Abstract
The distribution of FMRFamide (FMRFa)-like immunoreactivity (LI) was studied in the brain and subesophageal ganglion of Triatoma infestans, the insect vector of Chagas’ disease. The neuropeptide displayed a widespread distribution with immunostained somata in the optic lobe, in the anterior, lateral, and posterior soma rinds of the protocerebrum, and around the antennal sensory and mechanosensory and motor neuropils of the deutocerebrum. FMRFa-immunoreactive profiles of the subesophageal ganglion were seen in the mandibular, maxillary, and labial neuromeres. Immunostained neurites were detected in the medulla and lobula of the optic lobe, the lateral protocerebral neuropil, the median bundle, the calyces and the stalk of the mushroom bodies, and the central body. In the deutocerebrum, the sensory glomeruli showed a higher density of immunoreactive processes than the mechanosensory and motor neuropil, whereas the neuropils of each neuromere of the subesophageal ganglion displayed a moderate density of immunoreactive neurites. Colocalization of FMRFa-LI and crustacean pigment-dispersing hormone-LI was found in perikarya of the proximal optic lobe, the lobula, the sensory deutocerebrum, and the labial neuromere of the subesophageal ganglion. The distribution pattern of small cardioactive peptide B (SCPB)-LI was also widespread, with immunolabeled somata surrounding every neuropil region of the brain and subesophageal ganglion, except for the optic lobe. FMRFa- and SCPB-LIs showed extensive colocalization in the brain of this triatomine species. The presence of immunolabeled perikarya displaying either FMRFa- or SCPB-LI confirmed that each antisera identified different peptide molecules. The distribution of FMRFa immunostaining in T. infestans raises the possibility that FMRFa plays a role in the regulation of circadian rhythmicity. The finding of immunolabeling in neurosecretory somata of the protocerebrum suggests that this neuropeptide may also act as a neurohormone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptides with a FMRFamidated-C-terminal sequence (FMRFa) are present throughout higher invertebrate groups (Greenberg and Price 1992; Orchard et al. 2001) suggesting that they play important conserved roles in physiology. They form part of a larger peptide family containing an -RFamide-C terminus, commonly known as FaRPs (O’Brien and Taghert 1994). The FMRFa gene of Drosophila melanogaster was one of the first cloned insect-neuropeptide genes (Nambu et al. 1988; Schneider and Taghert 1988), and various molecules and mechanisms account for the regulation of the expression of this gene, whether in a specific cell type or during development (Benveniste and Taghert 1999; Taghert 1999). G-protein-coupled FMRFa receptors have been characterized in D. melanogaster (Cazzamali and Grimmelikhuijzen 2002; Meeusen et al. 2002) and Anopheles gambiae (Duttlinger et al. 2003); structural comparisons of these receptors have revealed a high degree of sequence conservation.
The distribution of FMRFa-like immunoreactivity (LI) has been reported in the central nervous system (CNS) of several insect species (Verhaert et al. 1985; Remy et al. 1988; Homberg et al. 1991a; Tsang and Orchard 1991). Various cell types including motoneurons, neuroendocrine cells, and interneurons display FMRFa-LI, and a role for FMRFa and related neuropeptides in the control of hemolymph circulation, feeding, and digestion has been postulated (Duve et al. 1993; Elia et al. 1993; Nichols et al. 1999; Duttlinger et al. 2002; Harshini et al. 2002; Predel et al. 2004).
Triatoma infestans is the main insect vector of Chagas’ disease in southern cone countries (WHO 2002). This hematophagous heteropteran insect feeds exclusively on warm-blooded vertebrates. Despite the epidemiological importance of this insect species, information on neural structures regulating host-related behavior is limited. Because of the reported actions of FMRFa in feeding and digestion, knowledge concerning the distribution and function of this molecule and its interactions with other neurotransmitters may contribute to the development of safe and highly specific control methods (Orchard et al. 2001; Maule et al. 2002).
The coexistence of neuroactive molecules in the CNS of Triatoma infestans has been previously reported. Thus, nitric oxide synthase has been found colocalized with cholecystokinin in somata of the brain and subesophageal ganglion (SOG; Villar et al. 1994). Furthermore, coexpression of either neuropeptide Y or its Y1 receptor with cholecystokinin has been detected in cephalic and thoracic ganglia (Settembrini et al. 2003), suggesting the possibility of considerable coexistence of neurotransmitters in this triatomine species. Several studies have reported the coexpression of FMRFa-LI with other neuroactive molecules (Orchard et al. 2001). Among them, colocalization with insect pigment-dispersing factor (PDF)-LI in the optic lobe (OL) has been reported as a frequent event. In several arthropod species, the distribution patterns of PDFs are well documented, and the projection areas of PDF-immunoreactive (IR) neurons have been thoroughly traced. Furthermore, in D. melanogaster and Leucophaea maderae, PDFs have been implicated as messengers of the biological clock (Petri and Stengl 1997; Renn et al. 1999). In the search for a food source, T. infestans relies mainly on olfactory cues (Taneja and Guerrin 1995). Some host odours that stimulate olfactory receptors of basiconic and grooved-peg sensilla have been shown to modify the walking speed of T. infestans (Guerenstein and Guerin 2001). The locomotor activity of these bugs displays a circadian nocturnal endogenous rhythm (Settembrini 1984; Lazzari 1992); however, the anatomical location of the circadian pacemaker (s) controlling this activity is still unknown. We have therefore studied the possible coexpression of β-PDH (PDH)- and FMRFa-LIs in the CNS of T. infestans by using an antiserum directed against crustacean pigment-dispersing hormone (βPDH), which has an amino acid sequence closely related to insect PDFs.
In some insect species, the distribution of FMRFa-LI has been studied together with that of the molluscan small cardioactive peptide B (SCPB), and their coexpression has been reported in neurosecretory cells and interneurons (Homberg and Hildebrand 1989; Homberg et al. 1990, 1991a). Moreover, SCPB-IR elements have been proposed as constituting a subset of those displaying FMRFa-LI in some arthropod species. We have consequently examined the distribution pattern of SCPB-LI and its colocalization with FMRFa-LI.
In the present study, we describe the distribution and colocalization of FMRFa-, PDH-, and SCPB-LIs in the brain and SOG of Triatoma infestans by means of immunocytochemical methods. Preliminary reports of these investigations have been published in abstract form (Settembrini et al. 2000, 2001).
Materials and methods
Insects
Adult male Triatoma infestans, free of Trypanosoma cruzi and Blastocrithidia triatomae, were used in this study. The insects were derived from stocks provided by the Center for the Control of Chagas’ disease (Santa María, Córdoba, Argentina) and were reared under controlled conditions of light, temperature, and relative humidity (Settembrini 1984). The bugs were fed every 15 days on the shaved thorax of pigeons for 1 h under dim light.
