Abstract
The small hive beetle, Aethina tumida, is an emerging pest of social bee colonies. A. tumida shows a specialized life style for which olfaction seems to play a crucial role. To better understand the olfactory system of the beetle, we used immunohistochemistry and 3-D reconstruction to analyze brain structures, especially the paired antennal lobes (AL), which represent the first integration centers for odor information in the insect brain. The basic neuroarchitecture of the A. tumida brain compares well to the typical beetle and insect brain. In comparison to other insects, the AL are relatively large in relationship to other brain areas, suggesting that olfaction is of major importance for the beetle. The AL of both sexes contain about 70 olfactory glomeruli with no obvious size differences of the glomeruli between sexes. Similar to all other insects including beetles, immunostaining with an antiserum against serotonin revealed a large cell that projects from one AL to the contralateral AL to densely innervate all glomeruli. Immunostaining with an antiserum against tachykinin-related peptides (TKRP) revealed hitherto unknown structures in the AL. Small TKRP-immunoreactive spherical substructures are in both sexes evenly distributed within all glomeruli. The source for these immunoreactive islets is very likely a group of about 80 local AL interneurons. We offer two hypotheses on the function of such structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The small hive beetle Aethina tumida (Murray 1867, Coleoptera: Nitidulidae) is a parasite and scavenger of colonies of social bees (honeybees: Apis mellifera: cf. Neumann and Elzen 2004; bumblebees: Bombus impatiens: Spiewok and Neumann 2006; stingless bees: Trigona carbonaria: cf. Greco et al. 2010 and Austroplebeia australis: cf. Halcroft et al. 2011). Both larvae and adults of the A. tumida feed on pollen, honey and bee brood, leading to fermentation of the honey and devastation of the combs, often resulting in the full structural collapse of the entire nest (Lundie 1940; Schmolke 1974; Neumann and Elzen 2004). In its native range in sub-Saharan Africa, A. tumida is a rather harmless parasite, mostly affecting weak and stressed colonies (Lundie 1940; Hepburn and Radloff 1998; Neumann and Elzen 2004; Neumann and Ellis 2008). However, A. tumida has become an invasive species. It was introduced into the USA (1996), Egypt (2000), Australia (2001) and into Europe twice (2004 and 2014; see Neumann and Ellis 2008; Mutinelli et al. 2014) and now has well-established new populations in North America and Australia (Neumann and Elzen 2004; Neumann and Ellis 2008). In these areas, A. tumida can be considered a significant pest of managed honeybees (Neumann and Elzen 2004) and possibly of wild bees (Neumann 2015).
To control this emerging pest, Neumann and Elzen (2004) speculated about the possibility of a A. tumida pheromone that could be used for trapping systems. Indeed, male-produced aggregation pheromones of other species in the family Nitidulidae are known from Carpophilus obsoletus and are used for pest control (Petroski et al. 1994). Today, a great variety of insect pheromones (especially for Lepidoptera and Coleoptera) are known and used for trapping systems (www.pherobase.com). Although pheromone communication has not yet been demonstrated in the small hive beetle, it has been shown that A. tumida is highly attracted to volatiles emitted by adult honey bees (A. mellifera), bumble bees (Bombus impatiens), stored pollen, wax, brood, and honey (Suazo et al. 2003; Graham et al. 2011; de Guzman et al. 2011). Furthermore, A. tumida prefer to fly before or after dusk (Schmolke 1974) suggesting that visual cues are less important than olfactory cues when it comes to locating beehives for mating. Altogether, understanding the olfactory system of A. tumida might be instrumental in controlling this pest.
In insects, olfactory information is detected by olfactory sensory neurons (OSNs) housed in olfactory sensilla on the antenna and the maxillary palps (reviewed in Hansson and Stensmyr 2011). They pass the information on to the neuronal network in the antennal lobes (AL), the first integration centers for odor information in the insect brain. Typically, AL are substructured in spheroidal compartments, the olfactory glomeruli. Typically, OSNs that express the same specific odorant receptor converge onto the same glomerulus (Vosshall et al. 2000). The glomerulus number ranges between about 40 in Diptera up to several hundred in Hymenoptera (Schachtner et al. 2005; Mysore et al. 2009; Kuebler et al. 2010). In various orders of neopteran insects, including Coleoptera, Dictyoptera, Diptera, Hymenoptera and Lepidoptera, sexual dimorphic glomeruli have been described (Kondoh et al. 2003; Kleineidam et al. 2005; Schachtner et al. 2005; Hu et al. 2011). Such glomerular dimorphism may have been evolved independently where it was needed, e.g., for long-distance pheromone detection or for the detection of specific odors like host plant volatiles or trail pheromone (Hansson and Stensmyr 2011). In the AL, olfactory information is processed by local interneurons (LN) and relayed to projection neurons (PN) that connect to other brain areas including the mushroom bodies (MB) or the lateral horn (LH). Additionally, the AL receives innervation from a few unique centrifugal neurons (CN) that provide efferent input from other brain areas (reviewed in Schachtner et al. 2005).
Antennal lobes across insect species contain a wide range of neuromediators including excitatory and inhibitory transmitters like acetylcholine and GABA (e.g., Bicker 1999; Homberg 2002; Schachtner et al. 2005; Berg et al. 2009; Fusca et al. 2015). In addition, AL neurons contain neuromediators like biogenic amines, gaseous signaling molecules like NO and a large variety of neuropeptides, suggesting important involvement for proper olfactory behavior (e.g., Schachtner et al. 2005; Berg et al. 2007; Utz et al. 2008; Carlsson et al. 2010; Binzer et al. 2014; Siju et al. 2014; Fusca et al. 2015). For example, in moths and flies, serotonin (5HT) is able to modulate the sensitivity of odors and sex pheromones (Linn and Roelofs 1986; Gatellier et al. 2004; Hill et al. 2003; Kloppenburg and Hildebrand 1995; Dacks et al. 2009). Another example are the tachykinin-related neuropeptides (TKRP), controlling olfactory sensitivity and locomotor activity in the fruit fly Drosophila melanogaster (Ignell et al. 2009; Winther et al. 2006; Winther and Ignell 2010).
Typically, neuromediators are distributed across all glomeruli of the AL (Schachtner et al. 2005; Carlsson et al. 2010; Binzer et al. 2014; Neupert et al. 2012; Siju et al. 2014). However, there are exceptions in which only one or several glomeruli receive innervations by neurons that express specific neuromediators like serotonin in the ant (Camponotus laevigatus; Dacks et al. 2006), short neuropeptide F (sNPF) in the mosquito (Aedes aegypti; Siju et al. 2014) and in the fly (Drosophila melanogaster; Carlsson et al. 2010), or serotonin and several neuropeptides in collembolans (Kollmann et al. 2011a).
The life cycle of A. tumida involves long-distance dispersal to new food sources (Neumann et al. 2012), preferentially after dusk (cf. Neumann and Elzen 2004). Therefore, olfaction seems to play a pivotal role for the adult beetles (reviewed in Neumann and Elzen 2004). Given that olfaction is that important for the behavior of the animal, the anatomy of the brain, especially of the central olfactory pathway, might very likely reflect this importance. We hypothesize that brain neuropils involved in processing of olfactory information should be enlarged in relationship to other brain areas. We also hypothesize that a sequential invasion of bee colonies, as was postulated by Elzen et al. (2000), with males first and females following could be reflected by specialized glomeruli, e.g., sexual dimorphic glomeruli as described for several other insect species. In addition, we are looking for any specialization in the central olfactory pathway that could reflect the special life style of A. tumida.
