Abstract
The carotid body (CB) is the major arterial chemoreceptor responsible for the detection of acute decreases in O2 tension (hypoxia) in arterial blood that trigger hyperventilation and sympathetic activation. The CB contains O2-sensitive glomus (chief) cells, which respond to hypoxia with the release of transmitters to activate sensory nerve fibers impinging upon the brain respiratory and autonomic centers. During exposure to sustained hypoxia (for weeks or months), the CB grows several-fold in size, a response associated with acclimatization to high altitude or to medical conditions presenting hypoxemia. Here, I briefly present recent advances on the mechanisms underlying glomus cell sensitivity to hypoxia, in particular the role of mitochondrial complex I in acute oxygen sensing. I also summarize the properties of adult CB stem cells and of glomus cell–stem cell synapses, which contribute to CB hypertrophy in chronic hypoxia. A note on the relationship between hypoxic CB growth and tumorigenesis is included. Finally, the medical implications of CB pathophysiology are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
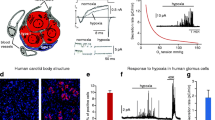
Oxygen (O2) sensing is fundamental for life of aerobic organisms and particularly for mammals, as it is necessary for their adaptation to environments with changing O2 tension (PO2) or pathophysiological conditions presenting a decrease in blood PO2 (hypoxemia). Acute changes in PO2 are detected by chemoreceptor, O2-sensitive, cells in organs of the homeostatic O2-sensing system (Weir et al. 2005). Among these organs, the carotid body (CB) has a special significance, as it is strategically located at the bifurcation of the carotid artery (Fig. 1a) and functions as the main arterial chemoreceptor activated by hypoxemia, hypercapnia (rise of blood PCO2), acidic pH, hypoglycemia and other stimuli (see Lopez-Barneo et al. 2016a). The CB is composed of clusters of cells (glomeruli) in close contact with capillaries and nerve fibers. Neuron-like glomus or type I cells (most frequently named in the medical literature as “chief” cells), the most abundant in the glomeruli, are highly dopaminergic (can be stained with antibodies against tyrosine hydroxylase; TH) and also contain ATP, neuropeptides and several other neurotransmitters (Fig. 1b). Upon activation, glomus cells release transmitters, which activate afferent fibers terminating at the respiratory and autonomic centers of the brainstem, thereby eliciting hyperventilation and sympathetic activation (Fig. 1c). Glomus cells are enveloped by glia-like type II, or sustentacular, cells (Fig. 1b), which have classically been ascribed a supportive role, although recent studies have unraveled their function as quiescent stem cells (Pardal et al. 2007; Macias et al. 2014; Navarro-Guerrero et al. 2016). CB glomeruli are highly complex structures with sophisticated autocrine and paracrine interactions among the different cell classes (see Nurse 2014).
Acute oxygen sensing by carotid body glomus cells. a The human carotid bifurcation after removal of the fat and connective tissues. The carotid body (CB) is indicated by an arrow. IC internal carotid artery. Scale bar 1 cm. Modified from Ortega-Saenz et al. (2013). b Schematic of the main cellular components in the CB glomerulus. c Hypoxic ventilatory response in a normal mouse. d, d’ Secretory responses to hypoxia (PO2, ~10 mmHg) and hypercapnia (20% CO2) of glomus cells in slices from normal (control) and Ndufs2-deficient (TH-NDUFS2) mice. e, e’, f, f’ Changes in NAD(P)H autofluorescence (e, e’) and reactive oxygen species in cytosol (f, f’) during exposure to hypoxia recorded from glomus cells from control and Ndufs2-null (TH-NDUFS2) mice. c–f’ Modified from Fernandez-Aguera et al. (2015)
In the last decade, the CB, with a well-established role in the regulation of respiration, has also drawn special medical attention due to its possible contribution to the pathogenesis of highly prevalent human diseases. Alterations of CB development have been associated with respiratory disturbances (congenital central hypoventilation or sudden infant death syndromes) in the newborn (Cutz et al. 1997; Gauda et al. 2004; Perez and Keens 2013) and, more recently, CB over-activation has been suggested to contribute to the exaggerated sympathetic outflow existing in sleep apnea (Del Rio et al. 2016), cardiac failure (Marcus et al. 2014) and metabolic (Ribeiro et al. 2013) syndromes. Indeed, CB resection has been proposed for the treatment of refractory neurogenic hypertension (McBryde et al. 2013), although this procedure does not seem to be exempt from adverse effects (Limberg et al. 2015). In addition to dopamine, CB glomus (chief) cells contain high levels of glial cell line-derived neurotrophic factor (GDNF), an agent that promotes survival of central dopaminergic neurons (Lin et al. 1993; Hidalgo-Figueroa et al. 2012), hence CB transplantation has been suggested as a potential therapeutic approach to Parkinson’s disease (Minguez-Castellanos et al. 2007).
