Abstract
It is well established that most of the human genome and those of other mammals and plants are transcribed into RNA without protein-coding capacity, which we define as non-coding RNA. From siRNA to microRNA, whose functions and features have been well characterized, non-coding RNAs have been a popular topic in life science research over the last decade. Long non-coding RNAs (lncRNAs), however, as a novel class of transcripts, are distinguished from these other small RNAs. Recent studies have revealed a diverse population of lncRNAs with different sizes and functions across different species. These populations are expressed dynamically and act as important regulators in a variety of biological processes, especially in gene expression. Nevertheless, the functions and mechanisms of most lncRNAs remain unclear. In this review, we present recent progress in the identification of lncRNAs, their functions and molecular mechanisms, their roles in human diseases, their potential diagnostic and therapeutic applications as well as newer technologies for identifying deregulated lncRNAs in disease tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of next generation sequencing methods and DNA tiling arrays, it has become possible to sequence the complete human transcriptome. Numerous studies have revealed that the human genome consisted of 20,500 of coded genes, which is only less than 2 % of the genome (Archer et al. 2015). Not surprisingly, at least 90 % of the genome is actively transcribed into non-coding RNAs (ncRNAs), which have no protein coding potentiality (Birney et al. 2007). ncRNAs can be approximately classified into two groups: (1) classical ‘housekeeping’ RNAs, such as rRNAs, tRNAs, small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs), which are constitutively expressed and play critical roles in protein biosynthesis; and (2) ‘regulatory’ RNAs that include small regulatory RNAs (e.g., microRNAs, siRNAs and piRNAs) and long non-coding RNAs (lncRNAs). The functions of most small RNAs have been clarified, however, our understanding of lncRNAs is still obscure.
LncRNAs are defined as all transcripts that are greater than 200 nucleotides in length but lack of protein-coding capacity (Iyer et al. 2015). LncRNAs are mainly transcribed by RNA polymerases (RNAP) II or III in mammal animals, and additionally, by RNAP IV and V in plants (Struhl 2007; Bierhoff et al. 2010). Due to the wide applications of high throughput RNA-sequencing (RNA-seq) approaches, thousands of lncRNAs in many organisms, such as mammal animals and plants, have been identified. For instance, about 6480 lncRNAs were identified from 200 Arabidopsis thaliana transcriptomic data sets, with either organ-specific or stress-induced expression profiles (Liu et al. 2012). Nearly 50,000 annotated lncRNA genes have been discovered in the human genome (Iyer et al. 2015). LncRNAs are processed by splicing or non-splicing, polyadenylation or non-polyadenylation, and can be located in the nucleus or cytoplasm (Wierzbicki 2012), which play cis- or trans-regulators of gene transcription, and are involved in a wide range of biological processes. This review summarizes recent progress in the research of lncRNAs, with an emphasizing on their role in gene expression regulation and human diseases.
Classification of lncRNAs
According to the current literature, there are several different classification methods. LncRNAs can be categorized as sense, antisense, intronic, intergenic and bidirectional lncRNAs based on their genomic proximity to protein coding genes (Ponting et al. 2009; Rinn and Chang 2012). Another classification includes “high abundance” and“low abundance” lncRNAs. Some publications have divided lncRNAs into cis- and trans-acting lncRNAs by function (Wang et al. 2011b). Recently, Di Gesualdo et al. (2014) provided an updated classification of lncRNAs: (1) lncRNAs that are transcribed from loci distinct from sense transcript-encoding gene loci are termed long intergenic non-coding RNAs (lincRNAs); (2) exceptionally long lncRNAs are termed macroRNAs and very long intergenic non-coding RNAs (vlincRNAs); (3) lncRNAs that are bidirectionally transcribed from the enhancer and promoter regions are referred to as enhancer-associated RNAs (eRNAs) and promoter-associated long RNAs (PALRs), respectively; (4) transcripts on the antisense strand can generate natural antisense transcripts (NATs) with varying degrees of overlap, including divergent and convergent NATs.
Functions of lncRNAs
Due to their low sequence conservation between species, lncRNAs were initially thought to be non-functional and even spurious transcriptional noise arising from low RNA polymerase fidelity (Struhl 2007; Bierhoff et al. 2010). In recent years, some functions of lncRNAs have been found, including participating in the formation of RNA–protein complexes or subcellular structures as scaffolds, modulating protein activity and localization by binding with specific proteins, regulating gene expression and controlling genomic imprinting and X-inactivation (Clemson et al. 2009; Kornienko et al. 2013).
lncRNAs act as scaffolds
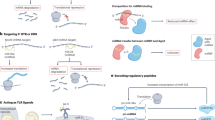
The formation of diverse RNA–protein complexes or subcellular structures is the common function of many lncRNAs, and it is within these structures where lncRNAs carry out scaffolding functions (shown in Fig. 1a). The earliest evidence of this phenomenon came from the discovery of telomerase (Greider and Blackburn 1985).