Materials and Methods
Experimental animals
Adult Aethina tumida were collected from naturally infested colonies of the African honeybee subspecies Apis mellifera scutellata at the experimental farm of the Department of Zoology and Entomology, University of Pretoria, South Africa. After collection, the beetles were immediately sexed following a routine procedure (Neumann et al. 2013). According to European legislation, import of live A. tumida to Germany is illegal (Commission Decisions EC No 2003/881 and Commission Regulation (EC) N° 1398/2003). Therefore, the beetles were decapitated in Pretoria and the heads were fixed for 12 h at 4 °C in 4 % FA (formaldehyde FA; Roth, Karlsruhe, Germany) in PBS (phosphate-buffered saline, 0.01 M, pH 7.4). They were rinsed for 15 min in PBS and afterwards stored in PBS in glass vials in a customized cooling device and then sent by express delivery to the Philipps University of Marburg (Marburg, Germany).
Immunohistochemistry
Primary antibodies
In the current study, we used antibodies against the synaptic vesicle protein synapsin, the biogenic amine serotonin and an antiserum recognizing tachykinin-related peptides (summarized in Table 1).
The monoclonal antibody from mouse against a fusion protein consisting of a glutathione-S-transferase and the first amino acids of the presynaptic vesicle protein synapsin I coded by its 5′-end (SYNORF1; 3C11, #151101) was used to selectively label neuropilar areas. It was used in combination with one additional primary antibody raised in rabbit. The synapsin antibody was kindly provided by Dr. Erich Buchner (University of Würzburg, Germany). This antibody was first described by Klagges et al. (1996) and has been used in many insect studies to label neuropilar areas (e.g., Utz et al. 2008; Heuer et al. 2012; Binzer et al. 2014). The antibody was used at a dilution of 1:100.
The polyclonal antiserum against serotonin (5HT) was raised in rabbit against paraformaldehyde-coupled conjugates of BSA (bovine serum albumin) and 5HT (DiaSorin, Dietzenbach, Germany). Its specificity for the insect nervous system has been shown in several studies (e.g., Dacks et al. 2006). It was used at a dilution of 1:5000.
The polyclonal TKRP antiserum was kindly provided by Dr. H. Agricola (University of Jena, Germany). It was raised in rabbits against locustatachykinin-2 (Lom-TK II, APLSGFYGVRamide) glutaraldehyde-conjugated to bovine thyroglobulin (Veenstra et al. 1995). The antiserum is also known to detect tachykinin-related peptides (TKRPs; consensus sequence FXGXRamide) in other insects (e.g., Vitzthum and Homberg 1998; Heuer et al. 2012; Binzer et al. 2014). In beetles, specificity for the anti-TKRP antiserum has so far been confirmed in Tribolium castaneum by preabsorption of the antiserum with synthetic Lom-TK II (Binzer et al. 2014). In the current study, we used the anti-TKRP antiserum to reveal morphological structures of the brain of A. tumida. It was used at a dilution of 1:2000.
Secondary antibodies
Goat anti-mouse antibodies conjugated to Cy5 (GAM-Cy5) and goat anti-rabbit antibodies conjugated to Cy3 (GAR-Cy3) were used as secondary antibodies (each 1:300; Jackson ImmunoResearch, Westgrove, PA, USA).
Whole mount double immunostainings
Brains of A. tumida were dissected out of the head capsule, fixed overnight at 4 °C in 4 % FA in PBS, followed by rinsing (4 × 10 min) with PBS at RT (room temperature). Afterwards, brains were preincubated for 2 days in PBT (PBS containing 0.3 % Triton-X 100; Sigma Aldrich, Steinheim, Germany) with 5 % NGS (normal goat serum; Jackson ImmunoResearch). The primary antibody anti-synapsin (1:100) was used in combination with the anti Lom-TK II (1:5000) or anti 5HT antiserum (1:2000) diluted in PBT with 1 % NGS. Brains were incubated for 2 days at 4 °C. After rinsing (4 × 10 min) with PBT at RT, brains were incubated in secondary antibodies (GAM-Cy5 and GAR-Cy3; 1:300) in PBT with 1 % NGS at 4 °C for 2 days in the dark. After rinsing (6 × 10 min) with PBT at RT and washing in distilled H2O for 10 min, brains were dehydrated in an ascending alcohol series (30, 50, 70, 90, 95 %, 2 × 100 % ethanol, 5 min each). Followed by clearing the tissue in methyl salicylate (10 min; Merck, Darmstadt, Germany) the brains were finally mounted in resin (Permount; Fisher Scientific, Pittsburgh, PA, USA). During the immunostaining procedure, all washing and incubation steps were performed on a laboratory orbital shaker (MS 3 digital; IKA, Staufen, Gemany).
Data processing
Fluorescence was analyzed with a confocal laser scanning microscope (Leica TCS SP5 Microsystems; Leica, Wetzlar, Germany), with the object lenses ×20 oil objective (HCX PL APO lambda blue ×20/ NA = 0.70 Imm UV, working distance: 260 μm; Leica), ×40 oil objective (HCX PL APO lambda blue ×40/ NA = 1.25 Oil UV, working distance: 100 μm; Leica), and ×63 glycerol objective (HCX PL APO ×63/ NA = 1.30 Glyc 21 °C CS working distance: 260 μm; Leica). We scanned with a resolution of 1024 × 1024 or 512 × 512 pixels, a line average of 2, speed of 200 Hz, a digital zoom of 1–2, and z-steps varying from 0.5 to 1.0 μm for detailed scans and from 3.0 to 5.0 μm for overview scans.
Image segmentation, reconstruction and visualization
Brain structures were 3-D reconstructed using AMIRA 5.2 (Visage Imaging, Berlin, Germany). Segmentation and reconstruction were performed according to Kurylas et al. (2008) and El Jundi et al. (2009). In short, data of the CLSM image stacks were opened in the segmentation editor of AMIRA. From all three spatial directions (lateral to lateral, anterior to posterior and dorsal to ventral) of the respective structure, 3–12 layers (depending on the size of the structure) were labeled and finally wrapped to obtain a voxel-based 3-D model. By using the tool “SurfaceGen”, we transferred the voxel-based 3-D model into a polygonal surface model. Standard color codes were used for the reconstructed neuropils (Brandt et al. 2005). For further global processing (i.e., contrast and brightness optimization) and final figure arrangements, snapshots were taken in AMIRA and subsequently processed in Corel Draw 13 (Corel, Ottawa, ON, Canada). Diagrams generated with Excel XP (Microsoft, Redmond, WA, USA) were imported and revised in Corel Draw 13 without any further modification. For statistical analyses, we used a two-tailed t test in Origin 6.0 (OriginLab, Northampton, MA, USA) and Excel XP.
Results
General organization of the brain
A 3-D reconstruction of the brain of Aethina tumida was created based on confocal sections of an adult female stained with anti-synapsin antibody (Fig. 1) (movie of a rotating 3-D reconstruction and a camera path through the synapsin staining of a brain can be found in the digital supplements, ESM 1 and 2; 3-D reconstructions of single male and female AL can be found in ESM 3). The brain contains all typical neuropils known from most insects including neuropils of the optic lobes, the antennal lobes, mushroom body and neuropils of the central complex (e.g., Drosophila melanogaster, Rein et al. 2002; honeybee Apis mellifera, Brandt et al. 2005; desert locust Schistocerca gregaria, Kurylas et al. 2008; sphinx moth Manduca sexta, el Jundi et al. 2009; red flour beetle Tribolium castaneum, Dreyer et al. 2010). We reconstructed all neuropils that were clearly identifiable and separable (8 paired and 3 unpaired neuropils).
3-D reconstruction of the female brain of Aethina tumida in a anterior, b dorsal, and c posterior view. The neuropils were reconstructed with the AMIRA tools SurfaceGen and SurfaceView. The color code of the labeled neuropils is consistent with Brandt et al. (2005). AL antennal lobe, Ca Calyx, CBL lower unit of the central body, CBU upper unit of the central body, aMe accessory medulla, Lo lobula, LoP lobula plate, Me medulla, No noduli, PB protocerebral bridge and Pe pedunculus with lobes. Orientation bars: a = anterior, d = dorsal, m = median, p = posterior, v = ventral. Scale bar 100 μm
In the optical lobes, we reconstructed the paired medulla (Me), lobula (Lo), lobula plate (LoP) and accessory medulla (aMe). The central complex is located in the center of the protocerebrum. Its reconstruction includes the unpaired upper and lower unit of the central body (CBU, CBL), the paired Noduli (No) and the dorso-posteriorly located unpaired protocerebral bridge (PB).