In this article, I will briefly summarize our current understanding of the mechanisms underlying acute O2 sensing by CB glomus (chief) cells and their responsiveness to hypoxia. I will also discuss the process of CB growth during sustained hypoxia, an intriguing property that is not normally seen in other structures of the peripheral nervous system (see, however, Pan et al. 2016). I will describe the properties of adult CB stem cells and the mechanisms whereby, during exposure to hypoxia, mature O2-sensitive glomus cells induce stem cells to proliferate and differentiate to generate new glomus cells.
Mechanisms of acute O2 sensing by carotid body chemoreceptor cells
A principal characteristic of CB chemoreceptor cells is that they contain several classes of K+ channels, the open probability of which is inhibited during hypoxia, thereby leading to cell depolarization, Ca2+ influx and transmitter release. These “O2-sensitive” K+ channels, initially identified in glomus (chief) cells, have also been described in other preparations such as pulmonary arterial myocytes (see Lopez-Barneo et al. 1999; Weir et al. 2005) or adrenal medulla (AM) chromaffin cells (Thompson et al. 1997; Keating et al. 2005). In rodent glomus cells, background K+ channels (most likely Task1/Task3 heteromers) seem to be the most important for the initiation of the hypoxic depolarization (Buckler et al. 2000; Kim et al. 2009), which may be potentiated by inhibition of O2-sensitive voltage-gated K+ channels (see Ortega-Saenz et al. 2010). Activation of Ca2+ permeable cationic channels has also been suggested to contribute to the hypoxic response in glomus (Kang et al. 2014) and chromaffin (Inoue et al. 1998) cells.
Although the “membrane model” of chemosensory transduction is generally accepted, the mechanisms whereby decreases in PO2 result in altered ion channel activity have been a matter of much debate and discussion (see Peers 2015; Nurse 2017). Several molecules that directly or indirectly modify ion channel function have been proposed to function as O2 sensors; however, none of them has received robust experimental support (see Lopez-Barneo et al. 2016b). On the other hand, as CB cells are strongly activated by cyanide and other electron transport chain (ETC) inhibitors, a classical view is that mitochondria have an important role in hypoxic CB activation (Mills and Jobsis 1972; Mulligan and Lahiri 1982; Duchen and Biscoe 1992). Rotenone, a mitochondrial complex (MC) I blocker, can selectively occlude sensitivity to hypoxia in glomus cells without affecting responsiveness to hypoglycemia (Garcia-Fernandez et al. 2007); therefore, a rotenone binding molecule has been suggested to be essential for acute O2 sensing (Ortega-Saenz et al. 2003; Keating et al. 2005; Thompson et al. 2007). To test this hypothesis, we generated genetically modified mice with ablation of the Ndufs2 gene, which encodes the 49-kD subunit of the ubiquinone (CoQ)/rotenone binding site at the catalytic core of MCI. Mice with Ndufs2 deficiency restricted to catecholaminergic cells (TH-NDUFS2 mice) can survive 3–4 months but show a complete abolition of the hypoxic ventilatory response with normal responsiveness to hypercapnia (Fernandez-Aguera et al. 2015). In agreement with this systemic phenotype, Ndufs2-null glomus cells are insensitive to hypoxia, although they respond normally to hypercapnia, hypoglycemia and direct depolarization with high extracellular K+ (Fig. 1d, d’). CBs from Ndufs2-deficient mice are histologically normal, which suggests that glomus cells survive without a functional MCI and rely on the MCII–MCIV pathway for oxidative phosphorylation. This idea is supported by the high levels of succinate found in the CB, in comparison with other central and peripheral neural organs (Fernandez-Aguera et al. 2015) and the marked cell loss in CB from MCII-deficient mice (Diaz-Castro et al. 2012). Recently, a “signature gene expression profile”, characterized by high levels of pyruvate carboxylase (Pcx) and of three atypical mitochondrial subunits (Ndufa4l2, Cox4i2 and Cox8b) as well as down-regulation of Phd3 and up-regulation of Hif2α, has been reported for acute O2-sensing chemoreceptor cells (Gao et al. 2017). CB cells also contain unusually high levels of biotin, the essential cofactor of carboxylases (Ortega-Saenz et al. 2016). Taken together, these findings suggest that specific metabolic specializations confer CB glomus cells with their special sensitivity to hypoxia. The overexpression of Pcx is probably required for TCA cycle anaplerosis and the accumulation of high levels of reduced ubiquinone (CoQH2). The presence of the three atypical mitochondrial subunits could make cytochrome c oxidase activity highly sensitive to decreases in PO2, such that even relatively mild hypoxia would cause backup of electrons in the ETC and a further increase in the CoQH2/CoQ ratio, thereby leading to reactive oxygen species (ROS) and NADH production in MCI. This comprehensive model of acute O2 sensing fits well with recent experiments showing reversible increases in mitochondrial ROS and NADH, which can modulate membrane ion channels-, during hypoxia and the disappearance of both signals in Ndufs2-deficient glomus cells (Fig. 1e, e’, f, f’) (Fernandez-Aguera et al. 2015). In addition, responsiveness to acute hypoxia in glomus cells is abolished by pharmacological and genetic inhibition of succinate dehydrogenase (Gao et al. 2017). Moreover, increases in mitochondrial ROS have also been associated with acute hypoxic pulmonary vasoconstriction (HPV) (Waypa et al. 2001) and ablation of the Cox4i2 subunit inhibits acute HPV and activation by hypoxia of single pulmonary artery smooth muscle cells (Sommer et al. 2017).