Dissection and fixation
Insects were processed for immunocytochemistry 1 week after feeding (Settembrini and Villar 1999). Briefly, bugs were cold-anesthetized, and their legs were secured to a wax lamina. The dorsal cuticle of the head was removed, and the tissues were immediately flushed with cold fixative, viz., either a mixture of 4% paraformaldehyde and 0.4% picric acid in 0.16 M sodium phosphate buffer (PB, pH 6.9) or 4% paraformaldehyde in 0.1 M PB, pH 7.3. Dissection of the brain and SOG out of the head capsule was performed with the tissues bathed in cold fixative. Samples remained in the fixative for 6 h at 4°C, following which they were rinsed in 0.01 M PB saline (PBS, pH 7.4) and kept at 4°C until processed for immunocytochemistry.
Immunocytochemistry
Table 1 shows the source, working dilutions, and references detailing the first characterization of the antisera used in single- and double-labeling experiments. Only clearly immunostained somata that appeared in all the brain and SOG samples were considered in the results. Anti-FMRFa (PT2) was a rabbit antiserum generated against authentic FMRFa conjugated to thyroglobulin. Anti-β-PDH was raised in rabbits against β-pigment-dispersing hormone of Uca pugilator, and anti-SCPB was a mouse monoclonal antiserum against synthetic molluscan SCPB.
Single-labeling experiments
By means of indirect immunofluorescence or avidin-biotin immunoperoxidase (ABC) techniques, single-labeling experiments were performed either in whole-mounted specimens (n=6) or in 18-μm serial cryostat sections (Microm, Walldorf, Germany). Before sectioning, the tissues were cryoprotected by immersion in PBS containing 15% sucrose, 0.02% bacitracin (Sigma, St. Louis, Mo., USA), and 0.01% sodium azide (Merck, Darmstadt, Germany), for at least 48 h. Briefly, to study the distribution of FMRFa (n=16 insects), the sections were incubated for 24 h at 4°C with FMRFa antiserum (1:1,000), rinsed in 0.01 M PBS, and incubated for 30 min at 37°C with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibodies (1:80 in PBS; Boehringer, Mannheim, Germany). Subsequently, the sections were rinsed, mounted in 80% glycerol in PBS, and examined with a Nikon Eclipse 800 epifluorescence microscope (Nikon, Tokyo, Japan) equipped with filter combinations for FITC-induced fluorescence. Kodak TriX black-and-white film was used for photography. Other experiments were performed with β-PDH antiserum (n=8 insects) diluted 1:1,000, followed by lissamine rhodamine B sulfonyl chloride (LRSC)-conjugated goat anti-rabbit antibodies (1:80, Jackson ImmunoResearch Labs, Pa., USA).
For the ABC method (Hsu et al. 1981), the sections were incubated with antisera either against FMRFa (n=30 insects) diluted 1:2,000 or against β-PDH (n=10 insects) at a dilution of 1:4,000. The slides were then rinsed in PBS, incubated at room temperature (RT) for 30 min in biotinylated goat anti-rabbit secondary antibodies (1:100, Vector Laboratories, Burlingame, Calif., USA), rinsed again in PBS, and further incubated for 1 h in ABC reagent (Vectastain Elite kit, Vector Laboratories). Peroxidase activity was revealed by reaction with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) by using glucose oxidase (Sigma) and nickel salts for enhancement of the reaction product (Shu et al. 1988). The sections were mounted with Permount (Fluka, Buchs, Switzerland) and photographed with Agfapan APX 25 (Agfa Gevaert, Leverkusen, Germany). To study the distribution of SCPB-LI (n=20 insects), anti-SCPB mouse monoclonal supernatant (1:250) and biotinylated horse anti-mouse secondary antibodies (1:100, Vector Laboratories) were used following the protocol given above.
Double-labeling experiments
Colocalization of FMRFa- and PDH-LIs (n=16 insects) was investigated by combining conventional indirect immunofluorescence with the tyramide signal amplification method (TSA Plus, NEN Life Science Products, Boston, Mass., USA). Cryostat sections of the brain and SOG were incubated overnight in a humid chamber at 4°C with anti-FMRFa antibodies (see Table 1), rinsed in TNT buffer kit (0.1 M TRIS-HCl pH 7.5, 0.15 M NaCl, 0.05% Tween 20), blocked with TNB buffer kit (0.1 M TRIS-HCl pH 7.5, 0.15 M NaCl, 0.05% DuPont Blocking Reagent) for 30 min, incubated with biotinylated goat anti-rabbit antibodies (Vector Laboratories) diluted 1:100 in TNB for 1 h, rinsed in TNT, and then incubated for 30 min in streptavidin-horseradish peroxidase 1:100 in TNB. The slides were then rinsed in TNT and incubated in biotinylated tyramide diluted 1:50 in amplification diluent for about 10 min. The slides were rinsed in TNT, followed by incubation with streptavidin-conjugated FITC (diluted 1:500 in TNB) for 30 min, and rinsed again in TNT and PBS. Subsequently, the sections were incubated overnight at 4°C with β-PDH antiserum (see Table 1) followed by staining with LRSC-conjugated goat anti-rabbit antibodies (1:50). The sections were dehydrated in ascending ethanol concentrations, rehydrated, and passed through solutions of 20%–80% glycerol in 0.05 M sodium carbonate-bicarbonate buffer pH 7.0. They were examined and photographed with a Nikon Eclipse 800 microscope equipped with filter combinations for FITC- and LRSC-induced fluorescence. TriX black-and-white film was used for photography.
To investigate the coexistence between FMRFa- and SCPB-LIs, whole-mount preparations of the brain and SOG (n=5 insects) were transferred to 1% Triton X-100 PBS (PBST) for at least 48 h and then immersed in PBS containing 10% normal donkey serum for 1 h to diminish non-specific binding. The same solution was also used for the dilution of the primary and secondary antisera. Subsequently, the samples were incubated with a mixture of rabbit anti-FMRFa and mouse monoclonal anti-SCPB antibodies (see Table 1) for 24 h at 4°C and then in a mixture of FITC-conjugated goat anti-rabbit (1:40) and tetramethyl-rhodamine isothiocyanate (TMRITC)-conjugated goat anti-mouse (1:40, Jackson ImmunoResearch Labs) secondary antibodies for 30 min at 37°C. Brains and SOGs were mounted in 80% glycerol as stated above and viewed with a Nikon PCM 2000 laser-scanning confocal microscope mounted on an E800 microscope equipped with green He/Ne (543 nm), red He/Ne (633 nm), and argon (488 nm) lasers and the appropriate filters. Stacks of digitized images were merged and processed by using Simple PCI 3.2 software (Compix, Cranberry Township, Pa., USA) for image acquisition. When needed, the digitized images were modified to enhance contrast only (Adobe Photoshop, Adobe Systems, San José, Calif., USA).