The paired mushrooms bodies are placed lateral to both sides of the central complex. We reconstructed calyx (Ca) and pedunculus (Pe) separately. The Pe contains the vertical lobe (vL) and medial lobe (mL). The Pe and the lobes are separated into an inner core region, which is densely stained with synapsin antibody (Fig. 1; non-transparent-shaped part of the MB) and a less densely anti-synapsin stained exterior region (Fig. 1; transparent-shaped part of the MB).
Organization of the antennal lobe
In total, we analyzed 12 ALs of 7 males and 9 ALs of 5 females. Characteristically for insects (Schachtner et al. 2005), in A. tumida the AL are organized in small, spherical substructures, the olfactory glomeruli, which are arranged around a central coarse neuropil. The small hive beetle possesses 72.0 ± 3.9 glomeruli per AL in males (n = 12 ALs) and 71.1 ± 3.4 glomeruli per AL in females (n = 9 ALs) (p = 0.588).
The average size of one glomerulus is 10.7 ± 1.8 μm3 in males (n = 12 ALs / n = 864 glomeruli) and 10.1 ± 1.8 μm3 in females (n = 9 ALs / n = 640 glomeruli). For approximation of the AL size, the volumes of all glomeruli within the AL were summed; extraglomerular space and central coarse neuropil were not included. Mean volume of male AL is 730.0 ± 120.5 μm3 (n = 12 ALs), compared to 719.2 ± 114.9 μm3 in females (n = 9 ALs). Taken together, male and female ALs of A. tumida were statistically indifferent in regard of glomeruli number (p = 0.588), overall glomeruli size (p = 0.875), or on the level of the AL volume (p = 0.997).
It is known from several insect species that conspicuously larger glomeruli appear in one of the sexes, typically at the entrance site of the antennal nerve (Schachtner et al. 2005; Hu et al. 2011). To verify whether this might also be true for the small hive beetle, we grouped the different-sized glomeruli of males (n = 12 ALs; n = 864 glomeruli) and females (n = 9 ALs; n = 640 glomeruli) according to their volume (0–999 μm3 = group 0; 1000–1999 μm3 = group 1; …35,000–35,999 μm3 = group 35), followed by analyzing the relative abundance of these different size groups (Fig. 2) (data of glomeruli from all ALs of one sex were pooled). No conspicuous sexual dimorphism could be seen. Middle-sized glomeruli with a volume between 4000 μm3 and 11,999 μm3 seemed to be the most common ones, while small glomeruli (between 1000 and 2999 μm3) and large glomeruli (between 17,000 and 20,999 μm3) were less abundant. In addition, a varying number of a few larger glomeruli (between 21,000 and 35,999 μm3) occur in both sexes. Analysis of the position of the three largest glomeruli per AL revealed that they are not clustered and that they distribute randomly within the anterior half of the AL, with no particular correlation to the site where the antennal nerve enters the AL.
Relative abundance of different sized glomeruli of both sexes of Aethina tumida. The x-axis represents the different glomerular volumes, shown as volume groups (0–999 μm3 = group 0; 1000–1999 μm3 = group 1; …35,000–35,999 μm3 = group 35). The y-axis represents the relative abundance in percent of the different size groups
In summary, we found no evidence for any sexual dimorphism in the AL on the level of AL size, glomerulus number and individual or overall glomerulus size.
Tachykinin-related peptides in the antennal lobes
Immunostaining with the anti TKRP antiserum in A. tumida revealed small, spherical substructures within all glomeruli (Fig. 3; see also ESM 4). In the anti-synapsin staining, these internal substructures are only slightly more strongly labeled in comparison to the surrounding neuropil of the glomerulus (Fig. 5b, c). Each AL contains about 230 of these substructures (males: 245.7 ± 14.3, n = 3 ALs; females: 224.6 ± 19.1, n = 5 ALs). We did not find a sexual dimorphism (p = 0.154). All observed glomeruli contain between one and ten substructures, which are never attached to the outer rim of a glomerulus but usually distributed evenly across the glomerular volume (Fig. 3; ESM 4). In both sexes, the number of substructures in a glomerulus correlates with the volume of the glomerulus (males: n = 3 ALs, n = 224 glomeruli, R 2 = 0.99; females: n = 5 ALs, n = 350 glomeruli, R 2 = 0.95 (Fig. 4).
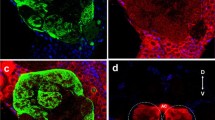
The antennal lobe (AL) of Aethina tumida stained with an antibody against synapsin (green) and Lom TKII (magenta). a Single optical section of an AL. Several individual glomeruli (G1–G6, dotted lines), containing TKRP-ir glomerular substructures. b, c 3-D reconstructions of glomerular substructures (b) and of the glomeruli (c) of the set of glomeruli outlined in (a). d–f Staining with antibodies against synapsin (e) and TKRP (f) and overlay of both (d; synapsin in green, TKRP in magenta) showing TKRP-ir local AL interneurons (arrowheads) and their axons (arrows) projecting into the core area of the AL (asterisk), from where they give rise to TKRP-ir substructures. Boxed areas in (d) and (f) are enhanced in brightness and contrast to better visualize fibers of the local AL interneurons. Orientation bars in (a) valid for all subfigures: l = lateral, d = dorsal. Scale bars 50 μm
Abundance of glomerular substructures (x-axis) in relation to glomerulus size (y-axis). Numbers at the base of the bars represent sample number (number of glomeruli). The diagram shows a linear relationship between glomerulus size and number of glomerular substructures, with a coefficient of determination of 0.95 for male and 0.99 for female animals
The staining of TKRP-ir glomerular substructures most likely originates from a set of about 80 ± 18 (n = 6 ALs) neurons located laterally in the AL (Fig. 3d–f, arrowheads), presumably exclusively local interneurons, which project their processes into the AL (Fig. 3d–f, arrows; see also electronic supplementary material, ESM 5). As the antennal nerve shows no immunoreactivity to the TKRP antibody, we exclude that the staining of TKRP-ir glomerular substructures originates from OSNs. We also did not find any TKRP-ir fibers leaving the AL or entering the AL from other brain regions, excluding that the TKRP-ir glomerular substructures originate from projection neurons (PN) or centrifugal neurons (CN).
Serotonin in the antennal lobes
All glomeruli of one AL are innervated by axons branching from one, brightly stained main fiber, entering the AL at its medio-ventral side (Fig. 5a, unfilled arrowheads). The origin of this 5HT-ir main fiber is most likely a single cell body, dorso-lateral to the AL at the contralateral side (as demonstrated in Fig. 5a; arrow). The primary neurite runs through the AL without obvious branching and exits the AL at its dorsal side (Fig. 5a, filled arrowheads). From here, the fiber runs dorsally and crosses to the contralateral hemisphere of the superior protocerebrum where a divergent branch forms putative dendritic arborizations. The main fiber continues ventrally, to enter the contralateral AL (Fig. 5a, unfilled arrowheads). The TKRP-ir glomerular substructures are not innervated by the 5HT-ir branches although some are touched just at their surface (Fig. 5b–d).