Carotid body growth in chronic hypoxia, stem cells and tumorigenesis
In addition to its function as an acute O2 sensor, the CB plays a fundamental role in acclimatization to sustained (chronic) hypoxia (see Joseph and Pequignot 2009). The CB has a high level of plasticity and in individuals living at high altitude with low atmospheric pressure or in patients suffering cardiopulmonary diseases who present with hypoxemia, it can grow to several-fold its normal size. This response, unusual for a neuronal organ, is characterized by angiogenesis and enlargement of the neural parenchyma, which leads to augmentation of the excitatory electrical signals that act on the brainstem respiratory center to produce hyperventilation. During acclimatization to hypoxia, the CB-mediated constant hyperventilation prevents an excessive fall in arterial PO2, while other mechanisms trigger angiogenesis and red blood cell proliferation to increase O2 supply to the tissues.
Although CB growth is a well-known classic response to hypoxia (Fig. 2a, a’) (Arias-Stella and Valcarcel 1976; McGregor et al. 1984) the underlying mechanisms have remained largely unstudied. Using genetic markers, we have shown that, as suggested before (Le Douarin 1986; Kameda 2005), the two main cell types in the CB (glomus, type I or chief cells and type II or sustentacular cells) derive from neural crest precursors (Pardal et al. 2007). In normoxic conditions, type II cells, which can be stained with antibodies against the glial fibrillary acidic protein (GFAP), are arranged with large processes enveloping glomus cells. However, in response to hypoxia, the GFAP staining progressively disappears in parallel with the appearance of proliferating nestin+ progenitors and new blood vessels, suggesting a change of phenotype in type II cells (Pardal et al. 2007). Although a population of TH+ CB cells can undergo mitosis (Paciga et al. 1999; Chen et al. 2007; Pardal et al. 2007), in vivo cell fate mapping experiments have shown that the nestin+-positive progenitors and many TH+ glomus cells newly generated during exposure of mice to chronic hypoxia derive from GFAP+ cells (Pardal et al. 2007). The CB contains a population of cells (~1% of the total cell number in CBs from rats or mice) that, once dispersed, behave as self-renewing multipotent stem cells able to form clonal colonies (neurospheres), composed of a core of proliferating nestin+ progenitors, which in a few days give rise to the appearance of blebs formed by differentiating TH+ cells budding out from the neurosphere (Fig. 2b–b”, c–c”). In rat preparations, the blebs (clusters of TH+ cell) can grow for several weeks in culture to reach the size of an entire CB. For unknown reasons, in preparations of mouse or human CB, this “regenerative potential” is, however, much smaller than in the rat (Ortega-Saenz et al. 2013; own unpublished observations). Newly generated glomus cells in vitro have a significant population of voltage-gated Ca2+ and K+ channels, numerous catecholaminergic secretory vesicles, which are released in response to hypoxia or hypoglycemia (Fig. 2d–e’) and high levels of GDNF (Fig. 2f). In addition to neuronal O2-sensitive glomus cells, CB stem cells can also give rise to actin-positive smooth muscle cells, a typical neural crest derivative, as well as endothelial cells (Pardal et al. 2007; Navarro-Guerrero et al. 2016; Annese et al. 2017). Therefore, the CB is a neurogenic niche in the peripheral nervous system, which shares many of the properties of neurogenic centers in the mammalian brain: the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus (see Kriegstein and Alvarez-Buylla 2009). In all these cases, quiescent stem cells with a glia-like phenotype can be activated to become proliferative nestin+ intermediate progenitors, which can eventually differentiate into neuroblasts and other cell types (Kokovay and Temple 2007). Multipotent glia-like stem cells have also been found in the adrenal medulla, where they seem to be able to generate new chromaffin cells and contribute to the plasticity of this organ (Rubin de Celis et al. 2015).