LncRNAs interact with proteins to function as scaffolds, influence protein activity and modulate protein localization. a LncRNAs can participate in the formation of diverse RNA–protein complexes or subcellular structures, where they carry out scaffolding functions. b LncRNAs can be involved in modulating the activity of proteins. ncRNACCND1 is induced upon DNA damage and enhances both the cleavage of TLS as well as its inhibition of CBP functions, thereby repressing CCND1 transcription. c LncRNAs can influence protein localization: lncRNA NRON binds to the transcription factor NFAT and limits the nuclear-cytoplasmic trafficking of NFAT, ultimate leading to the repression of NFAT target gene expression. POL II, RNA polymerase II; Ca2+, Calcium ion; CaM, calmodulin; Calcineurin, Ca2+∕calmodulin-dependent phosphatase calcineurin
Telomerase is a ribonucleoprotein enzyme that is able to add telomere repeats to the end of the chromosomes and which is expressed in most malignant tumor cells. The telomerase holoenzyme consists of a protein component, a reverse transcriptase termed telomerase reverse transcriptase (TERT) and an RNA primer, also known as telomerase RNA component (TERC) (Feng et al. 1995). TERC act as a template for TERT-catalyzed reverse transcription and as a molecular scaffold for the polymerase enzyme around the RNA (Lingner et al. 1997).
Another example of lncRNA scaffolding is the study of the composition of paraspeckles. Paraspeckles are a relatively new class of subnuclear bodies found in the interchromatin space of mammalian cells. These bodies are critical for the control of gene expression through the nuclear retention of RNA that contains double-stranded regions that have been subject to adenosine-to-inosine editing (A–I editing) (Fox and Lamond 2010). Clemson et al. (2009) have found that the nuclear enriched autosomal transcript 1 (NEAT1) RNA is associated with intense concentrations of PSP1 and P54, which demark paraspeckles in vivo and in vitro (Fox et al. 2002). These authors also proved that paraspeckles may initially form when NEAT1 RNA is transcribed and that their spread throughout the nucleus corresponds with the spread of NEAT1 RNA. Furthermore, an increase in NEAT1 expression causes a parallel increase in paraspeckle number, and knockdown of NEAT1 RNA leads to loss of paraspeckles (Clemson et al. 2009; Fox and Lamond 2010). Taken these results together, it is clear that NEAT1 is essential to the formation of paraspeckles and may function as, but is not necessarily limited to, a scaffold.
lncRNAs modulate protein activity and localization
Evidence is accumulating that lncRNAs, aside from their scaffolding function, are also involved in modulating the activity and localization of protein complexes. Wang et al. (2008) identified a series of single-stranded ncRNA transcripts with low copy number (known as ncRNACCND1) that are induced in response to DNA damage signals and tethered to the 5’ regulatory regions of the cyclin D1 (CCND1) gene. These authors found that ncRNACCND1 serves as a molecular “ligand” for translocation in liposarcoma (TLS), an RNA-binding protein that represses CCND1. ncRNACCND1 interacts with the C-terminus of TLS to enhance the binding and cleavage of TLS to CREB-binding protein (CBP), which can inhibit histone acetylation of the CCND1 promoter and repress its expression (Wang et al. 2008; Guttman and Rinn 2012) (shown in Fig. 1b).
Similarly, the lncRNA non-coding repressor of NFAT (NRON) was found to repress nuclear factor of activated T cells (NFAT), a Ca2+-regulated transcription factor that controls gene expression in many cell types (Sharma et al. 2011) (shown in Fig. 1c). The activation of NFAT protein is regulated by the phosphorylation status of the NFAT regulatory domain, which is necessary and sufficient for nuclear transport (Sharma et al. 2011). Phosphorylated NFAT proteins reside in the cytoplasm of resting cells, and dephosphorylated NFAT protein translocates to the nucleus to activate target gene expression when cells are stimulated by an increase in the intracellular Ca2+ concentration (Sharma et al. 2011). The lincRNA NRON can bind to NFAT proteins together with NFAT kinases and is involved in the maintenance of phosphorylated NFAT proteins (Sharma et al. 2011; Guttman and Rinn 2012). Consequently, the lncRNA NRON renders NFAT proteins inactive and inhibits their trafficking from cytoplasm to nucleus, ultimately leading to inhibition of NFAT target gene expression (Sharma et al. 2011; Guttman and Rinn 2012).