Antennal lobe (AL) of Aethina tumida stained with anti-synapsin (green) and anti-serotonin (magenta) antibodies. a The maximum projection shows the branching of a single serotonin immunoreactive (5HT-ir) fiber, entering the AL at the dorsal site (unfilled arrowheads). Dorso-lateral to the AL, a single 5HT-ir cell body can be observed (arrow) projecting dorsal (filled arrowheads) without any branching or varicosities out of the AL. b–d Single optical section of an AL. The glomerular substructures in the AL glomeruli are distinctly brighter stained with the synapsin antibody than the surrounding area of the glomeruli (b, c dotted lines). 5HT-ir fibers clearly stay outside the glomerular substructures (d). Orientation bars: m = median, d = dorsal. Scale bars 20 μm
Discussion
General organization of the A. tumida brain
The overall anatomy of the brain of A. tumida (Fig. 1) compares well to other beetle brains regarding major neuropils including the optical lobes (OL), antennal lobes (AL), mushroom bodies (MB) and central body complex (CBX) (e.g., Van Haeften 1993; Breidbach and Wegerhoff 1994; Larsson et al. 2004; Dreyer et al. 2010; Hu et al. 2011).
The OL of A. tumida contain the paired medulla (Me), lobula (Lo), lobula plate (LoP) and accessory medulla (aMe). The LoP has so far only been found in Ephemeroptera, Trichoptera, Coleoptera, Lepidoptera, Diptera (Strausfeld 2005) and Heteroptera (Settembrini and Villar 2005). The AL consists of about 70 glomeruli, which seems to be a typical number for beetles; there are about 70 glomeruli in the red flour beetle Tribolium castaneum (Dreyer et al. 2010), about 60 glomeruli in the scarab beetle Holotrichia diomphalia (Hu et al. 2011), and about 70 glomeruli in the cockchafer Melolontha hippocastani (third instar; Weissteiner et al. 2012). The AL will be discussed in more detail below. The paired MB contains the calyx (Ca) and the pedunculus (Pe), which is divided in the vertical and medial lobe (vL and mL). The Pe, vL and mL can be separated in a densely synapsin-stained core region and a less densely stained exterior region. This separation is in accordance with observations in the red flour beetle (Zhao et al. 2008; Binzer et al. 2014) and the African scarabid beetle Pachnoda marginata (Larsson et al. 2004). The medial part of the right mL is overlapping the medial part of the left mL, as has been observed in other beetles like T. castaneum (Dreyer et al. 2010) or the blind cave beetle Neaphaenops tellkampfii (Ghaffar et al. 1984). The unpaired central complex (CBX) can be separated into the protocerebral bridge (PB) and the central body (CB), which consists in the upper and lower unit (CBU and CBL), as well as the paired noduli (NO). This organization of the CBX is paralleled in other beetles like T. castaneum (Dreyer et al. 2010) or the mealworm beetle Tenebrio molitor (Breidbach and Wegerhoff 1994), as well as in many other insects (Homberg 2008).
Comparison with relative brain neuropil volumes of other insects reveals that the AL of A. tumida are comparably large, only surpassed by T. castaneum and the Madeira cockroach Rhyparobia maderae (Table 2). The AL of A. tumida take up about a fifth of the compared relative neuropil volumes, resembling the ratio found in T. castaneum, while the relative AL volume of R. maderae is even larger. T. castaneum is considered as an insect relying dominantly on olfactory cues (Dreyer et al. 2010), as are cockroaches (Periplaneta americana, Sakura and Mizunami 2001). In summary, this result supports the hypothesis that, for A. tumida, similar as stated for T. castaneum, olfactory cues are of major importance for their specialized behavior.
The relative volumes of the MB are remarkably smaller in A. tumida (11.5 %) compared to T. castaneum but still larger than in the majority of compared insects (Table 2). MBs are higher integrative centers of the insect brain that are best known for their involvement in olfactory learning (e.g., McGuire et al. 2001; Menzel 2001; Heisenberg 2003; Davis 2004). However, insect MB are not solely higher centers of the olfactory pathway but are involved in the integration and processing of a broad range of sensory modalities including processing of visual, gustatory and mechanosensory information as well as contributions to sleep regulation, place memory and temperature preference (reviewed in Heuer et al. 2012). Farris and Roberts (2005) demonstrated that generalist plant-feeding scarab beetles (Scarabaeidae) have larger MB, while specialist dung-feeding scarab beetles have smaller MB. Interestingly, this difference in MB volume is independent of size and glomerulus number of the AL, the primary input olfactory neuropil of the MB. This observation may offer a possible explanation for the difference in MB volume between feeding specialist A. tumida and feeding generalist T. castaneum, which evolved as saprophytic insects and naturally occur under the bark of trees, in rotten wood and infrequently in the nests of some Hymenoptera (Sokoloff 1977; Grimm 2001; Arnaud et al. 2005).
In A. tumida, the OLs are with about two-thirds of the relative neuropil volume, larger than the OLs of Tribolium. In insects, larger OLs typically correlate with larger complex eyes (e.g., Ghaffar et al. 1984; Gronenberg and Liebig 1999; Ehmer and Gronenberg 2004; Beutel et al. 2005; Kuebler et al. 2010). Tribolium has relative small compound eyes (80–83 ommatidia per eye; Friedrich et al. 1996) compared to other insect species including A. tumida.
The role of the central body is probably best described as a central coordinator in sensory and motor integration (for reviews, see Strauss 2002; Wessnitzer and Webb 2006; Homberg 2008). With 4.8 %, the relative volume of the central body of A. tumida is smaller than the same structure in Tribolium and Heliothis virescens but still larger than in the fly, honey bee, locust, cockroach or two Lepidoptera species. This suggests for the two beetles a more prominent function of the central complex than in most other insects. In this context, it would be interesting to have more comparable central complex volumes of other Coleoptera with different lifestyles, e.g., water beetles or non-flying beetles.
Olfactory driven behavior and sexual dimorphism
Males of the related beetle Carpophilus obsoletus release an aggregation pheromone that attracts both sexes (Petroski et al. 1994), leading to the hypothesis that a similar pheromone could guide A. tumida into host beehives that have already been parasitized (Elzen et al. 2000; Neumann and Elzen 2004). However, a sequential arrival of male and female A. tumida could not be observed (Spiewok and Neumann 2012). This does not rule out sex-specific differences in olfaction; females seem to be more responsive to beehive volatiles than males (Suazo et al. 2003) and this might be reflected in a sexual dimorphism of the olfactory system of A. tumida.
Sexual dimorphism has been described in various insect species on different levels of the olfactory pathway ranging from the periphery to the central nervous system including the antenna (e.g., beetles: Kaissling 1971; Âgren 1985; Allsopp 1990; Renou et al. 1998; Diptera: Clements 1999; Ruther et al. 2000; Stocker 2001; Hymenoptera: Streinzer et al. 2013; moths: Schneider 1992; Rospars and Hildebrand 2000; Huetteroth and Schachtner 2005), the specificity, number and/or distribution of olfactory receptors (e.g., moths: Miura et al. 2009; Nakagawa et al. 2005; or Diptera: Bohbot et al. 2007), the morphology and number of glomeruli in the AL (Kondoh et al. 2003; Schachtner et al. 2005; Hu et al. 2011; Kelber et al. 2010; Streinzer et al. 2013) and higher order brain structures (e.g., Drosophila: Cachero et al. 2010). Detailed analyses of the antennae of A. tumida are missing. So far, there is no evidence that demonstrates a sexual dimorphism at the level of the antenna of the small hive beetle and information on the distribution of olfactory receptors for beetles is rare. For T. castaneum, transcription analyses of female and male antenna show no sexual dimorphism in the expression of odorant receptors (Dippel et al., in preparation); data for the small hive beetle are so far lacking.
Differently sized sexual dimorphic glomeruli have been observed in a wide range of insects, including beetles, cockroaches, bees, ants, moths, flies, and mosquitoes (Schachtner et al. 2005; Vosshall and Stocker 2007 ; Hu et al. 2011). Typically, these are one up to five glomeruli of a so-called “macroglomerular complex” in males to detect sex pheromones or in ants to detect trail pheromones, or “female sex-specific glomeruli” to detect host plants for oviposition. Such glomeruli are normally positioned at the entrance area of the antennal nerve into the AL (Hansson 1997; Anton and Homberg 1999; Rospars and Hildebrand 2000; Schachtner et al. 2005; Kleineidam et al. 2005). Hu et al. (2011) demonstrated such a sexual dimorphism for the first time in a beetle, identifying a single macroglomerulus in the AL of the Korean black chafer (Holotrichia diomphalia). The current study addresses the AL morphology of A. tumida in detail but neither male macroglomeruli nor female sex-specific glomeruli could be found.