Carotid body stem cells. a, a’ Histological sections of carotid bodies from normal mouse and after exposure to hypoxia (10% O2) for 3 weeks. Scale bar 50 μm. b–b” A stem cell colony illustrating the formation of a typical clonal CB neurosphere. Scale bar 50 μm. c–c” Time course of rat CB neurosphere formation in vitro. Note the formation of the neurosphere core containing nestin+ progenitors and the subsequent appearance of blebs of TH+ glomus cells. Scale bar 50 μm. d A bleb of TH+ cells detached from a rat CB neurosphere. The carbon fiber electrode placed near the bleb was used to record the cellular secretory responses to hypoxia and hypoglycemia (e, e’). Scale bar 20 μm. f GDNF mRNA expression in rat CB and CB-derived neurospheres (NS). Note that GDNF is not expressed in the superior cervical ganglion (SCG). Modified from Pardal et al. (2007)
A question of interest is whether the proliferative potential of the CB is associated with the appearance of chemodectomas, a tumor subtype, which belongs to the group of paragangliomas affecting the peripheral nervous system. CB paragangliomas are mostly benign and have histological features that resemble those observed in the CB of individuals subjected to chronic hypoxemia (Heath et al. 1982; Arias-Stella and Valcarcel 1976; Kliewer et al. 1989). In addition, the incidence of CB tumors increases in high-altitude residents (Saldana et al. 1973; Arias-Stella and Bustos 1976; Astrom et al. 2003). There are regional variations in the prevalence of paragangliomas at high altitudes (e.g., extremely high in Mexico, probably much lower in the US Rocky Mountain states and in the Himalayas), which may result from regional differences in the prevalence of occult mutations of hereditary susceptibility genes (Cerecer-Gil et al. 2010). However, the relationship between CB tumorigenesis and the activity of the CB neurogenic niche has not been established. Mutations in the membrane anchoring subunit D of mitochondrial succinate dehydrogenase (SdhD) are the most frequent cause of congenital CB paraganglioma (Rustin et al. 2002; Baysal 2008). Affected individuals are heterozygous (contain a normal and a mutated allele) and the tumor appears after the loss of the normal allele (loss of heterozygosity) (Habano et al. 2003; Maier et al. 1999). Biallelic deletion of the succinate dehydrogenase subunits studied so far (SdhB and SdhD) are lethal at embryonic stages and heterozygous SdhD-deficient (+/−) mice up to 2 years of age do not develop tumors or any other obvious pathology, although they seem to have subtle CB alterations (Piruat et al. 2004). Moreover, conditional ablation of the Sdhd alleles in catecholaminergic cells of mice result in a marked cell loss in the CB, AM and superior cervical ganglion (SCG), as well as in mesencephalic dopaminergic neurons (Diaz-Castro et al. 2012). Therefore, it seems that SdhD ablation in mice, which can cause succinate accumulation, prolyl hydroxylase (PHD) inhibition and hypoxia inducible factor (HIF) stabilization (Selak et al. 2005; Millan-Ucles et al. 2014), is not sufficient to induce paragangliomas. A “multiple-hit” hypothesis and differential chromosomal arrangement have been suggested to explain the differences between CB tumorigenesis in humans and mice (Millan-Ucles et al. 2014). In any case, the data available do not support a direct relationship between the mechanism of hypoxic CB hypertrophy and the appearance of paragangliomas, which are tumors that can affect not only the CB but also tissues, such as the AM, without a hypertrophy response to hypoxia.