Controls
The specificity of the FMRFa antiserum was tested by overnight preincubation with each of the following peptides: Drosophila DPKQDFMRFamide, Drosophila DPKQDFMRFG-OH, and Aplysia myomodulin A (PMSMLRLamide) at a concentration of 10 μg/ml in the anti-FMRFa antibodies diluted 1:2,000. The specificity of the SCPB antibodies was tested by overnight preincubation of the sections with synthetic SCPB (Sigma) at 10 μg/ml, in SCPB antiserum diluted 1:250. After preadsorption of the antisera with the corresponding peptides, the sections were incubated with the preadsorbed antisera and processed following the ABC method as stated above. Other controls were performed by incubating the sections without either the first or the second antisera.
Results
Because of the massive development of the pharyngeal pump musculature, the brain and SOG of Triatoma infestans are located posteriorly in the cephalic capsule. The brain is composed of the protocerebrum (PC), deutocerebrum (DC), and tritocerebrum (TC), and the OLs. The PC hemispheres and the OL, which extend anterolaterally into the head capsule, are shown in a dorsal view in Fig. 1a–c. The DC (Fig. 1d–f, top) and the TC, which lie ventrally are concealed by the dome-shaped PC lobes. The SOG (Fig. 1d–f, bottom) is connected to the brain by short circumesophageal connectives and extends from the posterior edge of the PC lobes to the neck, where the cephalic connectives to the prothoracic ganglion arise.
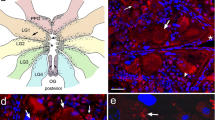
Drawings of dorsal views of Triatoma infestans protocerebrum with optic lobes (a–c), deutocerebrum (d–f top), and subesophageal ganglion (d–f bottom) showing FMRFa- (a, d), PDH- (b, e), and SCPB-LIs (c, f). Symbols represent the number of immunoreactive somata, and shadowing in the neuropils indicates the density of the immunolabeled processes (an antennal nerve, apn anterolateral protocerebral neuropil, c connectives, cb central body, ef esophageal foramen, la lamina ganglionaris, lmp lateromedial protocerebral neuropil, lo lobula complex, lpn lateral protocerebral neuropil, mb mushroom body, md mechanosensory and motor deutocerebrum, md-mx-lb mandibular, maxillary, and labial neuromeres of the subesophageal ganglion, me medulla, ol optic lobe, p lobula plate, sd sensory deutocerebral glomeruli, sog subesophageal ganglion). Bars 100 μm
Distribution of FMRFa-LI
Protocerebrum and optic lobes
Figure 1a depicts the distribution pattern of FMRFa-LI in the PC and OL as obtained from horizontal serial sections. Protocerebral FMRFa-IR somata of various sizes could be observed in the anterior, lateral, and the posterior soma rinds, which surrounded the immunostained neuropil. In the anterior soma rind, a group of medium-sized immunolabeled somata (11–15 μm) was found in the vicinity of the median furrow between the PC lobes (Fig. 2a), whereas a few small immunolabeled perikarya (6–10 μm) were detected lateral to this group (Fig. 2b). Other immunostained somata were present at the entry of the ocellar nerves and above the mushroom body (MB) calyces (Fig. 2e). In the lateral cell-body cortex, FMRFa-IR somata were detected forming clusters (Fig. 1a). A group of small (6–10 μm) to medium-sized (11–15 μm) perikarya was seen at the posterolateral edge (Fig. 2c,d). Immunopositive perikarya of the posterior soma rind were usually found forming small clusters of about 2–4 perikarya (Fig. 1a). FMRFa-IR cell-body clusters of the OL were largely observed in the medial layer (Fig. 1a). In the proximal OL, several stained somata were seen in the medial and lateral soma layers (Fig. 2e–f). A cluster of small perikarya (6–10 μm) was present at the level of the lobula; other immunolabeled somata surrounded the anterior medulla and the internal chiasma neuropils. FMRFa-LI could not be detected in the lamina ganglionaris, either in perikarya or in neurites.
a–f Bright-field micrographs showing FMRFa-LI in the PC. a Immunolabeled perikarya of the anteromedial soma rind and the median furrow (arrows). Positive fibers (arrowheads) form a commissure. b Heavily immunostained somata of the anterior soma rind (arrows) and thick positive processes traversing the median furrow (arrrowheads). The central body (cb) shows numerous positive neurites, whereas the lobes (l) of the MB are unstained. c Immunostained perikarya in the posterolateral soma rind (straight arrows) and in the posterior protocerebral slope (curved arrows). Immunopositive neurites (arrowheads) are scattered in the protocerebral neuropil (ppn). d Immunolabeled neurites are present in the central-body complex (cb), calyces (ca) of the MB, median furrow (arrowhead), and lateromedial protocerebral neuropil (open arrow). An immunostained soma of the posterolateral soma rind (arrow) possesses a thick neurite. e Two groups of somata: immunostained profiles above the calyces (ca, straight arrows) and immunopositive perikarya (curved arrows) at the boundary of the OL with the anterior protocerebral neuropil (apn). f A group of immunoreactive perikarya (straight arrows) at the proximal OL (ol) and somata (curved arrow) of the lateral soma layer. Varicose positive neurites (arrowheads) lie at the boundary of the OL with the anterolateral protocerebral neuropil (apn). Bars 20 μm
The distribution FMRFa-IR processes in the PC and OL is also depicted in Fig. 1a. In the anterior PC, thin immunoreactive neurites were seen running across the median furrow of the PC (Fig. 2a); other thick immunostained fibers formed short commissures between the PC hemispheres (Fig. 2b). The central body housed a high density of varicose neurites (Fig. 2b), whereas the MB calyces and the stalk displayed a moderate density of immunolabeled processes (Fig. 2d, e). Immunostaining was not evident either in the α- or in the β-lobes of the MB (Fig. 2b, d). The lateral and the ventromedial PC neuropils showed scattered immunostained neurites (Fig. 2d). OL neuropils displayed immunostaining in the serpentine layer of the medulla and in the lobula, with a higher density in the lobula plate.