A difference in number of glomeruli between sexes is common among insects. Yellow fever mosquito females have one additional glomerulus (Ignell et al. 2005) and Pieris brassicae female butterflies have three glomeruli more than their male counterparts (Rospars 1983). In hymenopterans, female honeybee workers possess about 160 glomeruli compared to about 106 glomeruli in males (Arnold et al. 1985; Flanagan and Mercer 1989; Brockmann and Brückner 2001). In beetles, varying glomerulus numbers between sexes have so far not been described. In A. tumida, we found with about 70 glomeruli in both sexes no sexual dimorphism. In summary, the absence of a sexual dimorphism in the AL of A. tumida does not favor the work of Spiewok and Neumann (2012) or of Neumann and Elzen (2004) and can on this level of analysis not add to a better understanding of why female A. tumida respond more strongly to beehive volatiles than males (Suazo et al. 2003). A detailed analysis of the A. tumida antenna including distribution of olfactory sensillae and the identification and distribution of olfactory receptors would be necessary. Such insights could be helpful to create/optimize olfactory beetle traps. In addition, analysis of higher brain centers in the olfactory pathway including mushroom body and lateral horn might provide additional insights.
Serotonin-ir neuron in the AL
The observed innervation of all glomeruli of one AL by only one 5HT-ir cell body is common among insects (Dacks et al. 2006), as well as for ancestral hexapods, as observed in collembolans (Kollmann et al. 2011a). The described anatomy of the 5HT-ir neuron has been described for Coleoptera before and can also be found in Lepidoptera, Trichoptera, non-Schizophoran Diptera and Neuroptera (Dacks et al. 2006), and is very likely also true for aphids (Kollmann et al. 2011b). A side branch of the 5HT-ir neuron into the ipsilateral lateral protocerebrum as observed in many insects (including Coleoptera; Dacks et al. 2006) could not be found in A. tumida.
Glomerular substructures
Immunostaining with an antiserum recognizing tachykinin-related peptides (TKRPs) resulted in the discovery of small, spherical substructures, which are evenly distributed among all glomeruli, with no obvious difference between both sexes. These substructures seem to originate from a cluster of cell bodies, presumably local interneurons, lateral in the AL. Immunostaining with the same antiserum in two other beetles, Tenebrio molitor (Wegerhoff et al. 1996) and T. castaneum (Binzer et al. 2014), resulted in a cluster of local interneurons located in a similar position lateral in the AL. They provide the glomeruli with a dense meshwork of projections but did not give rise to spheroidal structures as described here. Similar to A. tumida, the antiserum did not reveal fibers in the antennal nerve or fibers belonging to either projection or centrifugal neurons (Wegerhoff et al. 1996; Binzer et al. 2014). Similarly, in all other insects where TKRP stainings were performed, the antisera labeled only local interneurons in the AL and no other neuron types (Schachtner et al. 2005; Carlsson et al. 2010; Neupert et al. 2012; Binzer et al. 2014; Siju et al. 2014). In summary and in contrast to all other insects examined before, the TKRP positive local neurons provide each glomerulus with particular islet-like projections.
In ALs of insects, all or only a subpopulation of glomeruli can be the target of individual neurons or of populations of neurons and a variety of innervation patterns of olfactory glomeruli by AL neurons (LNs, PNs) or CNs has so far been described either by filling of single neurons or by immunostaining (summarized in Fig. 6; reviewed in Schachtner et al. 2005; Seki and Kanzaki 2008; Husch et al. 2009; Carlsson et al. 2010; Chou et al. 2010; Seki et al. 2010; Neupert et al. 2012; Binzer et al. 2014; Siju et al. 2014). Comparing the different patterns of glomerulus supply via central neurons (CNs, LNs, PNs), innervation can occur (a) only at the surface, (b) scattered throughout the whole glomerulus, (c) or only through parts of the glomerulus, (d) up to massive dense innervation of the whole, or (e) a distinct area of a glomerulus, or (f) as described in this study, through islet-like projections, which are evenly distributed throughout the glomerulus (Fig. 6). From our analysis, we cannot distinguish, whether an individual islet structure is supplied via a single axon or by more axons and whether islets of a single glomerulus are innervated by several or only by one neuron as suggested in Fig. 6f.
Schematic drawing of the principal innervation pattern of glomeruli. a Glomeruli are just sparsely innervated at the surface. b The innervation is scattered through the whole glomerulus. c The innervation is just scattered through a distinct area of the glomerulus. d The whole glomerulus is densely innervated by fine branches, which appears in immunohistological stainings as a bright, uniform staining. e A distinct area of the glomerulus is densely innervated by fine branches. f Branching appears just in several, small, distinct areas of the glomerulus (glomerular substructures), which are evenly distributed throughout the glomerulus
The role of LNs in the AL network is to shape the olfactory representation within and between the olfactory glomeruli to eventually form the output profile of the PNs via complex inhibitory and excitatory interactions (Stopfer et al. 1997; Sachse and Galizia 2002; Wilson and Laurent 2005; Olsen et al. 2007, 2010; Root et al. 2007; Shang et al. 2007; Silbering and Galizia 2007; Olsen and Wilson 2008; Okada et al. 2009; Tanaka et al. 2009; Chou et al. 2010; Seki et al. 2010; Wilson 2013; Nagel et al. 2015). It is interesting that the small hive beetle developed, at least for a subpopulation of LNs, a morphologically different pattern of glomerulus innervation compared to other insects. With the available data, we can only speculate on the function of the islet-like innervation.
We offer two hypotheses. Our first hypothesis argues that the islet-like innervation is another effective way to provide the glomerular network with information carried by neuromediators, e.g., neuropeptides. Such neuromediators can act as paracrine neurohormones, affecting a broad area surrounding the release site, also known as “volume transmission”, in contrast to “wiring” (Agnati et al. 1995; Nässel 2002). Considering the facts that the islet number is linearly correlated to glomerulus volume (Fig. 4) and that they are evenly distributed in each glomerulus, the islet contents could affect the glomerulus network over prolonged periods of time or in an otherwise temporally unique fashion. Our second hypothesis interprets the islets as a specific adaptation of the beetles to their lifestyle to cope with the complex chemical communication in a beehive with an olfactory system of a beetle. The TKRP immunostaining unmasks a group of LNs, which are part of such a particular network. It is known that chemical communication mainly based on pheromones is very important for bees (Slessor et al. 2005; Trhlin and Rajchard 2011). To manage this olfactory task, worker bees have about 64,000 OSNs (Esslen and Kaissling 1976), a large number of glomeruli (152–166 per AL in workers; Arnold et al. 1985) and about 170 ORs (Robertson and Wanner 2006). For a parasitic insect living in a beehive survival and breeding success may highly depend on the ability to understand at least parts of the chemical communication of its host. As the repertoire of the beetle is restricted to about 70 glomeruli per AL, the islets could be part of a system that allows the beetle to compensate for this disadvantage by expanding the glomerular coding space. The genome sequence of another beetle, T. castaneum, revealed a much higher number of functional ORs than olfactory glomeruli and it is still enigmatic what role these surplus ORs could play. The islets could be innervated by OSNs carrying different ORs than the OSNs that principally innervate the glomerulus. Following this line, the islets would functionally stand as specific “glomeruli” within the ordinary glomeruli and thus exaggerate the potential of the olfactory system of the beetle to cope with a more complex odor environment. The synapsin immunostaining is slightly stronger than in the surrounding parts of the glomeruli, suggesting a higher synaptic density within the islets. This argues for specialized zones with high synaptic communication between the involved neurons. If these islet-like zones are targeted by specialized OSNs and/or LNs, PNs remain to be shown in the future by backfills from the antenna and a thorough analysis of intrinsic end extrinsic antennal lobe neurons.
