most studies in this field have focused on interactions between closely related species (Connell 1983; Diamond 1987; Gurevitch et al. 1992). Indeed, the idea that competition between sibling taxa will often be intense because of their close morphological and behavioural similarities dates from Darwin (1859). Nonetheless, competition may also occur between organisms that are related only distantly and, thus, share few phenotypic similarities (e.g. Alford 1999; Brown and Davidson 1977; Carpenter 1979; Hurlbert et al. 1986; Morin et al. 1988).
Because of logistical constraints, competitive effects are easiest to assess in small, discrete habitat patches with relatively low species diversity. Ephemeral ponds offer an ideal opportunity in this regard. The habitat itself is discrete, and the food resources within it constrain the development of aquatic larvae which must grow and metamorphose before the pond dries (Newman 1994). Two of the most abundant and conspicuous components of the fauna within such ephemeral habitats are tadpoles and mosquito larvae (Blaustein and Margalit 1994, 1996). Both are primarily herbivorous (Alford 1999; Bern and Dahl 2000; Mokany 2001; Peterson and Boulton 1999).
Herbivorous tadpoles consume a wide range of organic material (Farlowe 1928), including green algae (Jenssen 1967), unicellular chlorophytes (Kupferberg et al. 1994), cyanobacteria (Seale and Beckvar 1980), faecal pellets (Steinwascher 1979) and organic detritus (Seale and Wassersug 1979). Tadpoles use both suspension feeding and scraping of organic matter from surfaces (Alford 1999; Kenny 1969). They are effective grazers, capable of reducing algal abundance (Rosenfeld 1997). This can have both positive and negative effects on other organisms (Morin 1983). Positive effects have been demonstrated for dipteran larvae raised with tadpoles (Osborne and McLachlan 1985). The tadpoles consumed algae at the surface of the water column and made them available to the bottom-dwelling dipteran larvae through their faecal pellets. This effectively gave the dipteran larvae access to resources that were otherwise unavailable to them (McLachlan 1981, 1985). However, most studies on this topic have demonstrated negative effects of tadpoles feeding on other organisms. For example, intraspecific competition between tadpoles is more intense if food supply is reduced (Griffiths et al. 1993; Wilbur 1997). In addition, interphyletic competitive interactions between tadpoles and snails are caused by the tadpoles limiting the amount of food available (Bronmark et al. 1991).
Like tadpoles, mosquito larvae feed on a wide range of organic matter, including bacteria, detritus, protozoans and algae (Chessman 1986; Hart 1985; Walker et al. 1988, 1991). A recent study demonstrated that mosquito larvae did not discriminate between feeding on polystyrene beads versus planktonic algae (Bern and Dahl 2000). The feeding mechanisms of mosquito larvae are as diverse as their diets (Merritt and Craig 1987; Rashed and Mulla 1990). For example, Culex spp. are mainly planktotrophic, spending most of the time suspension-feeding with their siphons attached to the air-water interface (Widahl 1992). In contrast, Ochlerotatus spp. generally feed at the base of the water column, with younger larvae devoting less time to feeding than do later instars (Merritt et al. 1992).
Preliminary evaluation of the gut contents of the tadpoles (Limnodynastes peronii and Crinia signifera) and their co-occurring mosquito larvae (Culex quinquefasciatus and Ochlerotatus australis) have revealed that they have a high dietary overlap. Both these tadpoles and mosquito larvae consume largely algae and bacteria (Mokany 2001). Here, we investigated the potential ecological interactions between these species for food, using replicated artificial ponds within the laboratory. There was no evidence that the tadpoles used in this study consumed mosquito larvae (Mokany 2001) so that direct predation was unlikely to influence the results. Our primary aims in conducting this study were: (1) to determine whether or not these species negatively affect each other's rates of growth and survival, and (2) if so, to see whether such competitive interactions are consumptive (and, thus, mediated by food supply) or alternatively, involve other competition mechanisms.
Materials and methods
Study species
We studied two systems, one in freshwater ponds (L. peronii plus Culex quinquefasciatus), and one in brackish-water pools close to the high-tide line (Crinia signifera plus O. australis):
-
1.