Stem cell activation in the hypoxic carotid body
A simple model of the hypoxic CB growth postulated that activation of progenitor cell proliferation by lowering PO2 was due to inhibition of PHDs and stabilization of HIF (Pardal et al. 2007; Kokovay and Temple 2007), as it is known that HIF up-regulation can induce proliferation in several cell types. However, we found that hypoxia-induced CB hypertrophy in vivo is not mimicked by the systemic administration of dimethyloxalylglycine, although this drug (a potent PHD inhibitor) is able to induce HIF-dependent responses, such as red blood cell proliferation and the up-regulation of Vegf mRNA expression in the brain. In agreement with these observations, it has also been shown that the size of the core of CB neurospheres in vitro (an indication of the proliferation of CB progenitors) is unaltered by exposure to PO2 as low as 1% (Platero-Luengo et al. 2014). These results indicated that, as it occurs in other multipotent stem cells (Ezashi et al. 2005; Mohyeldin et al. 2010) and neural progenitors (d’Anglemont de Tassigny et al. 2015), CB stem cells are not intrinsically sensitive to hypoxia as they mainly rely on a non-oxidative metabolism. Moreover, the data also suggested that activation of hypoxic CB stem cells in vivo depends on the presence of the O2-sensitive glomus cells. In support of this concept, ultrastructural studies have demonstrated the existence of numerous synaptic-like contacts between O2-sensitive glomus cells and type II (stem) cells (Fig. 3a–c). In vitro and in vivo studies have also shown that CB stem cells are induced to proliferate by endothelin 1 (ET-1) released from glomus cells and that type II cells contain ET-1 receptors (Platero-Luengo et al. 2014). Therefore, it seems that the O2-sensitive glomus cells function as presynaptic elements in two types of synapses: (1) “chemosensory synapses”, formed between glomus cells and afferent sensory fibers, involved in acute O2 sensing as well as other sensory functions of the CB and (2) “chemoproliferative synapses” formed between glomus and stem cells, which trigger CB hypertrophy during exposure to hypoxia (Fig. 3d) (Platero-Luengo et al. 2014). In the context of the current discussion, it is relevant to recall that synapses (both chemical and electrical) may also occur between pairs of adjacent glomus cells (see Nurse 2014). Although HIF induction in stem cells is not sufficient to trigger CB growth in hypoxia, the PHD–HIF pathway is necessary for normal CB plasticity. Overexpression of HIF2α induces CB hypertrophy (Macias et al. 2014) and inducible down-regulation of the HIF2α gene reduces CB cell proliferation during sustained hypoxia (Hodson et al. 2016).
Glomus cell–stem cell synapse. a Ultrastructure of a carotid body glomerulus with indication of glomus (type I, red) and type II (stem, blue) cells. Scale bar 5 μm. b High-magnification photograph showing the ultrastructure of a glomus cell (type I, uncolored) surrounded by processes of type II cells (blue) or nearby type I cells (red). Numerous dense-core secretory vesicles in type I cells are located in front of the type II cell membrane. Scale bar 2 μm. c High-magnification photograph of a glomus cell (type I)–stem cell (type II) synapse. Note the dense core vesicles (arrowhead) near the type I (presynaptic) membrane, the synaptic cleft and a bundle of intermediate filaments characteristic of type II cells (asterisk). Scale bar 0.2 μm. d Scheme illustrating the “chemosensory” and “chemoproliferative” CB synapses. See text for details. Modified from Platero-Luengo et al. (2014)
Regulation of neural stem cell activation by the mature cells is a phenomenon not only seen in the CB but it has also been reported to occur in the central neurogenic niches (see Pardal and Lopez-Barneo 2016). In the SVZ, there are astrocyte-like neural stem cells (NSCs) that, upon activation, are converted into rapidly proliferating intermediate progenitors, which in turn give rise to neuroblasts that migrate to the olfactory bulb (Kriegstein and Alvarez-Buylla 2009). NSCs in the SVZ can also generate oligodendrocyte precursors and striatal neurons (Nait-Oumesmar et al. 2007; Kernie and Parent 2010). The basal area of the SVZ is innervated by axonal branches of neighboring dopaminergic fibers, which release transmitters detected as spillover by NSCs (Baker et al. 2004; Hoglinger et al. 2004). Serotonergic fibers originated in the raphe nuclei also innervate the SVZ and form synaptic-like contacts with apical processes of NSCs (Tong et al. 2014). Both of these transmitters, as well as GABA released from neuroblasts, can regulate NSC quiescence. In all mammals studied, including man, there are NSCs in the DG of the hippocampus, which, like their counterpart in the SVZ, can also generate neuroblasts which mature and integrate into the granule cell layer. The innervation by glutamatergic and serotonergic fibers has been reported to modulate NSC maturation or proliferation in hippocampal DG (Deisseroth et al. 2004; Brezun and Daszuta 2000). In addition to long-distance innervation, local release of GABA by hippocampal interneurons has been shown to regulate DG NSCs’ quiescence and neural maturation (Song et al. 2012).
Concluding remarks
The molecular mechanisms underlying the detection of acute changes in O2 tension by CB glomus (chief) cells have remained elusive for decades; however, our understanding of this process has recently advanced significantly thanks to the use of gene profiling techniques and genetically modified animal models. Glomus cells do not appear to posses a specific O2 sensor but their responsiveness to acute hypoxia seems to depend on metabolic and biophysical properties, which result from the regulated expression of a mix of genes encoding mitochondrial subunits, metabolic enzymes and ion channels. On the other hand, the cellular mechanisms responsible for CB hypertrophy in chronic hypoxia, a response associated with adaptation/survival in high altitude and cardiorespiratory pathologies limiting gas exchange in the lungs, have also been identified. The CB contains a population of adult neural crest-derived multipotent stem cells, which are quiescent in normoxic conditions but are activated by hypoxia to produce new glomus cells as well as smooth muscle and endothelial cells. The CB behaves as a germinal niche in the adult peripheral nervous system, which shares many properties with neurogenic centers existing in the mammalian brain. Activation of CB stem cells during hypoxia requires stimulation of the O2-sensitive glomus cells and the release of transmitters, which induce stem cell proliferation and differentiation. Ultrastructural studies indicate that mature glomus cells and stem cells establish numerous chemical synapses (“chemoproliferative synapses”) that work in parallel with those existing between glomus cells and afferent nerve fibers (“chemosensory synapses”) (see Nurse 2014; Platero-Luengo et al. 2014). Understanding CB function in acute and chronic hypoxia has direct medical impact. It may help to combat respiratory depression, a frequent pathology generated during anesthesia or opioid overdose, as well as the CB-mediated exaggerated sympathetic outflow that exists in highly prevalent disorders, such as cardiac failure or sleep apnea.