Summary
Analyzing the brain of A. tumida by means of immunohistochemistry and 3-D reconstruction revealed a basic brain neuroarchitecture comparable to other beetle and insect brains. In relationship to other brain areas and in comparison to other insects, the AL are relatively large, suggesting that olfaction is of major importance for the beetle. The AL of both sexes house about 70 glomeruli with no obvious size differences of the glomeruli between males and females. In accordance to what is typically found in other insects, staining with a 5HT antiserum revealed a large cell that projects from one AL to the contralateral AL to densely innervate all glomeruli. Immunostaining with an antiserum recognizing TKRPs revealed small spherical substructures, which are in both sexes evenly distributed among all olfactory glomeruli. The source for the TKRP-ir structures is very likely a group of about 80 local AL interneurons. The number of substructures ranges between one and ten and correlates linearly with the volume of the glomeruli. In total, one AL contains about 230 of these islets. For this unusual finding, we offer two hypotheses. First, these evenly distributed substructures could act as massive releasing sites to deliver the neuromediator throughout the particular glomerulus. Second, the islets act as specialized subcompartments that expand the functional coding space of the beetle’s olfactory system.
References
Agnati Z, Zoli M, Strömberg I, Fuxe K (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69:711–726
Âgren L (1985) Architecture of a lamellicorn flagellum (Phyllopertha horticola, Scarabeidae, Coleoptera, Insecta). J Morphol 186:85–94
Allsopp PG (1990) Sexual dimorphism in the adult antennae of Antitrogus parvulus Britton and Lepidiota negatoria Blackburn (Coleoptera: Scarabaeidae: Melolonthinae). J Aust Entomol Soc 29:261–266
Anton S, Homberg U (1999) Antennal lobe structure. In: Hansson BS (ed) Insect olfaction. Springer, Berlin, pp 97–124
Arnaud L, Brostaux Y, Lallemand S, Haubruge E (2005) Reproductive strategies of Tribolium flour beetles. J Insect Sci 5:33
Arnold G, Masson C, Budharugsa S (1985) Comparative study of the antennal lobes and their afferent pathway in the worker bee and the drone Apis mellifera. Cell Tissue Res 242:593–605
Berg BG, Schachtner J, Utz S, Homberg U (2007) Distribution of neuropeptides in the primary olfactory centre of the heliothine moth Heliothis virescens. Cell Tissue Res 327:385–398
Berg BG, Schachtner J, Homberg U (2009) Distribution of GABA and neuropeptides in the antennal lobe of the heliothine moth Heliothis virescens. Cell Tissue Res 335:593–605
Beutel RG, Pohl H, Hünefeld F (2005) Strepsipteran brains and effects of miniaturization (Insecta). Arthropod Struct Dev 34:301–313
Bicker G (1999) Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microsc Res Tech 45:174–183
Binzer M, Heuer CM, Kollmann M et al (2014) Neuropeptidome of Tribolium castaneum antennal lobes and mushroom bodies. J Comp Neurol 522:337–357
Bohbot J, Pitts RJ, Kwon HW, Rützler M, Robertson HM, Zwiebel LJ (2007) Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol 16:525–537
Brandt R, Rohlfing T, Rybak J, Krofczik S, Maye A, Westerhoff M, Hege HC, Menzel R (2005) Threedimensional average-shape atlas of the honeybee brain and its applications. J Comp Neurol 492:1–19
Breidbach O, Wegerhoff R (1994) FMRFamide-like immunoreactive neurons in the brain of the beetle, Tenebrio molitor L. (coleoptera – tenebrionidae): constancies and variations in development from the embryo to the adult. Int J Insect Morphol Embryol 4:383–404
Brockmann A, Brückner D (2001) Structural differences in the drone olfactory system of two phylogenetically distant Apis species, A. florea and A. mellifera. Naturwissenschaften 88:78–81
Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE (2010) Sexual dimorphism in the fly brain. Curr Biol 20:1589–1601
Carlsson MA, Diesner M, Schachtner J, Nässel D (2010) Multiple neuropeptides in the Drosophila antennal lobe suggest complex modulatory circuits. J Comp Neurol 518:3359–3380
Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L (2010) Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci 13:439–449
Clements AN (1999) The biology of mosquitoes: sensory reception and behaviour. CABI, Wallingford
Dacks AM, Christensen TA, Hildebrand JG (2006) Phylogeny of a serotonin-immunoreactive neuron in the primary olfactory center of the insect brain. J Comp Neurol 498:727–746
Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW (2009) Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J Neurogenet 23:366–377
Davis RL (2004) Olfactory learning. Neuron 44:31–48
De Guzman LI, Frake AM, Rinderer TE, Arbogast RT (2011) Effect of height and color on the efficiency of pole traps for Aethina tumida (Coleoptera: Nitidulidae). J Econ Entomol 104:26–31
Dreyer D, Vitt H, Dippel S, Goetz B, el Jundi B, Kollmann M, Huetteroth W, Schachtner J (2010) 3D standard brain of the red flour beetle Tribolium castaneum: a tool to study metamorphic development and adult plasticity. Front Syst Neurosci 4:3
Ehmer B, Gronenberg W (2004) Mushroom body volumes and visual interneurons in ants: comparison between sexes and castes. J Comp Neurol 469:198–213
el Jundi B, Huetteroth W, Kurylas AE, Schachtner J (2009) Anisometric brain dimorphism revisited: implementation of a volumetric 3D standard brain in Manduca sexta. J Comp Neurol 517:210–225
Elzen PJ, Baxter JR, Westervelt D, Randall C, Wilson WT (2000) A scientific note on observations of the small hive beetle, Aethina tumida Murray (Coleoptera Nitidulidae) in Florida, USA. Apidologie 31:593–594
Esslen J, Kaissling KE (1976) Zahl und Verteilung antennaler Sensillen bei der Honigbiene (Apis mellifera L.). Zoomorphologie 83:227–251
Farris SM, Roberts NS (2005) Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proc Natl Acad Sci U S A 102:17394–17399
Flanagan D, Mercer AR (1989) An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera: Apidae). Int J Insect Morphol Embryol 18:145–159
Friedrich M, Rambold I, Melzer RR (1996) The early stages of ommatidial development in the flour beetle Tribolium castaneum (Coleoptera; Tenebrionidae). Dev Genes Evol 206:136–146
Fusca D, Schachtner J, Kloppenburg P (2015) Colocalization of allatotropin and tachykinin-related peptides with classical transmitters in physiologically distinct subtypes of olfactory local interneurons in the cockroach (Periplaneta americana). J Comp Neurol 523:1569–1586
Gatellier L, Nagao T, Kanzaki R (2004) Serotonin modifies the sensitivity of the male silkmoth to pheromone. J Exp Biol 207:2487–2496
Ghaffar H, Larsen JR, Booth GM, Perkes R (1984) General morphology of the brain of the blind cave beetle, Neaphaenops tellkampfii Erichson (Coleoptera - Carabidae). Int J Insect Morphol Embryol 13:357–371
Graham JR, Ellis JD, Carroll MJ, Teal PEA (2011) Aethina tumida (Coleoptera: Nitidulidae) attraction to volatiles produced by Apis mellifera (Hymenoptera: Apidae) and Bombus impatiens (Hymenoptera: Apidae) colonies. Apidologie 3:326–336
Greco MK, Hoffmann D, Dollin A, Duncan M, Spooner-Hart R, Neumann P (2010) The alternative Pharaoh approach: stingless bees mummify beetle parasites alive. Naturwissenschaften 97:319–323
Grimm R (2001) Faunistik und Taxonomie einiger Arten der Gattung Tribolium Macleay, 1825, mit Beschreibung von drei neuen Arten aus Afrika. (Coleoptera, Tenebrionidae). Entomofauna 22:393–404