Deutocerebrum
The distribution pattern of FMRFa-LI in the DC is shown in Fig. 1d. FMRFa-IR somata were mainly detected in the lateral cell-body layer. Thus, medium-sized (11–15 μm) and intensely immunostained perikarya could be distinguished around the sensory glomeruli and in the anterolateral edge (Fig. 3a). Other immunolabeled cell profiles of the lateral soma rind were detected close to the neuropil of the antennal mechanosensory and motor center.
a–b FMRFa-LI in the DC (a) and SOG (b). a Bright-field micrograph of immunopositive somata of the lateral layer (straight arrows); a cell body (curved arrow) projects to the sensory deutocerebral glomeruli (g). A fiber tract (arrowheads) branches medial to the glomeruli (o esophagus). Positive neurites are also visible in the antennal mechanosensory and motor center (md). Bar 20 μm. b Immunofluorescence micrograph of the SOG (straight arrow a group of medial immunopositive somata, curved arrow cells in the lateral cell-body layer, arrowhead paired positive neurites running medially within the ganglion). Bar 50 μm
A moderate density of immunostained neurites could be observed in the sensory glomeruli of the DC, with some positive fibers at the inner border of the glomeruli. The central neuropil was unstained. The antennal mechanosensory and motor center contained blebby positive fibers (Fig. 1d). A fiber tract with a branching pattern was seen running medially to the glomeruli (Fig. 3a) and to the neuropil of the antennal mechanosensory and motor center.
Subesophageal ganglion
FMRFa-LI was detected in the lateral cell-body layer of the SOG, around the esophageal foramen and close to the cephalic connectives linking this ganglion with the prothoracic ganglion (Figs. 1d, 3b).
FMRFa-IR fibers were seen around the esophageal foramen, whereas a moderate density of immunostained neurites was observed in the mandibular, maxillary, and labial neuromeres (Fig. 1d). Bilateral fibers could be distinguished in the posterior part of the SOG and in the cephalic connectives (Fig. 3b).
Distribution of PDH-LI
PDH-LI was observed in a few somata of the brain and SOG (Fig. 1b,e). Single-immunolabeled perikarya were observed in the anteromedial, anterolateral, and lateromedial soma rinds of the PC (Fig. 1b). Medium-sized PDH-IR somata (11–15 μm) were seen at the boundary of the OL with the PC lobes (Fig. 4b). A cluster of eight ovoid small PDH-IR somata (6–10 μm) was present in the medial cell-body layer at the level of the lobula (Fig. 4d).
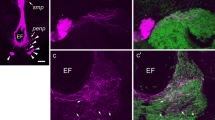
a–d Immunofluorescence micrographs of double-stained sections showing FMRFa-LI (a, c) and PDH-LI (b, d) in the proximal OL (a, b) and at the lobula (c, d). a, b Double-immunostained somata (arrows) in the medial layer of the proximal OL (open arrow in a perikaryon displaying only FMRFa-LI, arrowhead in b thick PDH-IR neurites at the margin of the lobula neuropil, arrowhead in a varicose FMRFa-IR neurite). c, d Double-immunostained somata (arrows) within a cell-body cluster located in the medial soma rind of the lobula (lo); one cell body (open arrow) shows only FMRFa-LI (arrowheads fibers that are either FMRFa-IR or PDH-IR). Bars 20 μm
Other immunoreactive perikarya were observed in the medial and lateral cell-body layers around the sensory glomeruli of the DC. Immunolabeled somata of the SOG could be detected around the esophageal foramen and in the labial neuromere, near the origin of the cephalic connectives (Fig. 1e).
In some areas of the brain, PDH-IR neurites were observed forming meshworks (Fig. 1b,e), whereas in other regions, the course of single-immunopositive processes could be followed. Thus, immunolabeled neurites were observed running from the OL to the anterior PC with emerging branches to the lateral horn and to the ventromedial PC neuropils. Positive varicose neurites from somata located at the level of the lobula could be followed up to the distal edge of the outer medulla after traversing the inner medulla and the serpentine layers. Furthermore, thick immunoreactive processes were seen at the medial border of the lobula neuropil (Fig. 4b). Scattered PDH-IR processes were observed in the SOG (Fig. 1e), and blebby neurites ran along the midline of the ganglion and in the cephalic connectives.
Colocalization of FMRFa- and PDH-LIs
The colocalization of FMRFa- and PDH-LIs is represented in Fig. 1b,e. In the OL, both markers were coexpressed in perikarya located in the medial soma rind at the level of the proximal OL (Fig. 4a,b) and at the anterior-most part of the lobula (Fig. 4c,d). Finally, somata in the medial layer of the sensory glomeruli and in the labial neuromere of the SOG also displayed both immunoreactivities (Fig. 1e).
Distribution of SCPB-LI
Protocerebrum and optic lobes
The distribution of SCPB-IR somata in the PC and OL is portrayed in Fig. 1c. In the PC lobes, scattered immunolabeled somata were observed in the anteromedial cell-body layer and above the MB calyces (Fig. 5a). At the anterolateral edge, some fusiform immunostained perikarya were observed close to the boundary of the PC with the OL (Fig. 5b). Single-immunopositive perikarya were also seen in the lateral soma rind (Fig. 1c). SCPB-IR cell bodies of the posterior PC usually formed clusters (Fig. 5a); thus, a cluster of about ten medium-sized (11–15 μm) immunopositive somata was detected at the posterior slope, whereas other immunolabeled profiles were found in a posteromedial position. In the proximal OL (Fig. 5b), three clusters of immunoreactive somata were observed; the larger one with the highest number of cells was located between the medial and lateral cell-body layers of the OL. No immunoreactive somata were detected in association with the lamina, the medulla, or the lobula neuropils.
a–d Bright-field micrographs of sections displaying SCPB-LI. a Section of the PC showing immunostained neurites in the calyces (ca) of the mushroom bodies, the central body (cb), and the lateromedial neuropil (arrowhead). Note the immunoreactive somata (curved arrows) of the anterior soma rind and the perikarya (straight arrows) of the posterior PC. b Immunolabeled somata (straight arrows) of the proximal OL (ol) and immunostained somata (curved arrows) of the anterior soma rind (arrowhead thick immunopositive neurites). c Section of the DC (curved arrows immunostained somata at the anterolateral edge, straight arrow large immunostained perikaryon close to the sensory glomeruli, arrowheads immunolabeled neurites of the antennal mechanosensory and motor center, g sensory glomeruli, md mechanosensory and motor center). d Section of the SOG (straight arrow medial immunostained cell body, curved arrow anterolateral immunoreactive perikarya, arrowheads medial immunoreactive neurites, c connectives). Bars 20 μm
The distribution pattern of SCPB-IR processes is also depicted in Fig. 1c. Immunolabeled neurites were observed along the median furrow, in the anterolateral PC, and in the central body (Fig. 5a). In the anterolateral PC, labeled neurites could be followed up to the OL. The upper division of the central body showed a lower density of immunoreactive processes than the lower part. The calyces of the MB and the stalk displayed a moderate density of immunolabeled neurites (Fig. 5a); some thick immunolabeled processes were detected in the external border of the calycal neuropil, whereas the α-lobe was unstained.