References
Arias-Stella J, Bustos F (1976) Chronic hypoxia and chemodectomas in bovines at high altitudes. Arch Pathol Lab Med 100(12):636–639
Arias-Stella J, Valcarcel J (1976) Chief cell hyperplasia in the human carotid body at high altitudes; physiologic and pathologic significance. Hum Pathol 7(4):361–373
Annese V, Navarro-Guerrero E, Rodriguez-Prieto I, Pardal R (2017) Physiological plasticity of neural-crest-derived stem cells in the adult mammalian carotid body. Cell Rep 19(3):471–478
Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE (2003) Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet 113(3):228–237
Baker SA, Baker KA, Hagg T (2004) Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci 20:575–579
Baysal BE (2008) Clinical and molecular progress in hereditary paraganglioma. J Med Genet 45:689–694
Brezun JM, Daszuta A (2000) Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci 12:391–396
Buckler KJ, Williams BA, Honore E (2000) An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol 525:135–142
Cerecer-Gil NY, Figuera LE, Llamas FJ, Lara M, Escamilla JG, Ramos R, Estrada G, Hussain AK, Gaal J, Korpershoek E, de Krijger RR, Dinjens WN, Devilee P, Bayley JP (2010) Mutation of SDHB is a cause of hypoxia-related high-altitude paraganglioma. Clin Cancer Res 16(16):4148–4154
Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S (2007) Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol 292:L1257–L1262
Cutz E, Ma TK, Perrin DG, Moore AM, Becker LE (1997) Peripheral chemoreceptors in congenital central hypoventilation syndrome. Am J Respir Crit Care Med 155(1):358–363
d’Anglemont de Tassigny X, Sirerol-Piquer MS, Gómez-Pinedo U, Pardal R, Bonilla S, Capilla-Gonzalez V, López-López I, De la Torre-Laviana FJ, García-Verdugo JM, López-Barneo J (2015) Resistance of subventricular neural stem cells to chronic hypoxemia despite structural disorganization of the germinal center and impairment of neuronal and oligodendrocyte survival. Hypoxia 3:15–33
Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42:535–552
Del Rio R, Andrade DC, Lucero C, Arias P, Iturriaga R (2016) Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension 68:436–445
Diaz-Castro B, Pintado CO, Garcia-Flores P, Lopez-Barneo J, Piruat JI (2012) Differential impairment of catecholaminergic cell maturation and survival by genetic mitochondrial complex II dysfunction. Mol Cell Biol 32(16):3347–3357
Duchen MR, Biscoe TJ (1992) Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol 450:13–31
Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102:4783–4788
Fernandez-Aguera MC, Gao L, Gonzalez-Rodriguez P, Pintado CO, Arias-Mayenco I, Garcia-Flores P, Garcia-Pergañeda A, Pascual A, Ortega-Saenz P, Lopez-Barneo J (2015) Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab 22(5):825–837
Gao L, Bonilla-Henao V, Garcia-Flores P, Arias-Mayenco I, Ortega-Saenz P, Lopez-Barneo J (2017) Gene expression analyses reveal metabolic specifications in acute O2-sensing chemoreceptor cells. J Physiol 595(18):6091–6120
Garcia-Fernandez M, Ortega-Saenz P, Castellano A, Lopez-Barneo J (2007) Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes 56(12):2893–2900
Gauda EB, McLemore GL, Tolosa J, Marston-Nelson J, Kwak D (2004) Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Semin Neonatol 9(3):181–194
Habano W, Sugai T, Nakamura S, Uesugi N, Higuchi T, Terashima M, Horiuchi S (2003) Reduced expression and loss of heterozygosity of the SDHD gene in colorectal and gastric cancer. Oncol Rep 10:1375–1380
Heath D, Smith P, Jago R (1982) Hyperplasia of the carotid body. J Pathol 138:115–127
Hidalgo-Figueroa M, Bonilla S, Gutierrez F, Pascual A, Lopez-Barneo J (2012) GDNF is predominantly expressed in the PV+ neostriatal interneuronal ensemble in normal mouse and after injury of the nigrostriatal pathway. J Neurosci 32:864–872
Hodson EJ, Nicholls LG, Turner PJ, Llyr R, Fielding JW, Douglas G, Ratnayaka I, Robbins PA, Pugh CW, Buckler KJ, Ratcliffe PJ, Bishop T (2016) Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway. J Physiol 594(5):1179–1195
Hoglinger GU, Rizk P, Muriel MP et al (2004) Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735
Inoue M, Fujishiro N, Imanaga I (1998) Hypoxia and cyanide induce depolarization and catecholamine release in dispersed guinea-pig chromaffin cells. J Physiol 507:807–818