Gronenberg W, Liebig J (1999) Smaller brains and optic lobes in reproductive workers of the ant Harpegnathos. Naturwissenschaften 86:343–345
Halcroft M, Spooner-Hart R, Neumann P (2011) Behavioural defence strategies of the stingless bee, Austroplebeia australis, against the small hive beetle, Aethina tumida. Insect Soc 58:245–253
Hansson BS (1997) Antennal lobe projection patterns of pheromone-specific olfactory receptor neurons in moths. In: Cardé RT, Minks AK (eds) Insect pheromone research. Springer, New York, pp 164–183
Hansson BS, Stensmyr MC (2011) Evolution of insect olfaction. Neuron 72:698–711
Heisenberg M (2003) Mushroom body memoir: from maps to models. Nat Rev Neurosci 4:266–275
Hepburn HR, Radloff SE (1998) Honeybees of Africa. Springer, Berlin
Heuer CM, Kollmann M, Binzer M, Schachtner J (2012) Neuropeptides in insect mushroom bodies. Arthropod Struct Dev 41:199–226
Hill ES, Okada K, Kanzaki R (2003) Visualization of modulatory effects of serotonin in the silkmoth antennal lobe. J Exp Biol 206:345–352
Homberg U (2002) Neurotransmitters and neuropeptides in the brain of the locust. Microsc Res Tech 56:189–209
Homberg (2008) Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct Dev 37(5):347–362
Hu JH, Wang ZY, Sun F (2011) Anatomical organization of antennal-lobe glomeruli in males and females of the scarab beetle Holotrichia diomphalia (Coleoptera: Melolonthidae). Arthropod Struct Dev 40:420–428
Huetteroth W, Schachtner J (2005) Standard three-dimensional glomeruli of the Manduca sexta antennal lobe: a tool to study both developmental and adult neuronal plasticity. Cell Tissue Res 319:513–524
Husch A, Paehler M, Fusca D, Paeger L, Kloppenburg P (2009) Distinct electrophysiological properties in subtypes of nonspiking olfactory local interneurons correlate with their cell type-specific Ca2+ current profiles. J Neurophysiol 29:11582.11592
Ignell R, Dekker T, Ghaninia M, Hansson BS (2005) Neuronal architecture of the mosquito deutocerebrum. J Comp Neurol 493:207–240
Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther ÅM (2009) Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci U S A 106:13070–13075
Kaissling KE (1971) Insect olfaction. In: Handbook of sensory physiology, vol 4. Springer, Berlin, pp 351–431
Kelber C, Rössler W, Kleineidam CJ (2010) Phenotypic plasticity in number of glomeruli and sensory innervation of the antennal lobe in leaf-cutting ant workers (A. vollenweideri). Dev Neurobiol 70:222–234
Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E (1996) Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci 16:3154–3165
Kleineidam CJ, Obermayer M, Halbich W, Rössler W (2005) A macroglomerulus in the antennal lobe of leaf-cutting ant workers and its possible functional significance. Chem Senses 30:383–392
Kloppenburg P, Hildebrand JG (1995) Neuromodulation by 5-hydroxytryptamine in the antennal lobe of the sphinx moth Manduca sexta. J Exp Biol 198:603–611
Kollmann M, Minoli S, Bonhomme J, Homberg U, Schachtner J, Tagu D, Anton S (2011a) Revisiting the anatomy of the central nervous system of a hemimetabolous model insect species: the pea aphid Acyrthosiphon pisum. Cell Tissue Res 343:343–355
Kollmann M, Huetteroth W, Schachtner J (2011b) Brain organization in Collembola (springtails). Arthropod Struct Dev 40:304–316
Kondoh Y, Kaneshiro KY, Kimura K, Yamamoto D (2003) Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc R Soc Lond B 270:1005–1013
Kuebler LS, Kelber C, Kleineidam CJ (2010) Distinct antennal lobe phenotypes in the leaf-cutting ant (Atta vollenweideri). J Comp Neurol 518:352–365
Kurylas AE, Rohlfing T, Krofczik S, Jenett A, Homberg U (2008) Standardized atlas of the brain of the desert locust, Schistocerca gregaria. Cell Tissue Res 333:125–145
Kvello P, Løfaldli BB, Rybak J, Menzel R, Mustaparta H (2009) Digital, three-dimensional average shaped atlas of the Heliothis virescens brain with integrated gustatory and olfactory neurons. Front Syst Neurosci 3:14. doi:10.3389/neuro.06.014.2009
Larsson MC, Hansson BS, Strausfeld NJ (2004) A simple mushroom body in an African scarabid beetle. J Comp Neurol 478:219–232
Linn CE, Roelofs WL (1986) Modulatory effects of octopamine and serotonin on male sensitivity and periodicity of response to sex pheromone in the cabbage looper moth, Trichoplusia ni. Arch Insect Biochem Physiol 3:161–171
Lundie AE (1940) The small hive beetle, Aethina tumida. Bulletin no 220, South African Department of Agriculture and Forestry, Pretoria
McGuire SE, Le PT, Davis RL (2001) The role of Drosophila mushroom body signaling in olfactory memory. Science 10:1126–1129
Menzel R (2001) Searching for the memory trace in a mini-brain, the honeybee. Learn Mem 8:53–62
Miura N, Nakagawa T, Tatsuki S, Touhara K, Ishikawa Y (2009) A male-specific odorant receptor conserved through the evolution of sex pheromones in Ostrinia moth species. Int J Biol Sci 5:319–330
Montgomery SH, Ott SR (2014) Brain composition in Godyris zavaleta, a diurnal butterfly, reflects an increased reliance on olfactory information. J Comp Neurol 523:869–891
Mutinelli F, Montarsi F, Federico G, Granato A, Ponti AM, Grandinetti G et al (2014) Detection of Aethina tumida Murray (Coleoptera: Nitidulidae.) in Italy: outbreaks and early reaction measures. J Apic Res 53:569–575
Mysore K, Subramanian KA, Sarasij RC, Suresh A, Shyamala BV, VijayRaghavan K, Rodrigues V (2009) Caste and sex specific olfactory glomerular organization and brain architecture in two sympatric ant species, Camponotus sericeus and Camponotus compressus (Fabricius, 1798). Arthropod Struct Dev 38:485–497
Nagel KI, Hong EJ, Wilson RI (2015) Synaptic and circuit mechanisms promoting broadband transmission of olfactory stimulus dynamics. Nat Neurosci 18:56–65
Nakagawa T, Sakurai T, Nishioka T, Touhara K (2005) Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307:1638–1642
Nässel DR (2002) Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68:1–84
Neumann P (2015) Small hive beetle in Italy: what can we expect in the future? In: Carreck NL (ed) The small hive beetle in Europe. International Bee Research Association, Groombridge
Neumann P, Ellis JD (2008) The small hive beetle (Aethinatumida Murray, Coleoptera: Nitidulidae): distribution, biology and control of an invasive species. J Apic Res 47:181–183
Neumann P, Elzen PJ (2004) The biology of the small hive beetle (Aethina tumida Murray, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species. Apidologie 35:229–247
Neumann P, Hoffmann D, Duncan M, Spooner-Hart R, Pettis JS (2012) Long-range dispersal of small hive beetles. J Agic Res 51:214–215
Neumann P, Evans J, Pettis JS, Pirk CWW, Schäfer MO, Tanner G, Ellis JD (2013) Standard methods for small hive beetle research. In: Dietemann V, Ellis JD, Neumann P (Eds) The COLOSS BEEBOOK, Volume II: standard methods for Apis mellifera pest and pathogen research. J Apic Res 52: 1–32
Neupert S, Fusca D, Schachtner J, Kloppenburg P, Predel R (2012) Towards a single-cell-based analysis of neuropeptide expression in Periplaneta americana antennal lobe neurons. J Comp Neurol 520:694–716
Okada R, Awasaki T, Ito K (2009) Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol 514:74–91
Olsen SR, Wilson RI (2008) Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452:956–960
Olsen SR, Bhandawat V, Wilson RI (2007) Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54:89–103
Olsen SR, Bhandawat V, Wilson RI (2010) Divisive normalization in olfactory population codes. Neuron 66:287–299