Deutocerebrum
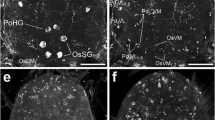
Around the sensory glomeruli, SCPB-IR perikarya could be observed in the anteromedial and anterolateral cell-body layers; the latter group was located above a large glomerulus (Fig. 5c). Scattered immunostained perikarya were also found in the lateral soma rind, whereas a large positive cell body (20 μm) was present at the level of the posterior glomeruli (Fig. 5c). SCPB-IR cell bodies of the antennal mechanosensory and motor center were located in both the lateral and medial cell-body layers (Fig. 1f).
The sensory glomeruli showed a low density of thin immunoreactive processes (Fig. 5c), whereas the neuropil of the antennal mechanosensory and motor center contained varicose neurites. A moderate density of stained neurites could be distinguished in the tritocerebral neuropil.
Subesophageal ganglion
Immunostaining was seen in perikarya around the esophageal foramen and in bilateral somata of the mandibular, maxillary, and labial neuromeres. The neuropil of the SOG displayed a low to moderate density of immunoreactive processes (Fig. 1f). Immunolabeled neurites could be detected around the esophageal foramen, close to the roots of the maxillary nerves and within the cephalic connectives (Fig. 5d).
Colocalization of FMRFa- and SCPB-LIs
The coexpression of SCPB- and FMRFa-LIs is portrayed in Fig. 1c,f. Colocalization of both markers was observed in somata of the proximal OL, in the lateral and posteromedial PC, and in the vicinity of the median furrow between the PC hemispheres (Fig. 6a,b). Other areas of coexpression included the lateral cell-body layer of the sensory glomeruli, the anterior soma layer of the SOG, and the lateral cell cortex at the level of the maxillary neuromere (Fig. 1f).
Confocal immunofluorescence micrographs of the PC, double-labeled with FMRFa antiserum (a) and SCPB (b) monoclonal antibodies (ao aorta, t tracheae). A soma displaying both immunoreactivities is indicated (curved arrows), whereas some perikarya (arrowhead) located close to the median furrow display only SCPB-LI and a straight arrow marks a cell body with only FMRFa-LI. Bar 100 μm
Discussion
FMRFa-LI distribution pattern
We have determined the distribution pattern of FMRFa-LI in the brain and SOG of Triatoma infestans by using an antiserum against authentic FMRFa. This antiserum, PT2, displays a high affinity for peptides sharing the C-terminal sequence of FMRFa (Wall and Taghert 1991). In insect tissues, antisera developed against FMRFa probably recognize the dominant epitope formed by the sequence -RFamide (Wall and Taghert 1991; Tsang and Orchard 1991). Thus, the possibility of cross-immunoreactions to a broad spectrum of FMRFa-related peptides cannot be ruled out. At least six factors contributing to FMRFa-LI, which may represent not only different FaRPs but also degradation products, have been detected by reversed-phase high-performance liquid chromatography in Rhodnius prolixus, a species akin to T. infestans (Tsang and Orchard 1991). In our control experiments, we preadsorbed PT2 antiserum with Drosophila DPKQDFMRFa, its intermediate unamidated peptide, and with PMSMLRLa (Aplysia myomodulin). The pattern of immunostaining was blocked only when DPKQDFMRFa was employed, suggesting that PT2 was specific for the FMRFa/-RFa C-terminus.
The widespread distribution of FMRFa-LI in the nervous tissue of adult triatomine bugs, revealed by both the large number of immunoreactive somata and the conspicuous network of positive neurites, is indicative of the outstanding physiological role of FaRPs in Triatoma infestans, acting either as neurotransmitters/neuromodulators or as neurohormones. A neurotransmitter role in T. infestans visual processing is indicated by the finding of FMRa-LI in OL interneurons. Six clusters of immunolabeled somata have been detected, three in the median layer and three in the proximal OL. Immnostained processes from OL somata have been followed up to the medulla and lobula neuropils. Comparisons of the immunostaining patterns have revealed a lower number of FMRFa-IR somata in the OL of T. infestans than in other insect groups (Nässel et al. 1988; Homberg et al. 1990; Pyza and Meinertzhagen 2003). An investigation as to whether this reduction in FaRP expression is related to a different visually guided behavior in T. infestans would be of interest. Coexpression of FMRFa- and PDH-LIs in a group of lobula neurons that, in other species have been proposed as constitutive elements of the circadian pacemaking system (Helfrich-Förster 1995), might indicate a modulatory role of FMRFa-like neuropeptides in the regulation of circadian rhythmicity. In this context, microinjections of molluscan FMRFa into the housefly OL counteract the effect of PDF in the circadian changes in axon caliber of two types of lamina interneurons (Pyza and Meinertzhagen 2003). Nevertheless, FMRFa- and PDH-LIs are also colocalized in neural elements of the triatomine CNS for which there is no evidence of a relationship with the circadian clock. Thus, the possible interaction of both molecules in controlling rhythmic events of T. infestans requires thorough physiological studies.
A neurohormonal role for FaRPs in T. infestans may be envisioned from the finding of FMRFa-IR somata located close to the median furrow. These cells, which belong to the median cell group of the pars intercerebralis, are known to project to the retrocerebral complex and to the aorta. FMRFa-like material synthesized by these cells might be discharged into the circulation when needed. Interestingly, the release of FaRPs from nervous tissue to the hemolymph at various times after a blood meal has been reported in R. prolixus (Elia et al. 1993) suggesting that FaRPs play a role in the feeding and digestion processes of hematophagous heteropterans by acting as a neurohormone.
In contrast to findings in R. prolixus, FMRFa-IR somata and neurites have been observed in the DC of T. infestans. The higher sensitivity of the ABC method may have allowed further detection of FMRFa-LI in this part of the brain of T. infestans. Although the role of FaRPs in olfactory processing is still unknown, FMRFa-LI has been reported in the antennal lobe of several insect species (Verhaert et al. 1985; Homberg et al. 1990; Breidbach and Wegerhoff 1994; Nässel 2002). In T. infestans, FMRFa-IR cell bodies have been mainly found in the lateral cell cortex of the sensory glomeruli, which houses both local and projection neurons. A high density of immunoreactive neurites has been observed in the basal margin of the glomeruli, suggesting that FaRPs might also be used as neurotransmitters of projection neurons. Several transmitter molecules have been recognized by immunocytochemical techniques in the sensory glomeruli of these triatomine bugs (Villar et al. 1994; Settembrini et al. 2003; Settembrini and Villar 2004), but none have been detected in the antennal nerve. These blood feeders rely on the perception of olfactory cues through specific receptors located in antennal sensilla that guide them toward the warm-blooded host. Therefore, the identification of neurotransmitters in antennal nerve fibers is important in order to develop specific pest-control strategies.