Joseph V, Pequignot JM (2009) Breathing at high altitude. Cell Mol Life Sci 66(22):3565–3573
Kameda Y (2005) Mash1 is required for glomus cell formation in the mouse carotid body. Dev Biol 283(1):128–139
Kang D, Wang J, Hogan JO, Vennekens R, Freichel M, White C, Kim D (2014) Increase in cytosolic Ca2+ produced by hypoxia and other depolarizing stimuli activates a non-selective cation channel in chemoreceptor cells of rat carotid body. J Physiol 592:1975–1992
Keating DJ, Rychkov GY, Giacomin P, Roberts ML (2005) Oxygen-sensing pathway for SK channels in the ovine adrenal medulla. Clin Exp Pharmacol Physiol 32(10):882–887
Kernie SG, Parent JM (2010) Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis 37:267–274
Kim D, Cavanaugh EJ, Kim I, Carroll JL (2009) Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol 587:2963–2975
Kliewer KE, Wen DR, Cancilla PA, Cochran AJ (1989) Paragangliomas: assessment of prognosis by histologic, immunohistochemical, and ultrastructural techniques. Hum Pathol 20(1):29–39
Kokovay E, Temple S (2007) Taking neural crest stem cells to new heights. Cell 131:234–236
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184
Le Douarin NM (1986) Cell line segregation during peripheral nervous system ontogeny. Science 231:1515–1522
Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, et al. (2015) Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65:1365–1371
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Lopez-Barneo J, Pardal R, Montoro RJ, Smani T, Garcia-Hirschfeld J, Urena J (1999) K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol 115:215–227
Lopez-Barneo J, Ortega-Saenz P, Gonzalez-Rodriguez P et al (2016a) Oxygen-sensing by arterial chemoreceptors: mechanisms and medical translation. Mol Asp Med 47-48:90–108
Lopez-Barneo J, Gonzalez-Rodriguez P, Gao L, Fernandez-Aguera MC, Pardal R, Ortega-Saenz P (2016b) Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am J Physiol Cell Physiol 310:C629–C642
Macias D, Fernandez-Aguera MC, Bonilla-Henao V, Lopez-Barneo J (2014) Deletion of the von Hippel-Lindau gene causes sympathoadrenal cell death and impairs chemoreceptor-mediated adaptation to hypoxia. EMBO Mol Med 6(12):1577–1592
Maier W, Marangos N, Laszig R (1999) Paraganglioma as a systemic syndrome: pitfalls and strategies. J Laryngol Otol 113:978–982
Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD (2014) Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592:391–408
McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA, Paton JF (2013) The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 4:2395
McGregor KH, Gil J, Lahiri S (1984) A morphometric study of the carotid body in chronically hypoxic rats. J Appl Physiol Respir Environ Exerc Physiol 57(5):1430–1438
Millan-Ucles A, Diaz-Castro B, Garcia-Flores P, Baez A, Perez-Simon JA, Lopez-Barneo J, Piruat JI (2014) A conditional mouse mutant in the tumor suppressor SdhD gene unveils a link between p21(WAF1/Cip1) induction and mitochondrial dysfunction. PLoS ONE 9(1):e85528
Mills E, Jobsis FF (1972) Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol 35(4):405–428
Minguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Mendez-Ferrer S, Martin-Linares JM, Katati MJ, Mir P, Villadiego J, Meersmans M, Perez-Garcia M, Brooks DJ, Arjona V, Lopez-Barneo J (2007) Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry 78(8):825–831
Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7:150–161
Mulligan E, Lahiri S (1982) Separation of carotid body chemoreceptor responses to O2 and CO2 by oligomycin and by antimycin a. Am J Phys 242(3):C200–C206
Nait-Oumesmar B, Picard-Riera N, Kerninon C et al (2007) Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci U S A 104:4694–4699
Navarro-Guerrero E, Platero-Luengo A, Linares-Clemente P, Cases I, Lopez-Barneo J, Pardal R (2016) Gene expression profiling supports the neural crest origin of adult rodent carotid body stem cells and identifies CD10 as a marker for mesectoderm-committed progenitors. Stem Cells 34(6):1637–1650
Nurse CA (2014) Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol 592:3419–3426
Nurse CA (2017) A sensible approach to making sense of oxygen sensing. J Physiol 595(18):6087–6088