The striped marsh frog (L. peronii) is a medium-sized frog (to 65 mm) common on the east coast of Australia (Cogger 2000). It mainly breeds from August to March, laying 700–1,000 eggs in a foam egg mass at the edge of water bodies such as freshwater creeks and ponds (Robinson 1996). The tadpoles of this species commonly co-occur with the mosquito species Culex quinquefasciatus in backyard ponds, creeks and streams (Mokany 2001). Culex quinquefasciatus has a global distribution and inhabits all states of mainland Australia (Russell 1993). This mosquito species also breeds from August to March, laying egg rafts on the surface of stagnant freshwater ponds. The larvae of this species can complete development in 7–12 days (Mpho et al. 2000).
-
2.
The common eastern toadlet (Crinia signifera) is a small (35 mm) terrestrial frog from a variety of habitats within Queensland, New South Wales, Victoria, South Australia and Tasmania. The larvae of this species inhabit both permanent and ephemeral ponds (Cogger 2000) and commonly co-occur with larvae of the mosquito O. australis in small coastal rock ponds (Mokany 2001). This mosquito inhabits vast areas of coastal Australia and breeds year-round, depositing its eggs above the high-tide mark on rock platforms of the coast (Russell 1993). Larvae of these mosquitoes are aggressive towards other organisms and feed on animal as well as plant material (Mokany 2001).
Competition experiments
From October to November 2000, we conducted a series of experiments in the laboratory using 1.5-l (10 cm×10 cm×15 cm) polyethylene containers as artificial ponds. These containers were well within the size range of ponds that naturally contain both tadpoles and mosquitoes (Mokany 2001). To each tub we added 1 l of aged tap water (Aqua-pet water ager), 100 g aquaria gravel, and floating fish pellets (I.M.P.S). We assigned three treatments, each with three food levels—low (one pellet per day), medium (two pellets per day) and high (three pellets per day)—to these ponds, in a Latin square formation.
The numbers of tadpoles and mosquito larvae used per container differed between the systems because of differences in their abundances in natural water bodies (Mokany and Shine 2002b). The three treatments for Crinia signifera and O. australis were: (1) 3 early-stage tadpoles (stage 25; Gosner 1960), (2) 20 first-instar mosquito larvae, and (3) 3 early-stage tadpoles plus 20 first-instar mosquito larvae. For L. peronii and Culex quinquefasciatus, the treatments were: (1) 5 early-stage tadpoles (Gosner stage 25), (2) 20 first-instar mosquito larvae, and (3) 5 early-stage tadpoles plus 20 first-instar mosquito larvae. Each treatment was replicated five times. We weighed and measured the tadpoles prior to experimentation. We established the wet weight of the tadpoles by transferring tadpoles via a sieve into a petri dish full of water, onto the pan of a digital balance. We measured the tadpoles with digital calipers (±0.01 mm) to record length (from snout to tail-tip) and maximum body width.
We checked the containers daily for mosquito pupae, and any pupae were transferred to a separate container to metamorphose. This removal of pupae should not influence competitive interactions because the pupae do not feed or excrete. We froze the metamorphosed adult mosquitoes to enable later measurement of wing sizes. We terminated the experiment after 25 days, as by this time most mosquito larvae had either died or pupated. We then re-weighed the tadpoles and recorded their final length and width. The measurements taken were converted to the mean percentage mass change, percentage length change and percentage width change of the three tadpoles since the beginning of the experiment.
At the end of experimentation, we calculated the percentage of mosquitoes that had pupated, the duration of their larval periods (day to pupation from the beginning of experimentation), their percentage survival and their wing sizes (separately for male and female mosquitoes). We measured wing size from the alular notch to the tip of the wing margin excluding wing scales.