PDH-LI distribution pattern
In T. infestans, antibodies generated against β-PDH reacted with a few perikarya located mainly in the PC and the OL. A few immunolabeled somata have also been detected in the DC and SOG. In the fly CNS, antiserum raised against β-PDH from Uca pugilator shows strong specific immunostaining of a few PDH-IR neurons (Nässel et al. 1993). This immunolabeling pattern is thought to represent the expression of a true PDF already sequenced in D. melanogaster (Park and Hall 1998). Furthermore, neurosecretory cells in the brain of insects have been shown to synthesize PDFs that are strongly cross-reactive with crustacean βPDH antisera (Rao 2001).
Variability exists in terms of the presence and number of PDH-IR somata within the heteropterans. Závodská et al. (2003) have described the distribution of this neuropeptide in Gerris palludum and Notonecta glauca, species belonging to the same order as T. infestans. Although there are differences among the three species in terms of habitat and behavior, including feeding preferences, PDH-LI have been observed in all of them in OL perikarya situated proximally to the medulla or close to the anterior part of the lobula. Cell-body clusters located between the medulla and lamina neuropils have not been detected in T. infestans, as reported for Phormia terranovae (Nässel et al. 1991), Manduca sexta (Homberg et al. 1991b), Antheraea pernyi (Sauman and Reppert 1996), D. melanogaster (Helfrich-Förster 1997), and Hierodula membranacea (Sehadová et al. 2003).
In D. melanogaster, the coexpression of specific clock genes with PDF has been observed in a group of OL neurons termed the ventral lateral neurons (LNv; Hall 1998). Moreover, recent studies have provided evidence that PDF acts as a chronobiological signaling substance per se (Helfrich-Förster et al. 2000). The group of PDH-IR somata located at the anterior-most part of the lobula may be considered as homologues to the LNv of D. melanogaster (Meinertzhagen and Pyza 1999). PDF is reported to affect the migration of the screening pigment granules of the photoreceptor terminals (Pyza and Meinertzhagen 1997). The compound eyes of T. infestans have to cope with rapid changes in the intensity of light occurring at dusk and dawn; such changes set the rhythm of the locomotor activity of these insects (Settembrini 1984; Lazzari 1992). Most of the activities related to the survival of these triatomine insects, i.e., orientation to the food source, biting, mating, and oviposition, which in turn are linked to the spread of Chagas’ disease, take place at night. These activities depend on rhythmic changes in eye sensitivity achieved in part by circadian movements in the screening pigments of the photoreceptor and pigment cells (Reisenman et al. 2002). PDH-IR terminals have not been found farther than the outer edge of the medulla. However, β-PDH released from outer medulla terminals may diffuse to the lamina neuropil (Meinertzhagen and Pyza 1996) and therefore affect the migration of the screening pigments.
In T. infestans, PDH-LI has also been detected in cell bodies and fibers of the PC, DC, and SOG. This subpopulation of PDH-IR somata might not express clock genes, as has been previously reported in D. melanogaster (Park et al. 2000), a finding that raises the possibility of additional functions for insect PDFs in this triatomine species. In this context, recent studies in locust abdominal neurons suggest a modulatory role of PDF in the musculature of the genitalia, when this molecule is released into the hemolymph (Persson et al. 2001).
SCPB-LI distribution pattern
The presence of SCPB-LI has been reported in the nervous tissue of higher invertebrate species, a finding that has led to the hypothesis of evolutionary conserved functions for this neuropeptide. Several roles have been proposed for this molecule including the stimulation of cardiac activity (Reich et al. 1997) and feeding behavior (Willows et al. 1988; Perry et al. 1999). Monoclonal antibodies against SCPB label somata and fibers in the brain and SOG of T. infestans. In this species, immunostaining has been observed in typical interneurons and in neurohemal terminals, suggesting that the peptide might function as neurotransmitter or neurohormone.
The colocalization of FMRFa- and SCPB-LIs has been frequently reported (Homberg and Hildebrand 1989; Homberg et al. 1990, 1991a; Davis et al. 1996). Furthermore, SCPB-IR elements are regarded as a subpopulation of those displaying FMRFa-LI. In the lobster Homarus americanus, SCPB antibodies have been suggested to label extended FaRPs (Arbiser and Beltz 1991). In T. infestans, double-labeling has shown the colocalization of immunoreactivity for both FMRFa and SCPB. Moreover, we have observed somata displaying only SCPB-LI and specific immunostaining in areas of the brain and SOG lacking FMRFa-LI. As proposed (Masinovsky et al. 1988), SCPB and FMRFa antisera probably stain different peptides coexisting within the same neuron in T. infestans.
In conclusion, the colocalization of neurochemicals in T. infestans nerve cells has been frequently observed (Villar et al. 1994; Settembrini et al. 2003). The amino acid sequences of most endogenous peptides are unknown in this species and in many other heteropterans. The present study provides a basis for future studies aimed at isolating and analyzing the function of neurotransmitters involved in feeding and digestion.