Ortega-Saenz P, Pardal R, Garcia-Fernandez M, Lopez-Barneo J (2003) Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol 548:789–800
Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J (2010) Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol 135(4):379–392
Ortega-Saenz P, Pardal R, Levitsky K, Villadiego J, Munoz-Manchado AB, Duran R, Bonilla-Henao V, Arias-Mayenco I, Sobrino V, Ordonez A, Oliver M, Toledo-Aral JJ, Lopez-Barneo J (2013) Cellular properties and chemosensory responses of the human carotid body. J Physiol 591:6157–6173
Ortega-Saenz P, Macias D, Levitsky KL, Rodriguez-Gomez JA, Gonzalez-Rodriguez P, Bonilla-Henao V, Arias-Mayenco I, Lopez-Barneo J (2016) Selective accumulation of biotin in arterial chemoreceptors: requirement for carotid body exocytotic dopamine secretion. J Physiol 594:7229–7248
Paciga M, Vollmer C, Nurse C (1999) Role of ET-1 in hypoxia-induced mitosis of cultured rat carotid body chemoreceptors. Neuroreport 10(18):3739–3744
Pan J, Bishop T, Ratcliffe PJ, Yeger H, Cutz E (2016) Hyperplasia and hypertrophy of pulmonary epithelial bodies, presumed airway hypoxia sensors, in hypoxia-inducible factor prolyl hydroxylase-deficient mice. Hypoxia 4:69–80
Pardal R, Lopez-Barneo J (2016) Mature neurons modulate neurogenesis through chemical signals acting on neural stem cells. Develop Growth Differ 58(5):456–462
Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J (2007) Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131(2):364–377
Peers C (2015) Acute oxygen sensing--inching ever closer to an elusive mechanism. Cell Metab 22:753–754
Perez IA, Keens TG (2013) Peripheral chemoreceptors in congenital central hypoventilation syndrome. Respir Physiol Neurobiol 185(1):186–193
Piruat JI, Pintado CO, Ortega-Saenz P, Roche M, Lopez-Barneo J (2004) The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol Cell Biol 24(24):10933–10940
Platero-Luengo A, Gonzalez-Granero S, Duran R, Diaz-Castro B, Piruat JI, Garcia-Verdugo JM, Pardal R, Lopez-Barneo J (2014) An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell 156(1–2):291–303
Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV (2013) Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62(8):2905–2916
Rubin de Celis MF, Garcia-Martin R, Wittig D, Valencia GD, Enikolopov G, Funk RH, Chavakis T, Bornstein SR, Androutsellis-Theotokis A, Ehrhart-Bornstein M (2015) Multipotent glia-like stem cells mediate stress adaptation. Stem Cells 33(6):2037–2051
Rustin P, Munnich A, Rötig A (2002) Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet 10:289–291
Saldana MJ, Salem LE, Travezan R (1973) High altitude hypoxia and chemodectomas. Human Pathol 4(2):251–263
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7(1):77–85
Sommer N, Hüttermann M, Pak O et al (2017) Mitochondrial complex IV subunit 4 isoform 2 is essential for acute pulmonary oxygen sensing. Circ Res 121(4):424–438
Song J, Zhong C, Bonaguidi MA et al (2012) Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489:150–154
Thompson RJ, Jackson A, Nurse CA (1997) Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol 498:503–510
Thompson RJ, Buttigieg J, Zhang M, Nurse CA (2007) A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience 145(1):130–141
Tong CK, Chen J, Cebrian-Silla A et al (2014) Axonal control of the adult neural stem cell niche. Cell Stem Cell 14:500–511
Waypa GB, Chandel NS, Schumacker PT (2001) Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 88(12):1259–1266
Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL (2005) Acute oxygen-sensing mechanisms. N Engl J Med 353(19):2042–2055
Acknowledgements
I am grateful to Dr. Patricia Ortega-Sáenz for her generous help in the preparation of the figures. I also thank the colleagues who have contributed to the experiments and ideas discussed in this review. The author’s laboratory is supported by grants from the Spanish Ministry of Economy and Innovation (SAF2012-39343, SAF2016-74990-R) and the European Research Council (ERC Advanced Grant PRJ201502629).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Barneo, J. Oxygen sensing and stem cell activation in the hypoxic carotid body. Cell Tissue Res 372, 417–425 (2018). https://doi.org/10.1007/s00441-017-2783-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2783-9