Data analysis
We analysed our results using the program Statview 5 (SAS Institute 1998). All percentage data were transformed by arcsin square root prior to analysis. Due to the large number of dependent variables that we measured, conducting separate statistical tests on each variable would raise issues of artificially "significant" results simply due to the number of tests conducted. Unfortunately, methods to remedy this situation (such as applying Bonferroni "correction factors") are themselves prone to considerable subjectivity (Cabin and Mitchell 2000; Snedecor and Cochran 1980). Therefore, we combined the data from both study systems and conducted multivariate ANCOVA (MANCOVA) separately for tadpoles and mosquito larvae. The probability values from these analyses offer robust evidence on the significance of results, because they are based on single comparisons that use all available data.
Results
Competition experiments
The MANCOVA for tadpoles included two factors: tadpole species and the presence/absence of mosquito larvae. Food level was included as a covariate (a continuous variable) rather than as a nominal factor, because of the clear quantitative relationship among treatment levels (one, two or three pellets per day). Analyses that included food level as a nominal rather than continuous variable yielded virtually identical conclusions. The dependent variables were proportional changes in the length, width and mass of tadpoles over the course of the experiment. The MANCOVA revealed that tadpole growth rates overall were higher when more food was available, and were depressed by the presence of mosquito larvae (Table 1). Overall growth rates did not differ significantly between the two species of tadpoles, but the two species responded differently to variations in food supply and the presence of mosquitoes (Table 1). Importantly, however, the growth rates of tadpoles were not significantly affected by the interaction between mosquito presence and food supply, nor by the three-way interaction between food level, mosquito presence and tadpole species (Table 1).
The MANCOVA for mosquito larvae included mosquito species and presence/absence of tadpoles as factors, food level as a covariate, and dependent variables of the number of days to pupation, the proportion of larvae pupating, the proportion surviving, and male wing sizes. No female mosquitoes emerged from some treatments, so that we could not include female wing size in the MANCOVA. As for the MANCOVA on tadpoles (above), the attributes of mosquitoes were affected by food supply and the presence of tadpoles, and differed between the two mosquito species (Table 1). The variables we measured on mosquitoes were also influenced by the three-way interaction between mosquito species, food supply and tadpole presence, and lower-level interaction terms were statistically significant as well (Table 1).
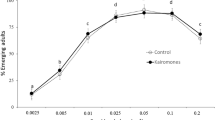
In summary, tadpoles in both study systems grew more rapidly when given more food, and less rapidly in the presence of mosquito larvae (Fig. 1). Effects on mosquitoes were more complex, and differed between the two study systems. In freshwater ponds (L. peronii and Culex quinquefasciatus), food supply affected some but not all traits of mosquito larvae (Fig. 2). The amount of food had no significant effect on the day to pupation, female wing size or percentage pupation, but male wing size and percentage survival were reduced in the low-food treatment compared to the high-food treatment (Fig. 2). The presence of tadpoles did not affect day to pupation or female wing size for Culex quinquefasciatus mosquitoes, but affected all other traits. For example, male wing size, percentage pupation and percentage survival were lower in the treatments raised with tadpoles (Fig. 2).
Effects of the level of food and presence of mosquito larvae on the growth rates of tadpoles (Limnodynastes peronii). The graphs show mean values (±SE) for percentage growth measured for L. peronii raised without mosquito larvae (unfilled histograms), and L. peronii tadpoles raised with Culex quinquefasciatus mosquito larvae (shaded histograms) at various food levels. The histograms represent: A percentage weight change; B percentage width change; C percentage length change of the tadpoles for the various treatments. The x-axis represents food level
Effects of food level and presence of tadpoles on the traits of the mosquito Culex quinquefasciatus. The graphs represent mean values (±SE) for traits measured for C. quinquefasciatus mosquito larvae raised without tadpoles present (unfilled histograms), and C. quinquefasciatus raised with Limnodynastes peronii tadpoles (shaded histograms). The histograms represent: A day to pupation; B female wing size; C male wing size; D percentage pupation; E percentage survival of the mosquito larvae for the experimental period. The x-axis represents the experimental food level
In brackish-water ponds (Crinia signifera and O. australis), food supply affected all of the measured traits of O. australis except for percentage survival (Figs. 3, 4). The mosquitoes in the low-food treatments pupated later, often failed to pupate and were smaller if they did so, than were mosquitoes in the high-food treatments (Fig. 4). The presence of tadpoles did not affect day to pupation or percentage pupation of the mosquitoes, but wing sizes and percentage survival were reduced when the mosquitoes were raised with tadpoles (Fig. 4).