References
Arbiser ZK, Beltz BS (1991) SCPB and FMRFamide-like immunoreactivities in lobster neurons: colocalization of distinct peptides or colabeling of the same peptide(s)? J Comp Neurol 306:417–424
Benveniste RJ, Taghert PH (1999) Cell type-specific regulatory sequences control expression of the Drosophila FMRF-NH2 neuropeptide gene. J Neurobiol 38:507–520
Breidbach O, Wegerhoff R (1994) FMRFamide-like immunoreactive neurons in the brain of the beetle, Tenebrio molitor L (Coleptera: Tenebrionidae): constancies and variations in development from embryo to the adult. Int J Insect Morphol Embryol 23:383–404
Cazzamali G, Grimmelikhuijzen CJ (2002) Molecular cloning and functional expression of the first insect FMRFamide receptor. Proc Natl Acad Sci U S A 99:12073–12078
Davis NT, Homberg U, Teal PEA, Altstein M, Agricola HJ, Hildebrand JG (1996) Neuroanatomy and immunocytochemistry of the median neuroendocrine cells of the subesophageal ganglion of the tobacco hawkmoth, Manduca sexta: immunoreactivities to PBAN and other neuropeptides. Microsc Res Tech 35:201–229
Duttlinger A, Berry K, Nichols R (2002) The different effects of three Drosophila melanogaster dFMRFamide-containing peptides on crop contraction suggest these structurally related peptides do not play redundant functions in gut. Peptides 23:1953–1957
Duttlinger A, Mispelon M, Nichols R (2003) The structure of the FMRFamide receptor and activity of the cardioexcitatory neuropeptide are conserved in mosquito. Neuropeptides 37:120–126
Duve H, Elia AJ, Orchard I, Johnsen AH, Thorpe A (1993) The effects of calliFMRFamide-related neuropeptides on the activity of the heart of the blowfly Calliphora vomitoria. J Insect Physiol 39:31–40
Elia AJ, Tebrugge VA, Orchard I (1993) The pulsatile appearence of FMRFamide-related peptides in the haemolymph and the loss of FMRFamide-like immunoreactivity from neurohaemal areas of Rhodnius prolixus following a blood meal. J Insect Physiol 39:459–469
Greenberg MJ, Price DA (1992) Relationships among the FMRFamide-like peptides. Prog Brain Res 92:25–37
Guerenstein PG, Guerin PM (2001) Olfactory and behavioural responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. J Exp Biol 204:585–597
Hall JC (1998) Molecular neurogenetics of biological rhythms. J Neurogenet 12:115–181
Harshini S, Nachman RJ, Sreekumar S (2002) In vitro release of digestive enzymes by FMRF amide related neuropeptides and analogues in the lepidopteran insect Opisina arenosella (Walk). Peptides 23:1759–1763
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci U S A 92:612–616
Helfrich-Förster C (1997) Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild type Drosophila melanogaster. J Comp Neurol 337:177–190
Helfrich-Förster C, Tauber M, Park JH, Mühlig-Versen M, Schneuwly S, Hofbauer A (2000) Ectopic expresion of the neuropeptide pigment-dispersing factor alters behavioral rhythms in Drosophila melanogaster. J Neurosci 20:3339–3353
Homberg U, Hildebrand JG (1989) Serotonin immunoreactivity in the optic lobes of the sphinx moth Manduca sexta and colocalization with FMRF amide and SCPB immunoreactivity. J Comp Neurol 288:243–253
Homberg U, Kingan TG, Hildebrand JG (1990) Distribution of FMRFamide-like immunoreactivity in the brain and subesophageal ganglion of the sphinx moth Manduca sexta and colocalization with SCPB-, BPP- and GABA-like immunoreactivity. Cell Tissue Res 259:401–409
Homberg U, Davis NT, Hildebrand JG (1991a) Peptide-immunocytochemistry of neurosecretory cells in the brain and retrocerebral complex of the sphinx moth Manduca sexta. J Comp Neurol 303:35–52
Homberg U, Würden S, Dircksen H, Rao KR (1991b) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Hsu SM, Raind O, Fanger H (1981) Use of avidin biotin peroxidase complex (ABC) in immunoperoxidase technique. A comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Lazzari CR (1992) Circadian organisation of locomotor activity in the haematophagous bug Triatoma infestans. J Insect Physiol 38:895–903
Masinovsky B, Kempf SC, Calloway JC, Willows AOD (1988) Monoclonal antibodies to the molluscan small cardioactive peptide SCPB: immunolabeling in diverse invertebrates. J Comp Neurol 273:500–512
Maule AG, Mousley A, Marks NJ, Day TA, Thompson DP, Geary TG, Halton DW (2002) Neuropeptide signaling systems—potential drug targets for parasite and pest control. Curr Top Med Chem 2:733–753
Meeusen T, Mertens I, Clynen E, Baggerman G, Nichols R, Nachman RJ, Huybrechts R, De Loof A, Schoofs L (2002) Identification in Drosophila melanogaster of the invertebrate G protein-coupled FMRFamide receptor. Proc Nat Acad Sci U S A 99:15363–15368
Meinertzhagen IA, Pyza E (1996) Daily rhythms in cells of the fly’s optic lobe: taking time out of the circadian clock. Trends Neurosci 19:285–291
Meinertzhagen IA, Pyza E (1999) Neurotransmitter regulation of circadian structural changes in the fly’s visual system. Microsc Res Tech 45:96–105
Nambu JR, Murphy-Erdosh C, Arews PC, Feistner GJ, Scheller RH (1988) Isolation and characterization of a Drosophila neuropeptide gene. Neuron 1:55–61
Nässel DR (2002) Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68:1–84
Nässel DR, Ohlsson LG, Johansson KU, Grimmelikhuijzen CJP (1988) Light and electron microscopic immunocytochemistry of neurons in the blowfly optic lobe reacting with antisera to RFamide and FMRFamide. Neuroscience 27:347–362
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-immunoreactive neurons and their relation to serotonergic neurons in the blowfly and cockroach visual system. Cell Tissue Res 66:511–523
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Nichols R, Mc Cormick J, Lim I, Cohen M, Jean C, Howe E, Paisley K, Rosario C (1999) Differential processing of neuropeptides influences Drosophila heart rate. J Neurogenet 13:35–40
O’Brien MA, Taghert PH (1994) The genetic analysis of neuropeptide signaling systems. Zool Sci 11:633–645
Orchard I, Lange AB, Bendena WG (2001) FMRFamide-related peptides: a multifunctional family of structurally related neuropeptides in insects. Adv Insect Physiol 28:267–329
Park JH, Hall JC (1998) Isolation and chronobiological analysis of a neuropeptide-pigment-dispersing factor gene in Drosophila melanogaster. J Biol Rhythms 13:219–228
Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbach M, Hall JC (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A 97:3608–3613
Perry SJ, Dobbins AC, Schofield MG, Piper MR, Benjamin PR (1999) Small cardioactive peptide gene: structure, expression and mass spectrometric analysis reveals a complex pattern of co-transmitters in a snail feeding neuron. Eur J Neurosci 11:655–662
Persson MGS, Eklund MB, Dircksen H, Muren JE, Nässel DR (2001) Pigment-dispersing factor in the locust abdominal ganglia may have roles as circulating neurohormone and central neuromodulator. J Neurobiol 48:19–41