Petroski RJ, Bartelt RJ, Vetter RS (1994) Male-produced aggregation pheromone of Carpophilusobsoletus (Coleoptera, Nitidulidae). J Chem Ecol 20:1483–1493
Rein K, Zöckler M, Mader MT, Grübel C, Heisenberg M (2002) The Drosophila standard brain. Curr Biol 12:227–231
Renou M, Tauban D, Morin J-P (1998) Structure and function of antennal poreplate sensilla of Oryctes rhinoceros (L.) (Coleoptera : Dynastinae). Int J Insect Morphol Embryol 27:227–233
Robertson HM, Wanner KW (2006) The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res 16:1395–1403
Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW (2007) Propagation of olfactory information in Drosophila. Proc Natl Acad Sci U S A 104:11826–11831
Rospars JP (1983) Invariance and sex-specific variations of the glomerular organization in the antennal lobes of a moth, Mamestra brassicae, and a butterfly, Pieris brassicae. J Comp Neurol 220:80–96
Rospars JP, Hildebrand JG (2000) Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem Senses 25:119–129
Ruther J, Reinecke A, Thiemann K, Tolasch T, Francke W, Hilker M (2000) Mate finding in the forest cockchafer, Melolontha hippocastani, mediated by volatiles from plants and females. Physiol Entomol 25:172–179
Sachse S, Galizia CG (2002) Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol 87:1106–1117
Sakura M, Mizunami M (2001) Olfactory learning and memory in the cockroach Periplaneta americana. Zool Sci 18:21–28
Schachtner J, Schmidt M, Homberg U (2005) Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea and Hexapoda). Arthropod Struct Dev 34:257–299
Schmolke MD (1974) A study of Aethina tumida: the small Hive Beetle, Project Report. University of Rhodesia, Harare
Schneider D (1992) 100 years of pheromone research: an essay on Lepidoptera. Naturwissenschaften 79:241–250
Seki Y, Kanzaki R (2008) Comprehensive morphological identification and GABA immunocytochemistry of antennal lobe local interneurons in Bombyx mori. J Comp Neurol 506:93–107
Seki Y, Rybak J, Wicher D, Sachse S, Hansson BS (2010) Physiological and morphological characterization of local interneurons in the Drosophila antennal lobe. J Neurophysiol 104:1007–1019
Settembrini BP, Villar MJ (2005) FMRFamide-like immunocyrochemistry in the brain and subesophageal ganglion of Triatoma infestans (Insecta: Heteroptera). Coexpression with ß-pigment-dispersing hormone and small cardioactive peptide. Cell Tissue Res 321:299–310
Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G (2007) Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128:601–612
Siju KP, Schachtner J, Reifenrath A, Scheiblich H, Neupert S, Predel R, Hansson B, Ignell R (2014) Neuropeptides in the antennal lobe of the yellow fever mosquito, Aedes aegypti. J Neurophysiol 522:592–608
Silbering AF, Galizia CG (2007) Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci 27:11966–11977
Slessor KN, Winston ML, Le Conte Y (2005) Pheromone communication in the honeybee (Apis mellifera L.). J Chem Ecol 31:2731–2745
Sokoloff A (1977) The biology of tribolium with special emphasis on genetic aspects. Oxford University Press, London
Spiewok S, Neumann P (2006) Infestation of commercial bumblebee (Bombus impatiens) field colonies by small hive beetles (Aethina tumida). Ecol Entomol 31:623–628
Spiewok S, Neumann P (2012) Sex ratio and dispersal of small hive beetles. J Agric Res 51:216–217
Stocker RF (2001) Drosophila as a focus in olfactory research: mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc Res Tech 55:284–296
Stopfer M, Bhagavan S, Smith BH, Laurent G (1997) Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390:70–74
Strausfeld NJ (2005) The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct Dev 34:235–256
Strauss R (2002) The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12:633–638
Streinzer M, Kelber C, Pfabigan S, Kleineidam CJ, Spaethe J (2013) Sexual dimorphism in the olfactory system of a solitary and a eusocial bee species. J Comp Neurol 521:42–55
Suazo A, Torto B, Teal PEA, Tumlinson JH (2003) Response of the small hive beetle (Aethinatumida) to honey bee (Apis mellifera) and beehive-produced volatiles. Apidologie 34:525–533
Tanaka NK, Ito K, Stopfer M (2009) Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci 29:8595–8603
Trhlin M, Rajchard J (2011) Chemical communication in the honeybee (Apis mellifera L.): a review. Vet Med 56:265–273
Utz S, Huetteroth W, Vömel M, Schachtner J (2008) Mas-allatotropin in the developing antennal lobe of the sphinx moth Manduca sexta: distribution, time course, developmental regulation and colocalization with other neuropeptides. Dev Neurobiol 68:123–142
Van Haeften T (1993) Location and function of serotonin in the central and peripheral nervous system of the Colorado potato beetle. PhD thesis, University of Wageningen, The Netherlands. (ISBN 1993 90 5485 141 4)
Veenstra JA, Lau GW, Agricola HJ, Petzel DH (1995) Immunohistochemical localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem. Cell Biol 104:337–347
Vitzthum H, Homberg U (1998) Immunocytochemical demonstration of locustatachykinin‐related peptides in the central complex of the locust brain. J Comp Neurol 390:455–469
Vosshall LB, Stocker RF (2007) Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci 30:505–533
Vosshall LB, Wong AM, Axel R (2000) An olfactory sensory map in the fly brain. Cell 102:147–159
Wegerhoff R, Breidbach O, Lobemeier M (1996) Development of locustatachykinin immunopositive neurons in the central complex of the beetle Tenebrio molitor. J Comp Neurol 375:157–166
Wei H, el Jundi B, Homberg U, Stengl M (2010) Implementation of pigment-dispersing factor- immunoreactive neurons in a standardized atlas of the brain of the cockroach Leucophaea maderae. J Comp Neurol 518:4113–4133
Weissteiner S, Huetteroth W, Kollmann M, Weißbecker B, Romani R, Schachtner J, Schütz S (2012) Cockchafer larvae smell host root scents in soil. PLoS ONE 7(10)
Wessnitzer J, Webb B (2006) Multimodal sensory integration in insects – towards insect brain control architectures. Bioinspir Biomim 1:63–75
Wilson RI (2013) Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci 36:217–241
Wilson RI, Laurent G (2005) Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci 25:9069–9079
Winther ÅME, Ignell R (2010) Local peptidergic signaling in the antennal lobe shapes olfactory behavior. Fly 4:167–171
Winther ÅME, Acebes A, Ferrús A (2006) Tachykinin-related peptides modulate odor perception and locomotor activity in Drosophila. Mol Cell Neurosci 31:399–406
Zhao X, Coptis V, Farri SM (2008) Metamorphosis and adult development of the mushroom bodies of the red flour beetle, Tribolium castaneum. Dev Neurobiol 68:1487–1502
Acknowledgments
We thank Dr. Agricola for kindly providing the Locusta migratoria Tachykinin II antibody, as well as Dr. Buchner for the supply of the Drosophila melanogaster Synapsin I antibody. We thank the Department of Zoology and Entomology of the University of Pretoria (Pretoria/Tshwane South Africa) for providing kind local support and laboratory facilities. We also want to thank Martina Kern for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kollmann, M., Rupenthal, A.L., Neumann, P. et al. Novel antennal lobe substructures revealed in the small hive beetle Aethina tumida . Cell Tissue Res 363, 679–692 (2016). https://doi.org/10.1007/s00441-015-2282-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2282-9