Effects of the level of food and presence of mosquito larvae on the growth rates of tadpoles (Crinia signifera). The graphs show mean values (±SE) for percentage growth measured for Crinia signifera raised without mosquito larvae present (unfilled histograms), and Crinia signifera tadpoles raised with Ochlerotatus australis mosquito larvae (shaded histograms) at various food levels. The histograms represent: A percentage weight change; B percentage width change; C percentage length change of the tadpoles for the various treatments. The x-axis represents food level
Effects of food level and presence of tadpoles on the traits of the mosquito Ochlerotatus australis. The graphs show mean values (±SE) for traits measured for O. australis mosquito larvae raised without tadpoles (unfilled histograms) and O. australis raised with Crinia signifera tadpoles (shaded histograms). The histograms represent: A day to pupation; B female wing size; C male wing size; D percentage pupation; E percentage survival of the mosquito larvae for the experimental period. The x-axis represents the experimental food level
Discussion
Increasing the supply of food in our experiments accelerated the growth of both the tadpoles and the mosquitoes with or without competitors. However, the presence of competitors depressed the growth rates of both the tadpoles and the mosquito larvae, even at high food levels. These results suggest that tadpoles and mosquito larvae can potentially compete for food, and that they do indeed suppress each other in laboratory "ponds". However, the mechanism for this suppression involves more than simple consumptive competition.
Tadpoles of both study species were affected by the level of food they were given, as well as by interactions with mosquito larvae. L. peronii and Crinia signifera tadpoles experienced reduced growth at low food levels, indicating (unsurprisingly) that the abundance of food can influence the growth and development of tadpoles (Figs. 1, 3). This finding is consistent with previous studies; tadpoles fed reduced amounts of food (Griffiths et al. 1993; Newman 1994, 1998) or low-quality food (Kupferberg 1997) grow at reduced rates compared to those tadpoles fed to satiation. Of more interest, growth rates of both L. peronii and Crinia signifera tadpoles were also reduced when the anurans were raised with mosquito larvae. These effects were seen at all food levels. However, our studies did not detect an interaction between the level of food and the presence of mosquito larvae for any of the variables that we measured for either species of tadpole. This result suggests that competition for food may not be the only mechanism involved in the interaction. Other mechanisms that might be involved include chemical (Wilbur 1997) and mechanical interference (Mokany and Shine 2002a).
Larvae of both mosquito species were negatively affected by low food supply and the presence of tadpoles (Figs. 2, 4). Culex quinquefasciatus mosquito larvae demonstrated reduced survival and male wing size in the low food treatments. The development time and adult wing size of O. australis were also affected by reduced food levels, but survival was not reduced. These interspecific differences may reflect ability to withstand starvation. Ochlerotatus spp. mosquitoes decrease their developmental rate when food levels are low, whereas Culex spp. display reduced survival (Barrera and Medialdea 1996).
Our study also showed that both mosquito species were significantly affected by their interactions with tadpoles. The survival rate and adult wing size of both Culex quinquefasciatus and O. australis were reduced in the presence of tadpoles. The significant interaction between the presence of tadpoles and the level of food in our MANCOVA for mosquito larvae suggests that consumptive competition may be involved. That is, the effects of tadpoles on mosquito larvae were most pronounced when food was limiting. However, this may not be the sole mechanism by which tadpoles influence mosquito larvae, because we saw substantial competitive effects even at high food levels (Figs. 2, 4).