Petri B, Stengl M (1997) Pigment-dispersing hormone phase shifts the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci 17:4087–4093
Predel R, Neupert S, Wicher D, Gundel M, Roth S, Derst C (2004) Unique accumulation of neuropeptides in an insect: FMRFamide-related peptides in the cockroach, Periplaneta americana. Eur J Neurosci 20:1499–1513
Pyza E, Meinertzhagen IA (1997) Circadian rhythms in screening pigment and invaginating organelles in photoreceptor terminals of the housefly’s first optic neuropile. J Neurobiol 32:517–529
Pyza E, Meinertzhagen IA (2003) The regulation of circadian rhythms in the fly’s visual system: involvement of FMRFamide-like neuropeptides and their relationship to pigment-dispersing factor in Musca domestica and Drosophila melanogaster. Neuropeptides 37:277–289
Rao KR (2001) Crustacean pigmentary-effector hormones: chemistry and functions of RPCH, PDH and related peptides. Am Zool 41:364–379
Rao KR, Riehm JP, Zahnov CA, Kleinholz LH, Tarr GE, Johnson L, Norton S, Landau M, Semmes OJ, Sattelberg RM, Jorenby WH, Hintz MF (1985) Characterization of a pigment dispersing hormone in eyestalks of the fiddler crab Uca pugilator. Proc Natl Acad Sci U S A 82:5319–5322
Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnson L, Tarr GR (1987) Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. J Biol Chem 262:2672–2675
Reich G, Doble KE, Greenberg MJ (1997) Protein phosphorylation in snail cardiocytes stimulated with molluscan peptide SCPB. Peptides 18:1311–1314
Reisenman CE, Insausti TC, Lazzari CR (2002) Light-induced and circadian changes in the compound eye of the haematophagous bug Triatoma infestans (Hemiptera: Reduviidae). J Exp Biol 205:201–210
Remy C, Guy J, Pelletier G, Boer HH (1988) Immunohistological demostration of a substance related to neuropeptide Y and FMRFamide in the cephalic and thoracic nervous systems of the locust Locusta migratoria. Cell Tissue Res 254:189–195
Renn SC, Park JH, Roshbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Sauman I, Reppert SM (1996) Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of period protein regulation. Neuron 17:889–900
Schneider LE, Taghert PH (1988) Isolation and characterization of a Drosophila gene that encodes multiple neuropeptides related to Phe-Met-Arg-Phe-NH2 (FMRFamide). Proc Natl Acad Sci U S A 85:1993–1997
Sehadová H, Sauman I, Sehnal F (2003) Immunocytochemical distribution of pigment-dispersing hormone in the cephalic ganglia of polyneopteran insects. Cell Tissue Res 312:113–125
Settembrini BP (1984) Circadian rhythms of locomotor activity in Triatoma infestans (Hemiptera: Reduviidae). J Med Entomol 21:204–212
Settembrini BP, Villar MJ (1999) Proctolin in the brain and ganglia of Triatoma infestans. J Morphol 240:39–47
Settembrini BP, Villar MJ (2004) Distribution of serotonin in the central nervous system of the blood-feeding heteropteran, Triatoma infestans (Heteroptera: Reduviidae). J Morphol 260:21–32
Settembrini BP, Compagnucci L, Villar MJ (2000) Distribution of FMRFamide-like immunoreactivity in the brain of Triatoma infestans. XXI International Congress of Entomology (Abstracts) p 632
Settembrini BP, Davis NT, Villar MJ (2001) Distribution of FMRFamide-like immunoreactivity in the central nervous of Triatoma infestans. Soc Neurosci Abstr 27 Program no. 726.3
Settembrini BP, Nowicki S, Hökfelt T, Villar MJ (2003) Distribution of NPY and npy-Y1 receptor-like immunoreactivities in the central nervous system of Triatoma infestans (Insecta: Heteroptera). J Comp Neurol 460:141–154
Shu S, Ju G, Fan I (1988) The glucose oxidase method in peroxidase histochemistry of the nervous system. Neurosci Lett 85:169–171
Taghert PH (1999) FMRFamide neuropeptides and neuropeptide-associated enzymes in Drosophila. Microsc Res Tech 45:80–95
Taghert PH, Schneider LE (1990) Interespecific comparison of a Drosophila gene encoding FMRFamide-related neuropeptides. J Neurosci 10:1929–1942
Taneja J, Guerrin PM (1995) Oriented responses of the triatomine bugs Rhodnius prolixus and Triatoma infestans to vertebrate odours on a servosphere. J Comp Physiol [A] 176:455–464
Tsang PW, Orchard I (1991) Distribution of FMRFamide-related peptides in the blood-feeding bug, Rhodnius prolixus. J Comp Neurol 311:17–32
Verhaert P, Grimmelikhuijzen CJ, De Loof A (1985) Distinct localization of FMRFamide- and bovine pancreatic polypeptide-like material in the brain, retrocerebral complex and suboesophageal ganglion of the cockroach Periplaneta americana L. Brain Res 348:331–338
Villar MJ, Settembrini BP, Hökfelt T, Tramezzani JH (1994) NOS is present in the brain of Triatoma infestans and is colocalyzed with CCK. NeuroReport 6:81–84
Wall JB, Taghert PH (1991) The timing of initial neuropeptide expression by an identified insect neuron does not depend on interactions with its normal peripheral target. J Neurobiol 22:935–956
WHO (2002) Special Program for Research and Training in Tropical Diseases Report. TDR Strategic Direction: Chagas’ disease. February, pp 1–6
Willows AOD, Lloyd PE, Masinovsky BP (1988) Multiple transmitter neurons in Tritonia hi. Modulation of central pattern generator controlling feeding. J Neurobiol 19:69–86
Závodská R, Šauman I, Sehnal F (2003) Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, eclosion hormone in the cephalic nervous system of insects. J Biol Rhythms 18:106–122
Acknowledgements
We are grateful to P.H. Taghert (Washington University School of Medicine, Saint Louis, Mo.) for his generous donation of the FMRFa antiserum and the peptides used in the preadsorption experiments. We thank K.R. Rao (University of West Florida) and N.T. Davis (Arizona Research Laboratories, Division of Neurobiology, University of Arizona) for their generous gifts of the βPDH and SCPB antisera. The invaluable suggestions and encouragement from N.T. Davis during the course of this study are deeply appreciated. The authors thank Dr. A. Oliva and Dr. F. Coronel for helpful comments on the manuscript, Dr. D. Canale and Dr. A. Stariolo (Center for the Control of Chagas’ disease) for providing the insects, and G. Ruffolo and S. Ruffolo for technical assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was sponsored by the Facultad de Ciencias Biomédicas, Universidad Austral. Part of this work was performed at the Division of Neurobiology, Arizona Research Laboratories (Tucson, Arizona) with the support of a Fulbright Research Award to B.P.S.
Rights and permissions
About this article
Cite this article
Settembrini, B.P., Villar, M.J. FMRFamide-like immunocytochemistry in the brain and subesophageal ganglion of Triatoma infestans (Insecta: Heteroptera). Coexpression with β-pigment-dispersing hormone and small cardioactive peptide B. Cell Tissue Res 321, 299–310 (2005). https://doi.org/10.1007/s00441-005-1147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-1147-z