The dramatic recent decline in amphibian populations in many parts of the world (Pechmann et al. 1991; Pyke and White 1999; Vitt et al. 1990) means that studies on competition between tadpoles and other taxa warrant urgent attention. In particular, our study suggests that tadpoles may substantially reduce the viability of mosquitoes by delaying their metamorphosis, reducing their larval survival and decreasing their adult body sizes (and hence, both disease-carrying potential and fecundity). The recent (and continuing) widespread decline in amphibian numbers may thus have severe ramifications, with frogs acting not only as indicator species for ecosystem function, but as direct contributors to major ecological processes with important implications for human health.
References
Alford RA (1999) Ecology: resource use, competition, and predation. In: McDiarmid RW, Altig R (eds) Tadpoles—the biology of anuran larvae. University of Chicago Press, Chicago, pp 240–278
Barrera R, Medialdea V (1996) Development time and resistance to starvation of mosquito larvae. J Nat Hist 30:447–458
Bern L, Dahl C (2000) Ingestion of algae and inert particles by larval Culex quinquefasciatus. Arch Hydrobiol 147:25–33
Blaustein L, Margalit J (1994) Mosquito larvae (Culiseta longiareolata) prey upon and compete with toad tadpoles (Bufo viridis). J Anim Ecol 63:841–850
Blaustein L, Margalit J (1996) Priority effects in temporary pools: nature and outcome of mosquito larva-toad tadpole interactions depend on order of entrance. J Anim Ecol 65:77–84
Bronmark C, Rundle SD, Erlandsson A (1991) Interactions between freshwater snails and tadpoles: competition and facilitation. Oecologia 87:8–18
Brown JH, Davidson DW (1977) Competition between seed eating rodents and ants in desert ecosystems. Science 196:880–882
Cabin RJ, Mitchell RJ (2000) To Bonferroni or not to Bonferroni: when and how are the questions. Bull Ecol Soc Am 81:246–248
Carpenter FL (1979) Competition between humming birds and insects for nectar. Am Zool 19:1105–1114
Chessman BC (1986) Dietary studies of aquatic insects from two Victorian rivers. Aust J Mar Freshwater Res 37:129–146
Cogger HG (2000) Reptiles and amphibians of Australia. Reed New Holland, Sydney
Connell JH (1983) On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat 122:661–696
Darwin C (1859) On the origin of species. Murray, London
Diamond J (1987) Competition among different taxa. Nature 326:241
Farlowe V (1928) Algae of ponds as determined by examination of the intestinal contents of tadpoles. Biol Bull 55:443–448
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Griffiths RA, Denton J, Wong ALC (1993) The effect of food level on competition in tadpoles—interference mediated by Protothecan algae. J Anim Ecol 62:274–279
Gurevitch J, Morrow LL, Wallace A, Walsh JS (1992) A meta-analysis of competition in field experiments. Am Nat 140:539–572
Hart DD (1985) Grazing insects mediate algal interactions in a stream benthic community. Oikos 44:40–46
Hurlbert SH, Loayza W, Moreno T (1986) Fish-flamingo-plankton interactions in Peruvian Andes. Limnol Oceanogr 31:457–468
Jenssen TA (1967) Food habits of the green frog, Rana clamitans, before and during metamorphosis. Copeia 1967:214–218
Kenny JS (1969) Feeding mechanisms in anuran larvae. J Zool Lond 157:225–246
Kupferberg SJ (1997) The role of larval diet in anuran metamorphosis. Am Zool 37:146–159
Kupferberg SJ, Marks JC, Power ME (1994) Effects of variation in natural algal and detrital diets on larval anuran (Hyla regilla) life-history traits. Copeia 1994:446–457
Lotka AH (1932) The growth of mixed populations: two species competing for a common food supply. J Wash Acad Sci 22:461–469
McLachlan AJ (1981) Interactions between insect larvae and tadpoles in tropical rain pools. Ecol Entomol 6:175–182
McLachlan AJ (1985) What determines the species present in a rain pool? Oikos 45:1–7
Merritt RW, Craig DA (1987) Larval mosquito (Diptera: Culicidae) feeding mechanisms: muco-substance production for capture of fine particles. J Med Entomol 24:275
Merritt RW, Dadd RH, Walker ED (1992) Feeding behaviour, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol 37:349–376
Mokany A (2001) Ecological and behavioural interactions between tadpoles and mosquito larvae. BSc Thesis, University of Sydney
Mokany A, Shine R (2002a) Pond attributes influence competitive interactions between tadpoles and mosquito larvae. Aust Ecol 27:396–404
Mokany A, Shine R (2002b) Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Aust Ecol (in press)
Morin PJ (1983) Predation, competition, and the composition of larval anuran guilds. Ecol Monogr 53:119–138
Morin PJ, Lawler SP, Johnson EA (1988) Competition between aquatic insects and vertebrates: interaction strength and higher order interactions. Ecology 69:1401–1409
Mpho M, Holloway GJ, Callaghan A (2000) Fluctuating wing asymmetry and larval density stress in Culex quinquefasciatus. Bull Entomol Res 90:279–283
Newman RA (1994) Effects of changing density and food level on metamorphosis of a desert amphibian, Scaphiophus couchii. Ecology 75:1085–1096
Newman RA (1998) Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with response to changing food level. Oecologia 115:9–16
Osborne PL, McLachlan AJ (1985) The effects of tadpoles on algal growth in temporary, rain-filled rock pools. Freshwater Biol 15:77–88
Pechmann JHK, Scott DE, Semlitsch RD, Caldwell JP, Vitt LJ, Gibbons JW (1991) Declining amphibian populations: the problem of separating human impact from natural fluctuations. Science 253:892–895
Peterson CG, Boulton AJ (1999) Stream permanence influences micro-algal food availability to grazing tadpoles in arid-zone springs. Oecologia 118:340–352
Pyke GH, White AW (1999) Dynamics of co-occurring frog species in three ponds utilised by the endangered Green and Golden Bell frog Litoria aurea. Aust Zool 32:230–239
Rashed SS, Mulla MS (1990) Comparative functional morphology of the mouth brushes of mosquito larvae (Diptera: Culicidae). J Med Entomol 27:429–439
Robinson M (1996) A field guide to the frogs of Australia, from Port Augusta to Fraser Island including Tasmania. Reed, Melbourne
Rosenfeld JS (1997) The effect of large macroinvertebrate herbivores on sessile epibenthos in a mountain stream. Hydrobiologia 344:75–79
Russell RC (1993) Mosquitoes and mosquito-borne disease in south-eastern Australia. Medical Entomology, Westmead
SAS Institute (1998) Statview 5. Cary, Ind
Seale DB, Beckvar N (1980) The comparative ability of anuran larvae (Genera: Hyla, Bufo and Rana) to ingest suspended blue-green algae. Copeia 1980:495–503
Seale DB, Wassersug RJ (1979) Suspension feeding dynamics of anuran larvae related to their functional morphology. Oecologia 39:259–272
Snedecor GW, Cochran WG (1980) Statistical methods. Iowa State University Press, Iowa
Steinwascher K (1979) Host-parasite interaction as a potential population regulating mechanism. Ecology 60:884–890
Vitt LJ, Caldwell JP, Wilbur HM, Smith DC (1990) Amphibians as harbingers of decay. Bioscience 40:418
Walker ED, Olds EJ, Merritt RW (1988) Gut content analysis of mosquito larvae (Diptera: Culicidae) using DAPI stain and epifluorescence microscopy. J Med Entomol 25:551–554
Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ (1991) Nutrient dynamics, bacterial populations and mosquito productivity in tree hole ecosystems and microcosms. Ecology 72:1529–1546
Widahl L (1992) Flow patterns around suspension feeding mosquito larvae. Ann Entomol Soc Am 85:91–95
Wilbur HM (1997) Experimental ecology of food webs: complex systems in temporary ponds. Ecology 78:2279–2302
Acknowledgements
We thank Mike Thompson and Angela Low for collecting tadpoles. The Australian Research Council provided financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokany, A., Shine, R. Competition between tadpoles and mosquito larvae. Oecologia 135, 615–620 (2003). https://doi.org/10.1007/s00442-003-1215-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1215-6