lncRNAs regulate gene expression
The most important function of lncRNAs is their role in regulating gene expression (Gutschner and Diederichs 2012; Ma et al. 2012). This function was confirmed first by loss-of-function studies on most intergenic lncRNAs (lincRNAs) expressed in mouse embryonic stem (ES) cells. This analysis revealed that knockdown of lncRNAs has significant consequences on gene expression patterns (Guttman et al. 2011). As we will show, lncRNAs have been demonstrated to regulate protein-coding gene expression through a variety of mechanisms, including epigenetic modifications, transcription and post-transcriptional processing (Mercer et al. 2009; Kornienko et al. 2013).
Epigenetic modification-dependent pre-transcriptional regulation
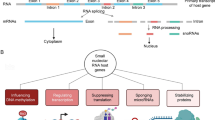
Studies from several research groups have shown that lncRNAs are involved in epigenetic regulations by recruiting chromatin-remodeling complexes, such as PRC1, PRC2, MLL and G9A, to specific locations in the genome (Nagano et al. 2008; Pontier and Gribnau 2011; Wang et al. 2011a) (shown in Fig. 2a).
LncRNAs regulate gene expression by multiple mechanisms. a LncRNAs can recruit chromatin-modifying complexes to specific genomic loci to regulate target gene expression. b LncRNAs influence the general output of mRNAs by directly affecting the loading and activity of RNAPII (middle panel) or general transcription factors (left panel). In addition, lncRNAs can act as co-factors or inhibitors to regulate the activity of a particular transcription factor (right panel). The right panel shows that the lncRNA Evf2 acts as a co-activator of the transcription factor DLX2 to regulate DLX5 and DLX6 gene transcription. c Antisense lncRNAs can mediate the alternative splicing of mRNA by forming RNA duplexes. LncRNA Zeb2 NAT can overlap the 5′splice site in an intron located in the 5′-untranslated region (UTR) of Zeb2 mRNA and prevent the splicing of the intron, causing an up-regulation of Zeb2 protein without significantly altering the level of Zeb2 mRNA
HOTTIP lncRNA is expressed in the HOXA cluster, whose higher-order structure is dependent on positional identity (Wang et al. 2011a; Kornienko et al. 2013). Wang et al. (2011a) have demonstrated a looped conformation within the 5′ HOXA locus and a linear conformation within the 3′ HOXA locus in anatomically distal cells, which is diametrically opposite to the pattern in anatomically proximal cells. Their research showed that chromosomal looping brings the HOTTIP gene into close proximity to the 5′ HOXA genes. Then, the HOTTIP gene produces HOTTIP lncRNA, which directly binds the adaptor protein WDR5 and targets the WDR5/MLL histone-modifying complexes to the 5′ HOXA locus, driving histone H3 lysine 4 trimethylation and transcription activation.
Except for HOTTIP,which acts in cis-regulation, there are many other lncRNAs regulated gene expression in trans, such as HOTAIR (Rinn et al. 2007). HOTAIR is transcribed from the HOXC locus and represses transcription in trans across 40 kb of the HOXD cluster by recruiting the PRC2 complexes (Rinn et al. 2007). The epigenetic regulatory-mechanism mediated by lncRNA may explain the question of how chromatin-remodeling complexes gain locus specificity (Mercer et al. 2009; Kornienko et al. 2013).
Recent evidence rasied the possibility that lncRNAs may interact with target genes through formation of RNA–DNA triplex structures, serving as a link between chromatin and chromatin modifiers. Very recently, Mondal et al. (2015) have demonstrated that lncRNA MEG3 regulates the TGF-β pathway genes through RNA–DNA triplex formation. Genome-wide mapping of MEG3 binding sites reveals that MEG3 modulates the activity of TGF-β pathway genes by binding to distal regulatory elements containing GA-rich sequences, which guide MEG3 to the chromatin through RNA–DNA triplex formation, and then contributing to PCR2 recruitment. O’Leary et al. (2015) named a new lncRNA PARTICLE (gene PARTICLE, promoter of MAT2A-antisense radiation-induced circulating lncRNA), which can forms a DNA-lncRNA triplex upstream of a MAT2A promoter CpG island and interacts with the transcription-repressive complex proteins G9a and SUZ12 (subunit of PRC2) to repress MAT2A via methylation (Yang et al. 2001). These findings implicate lncRNA play as a recruitment platform for gene-silencing machineries by RNA–DNA triplex formation.
Transcriptional regulation
As the first critical step in gene expression, the transcription of protein-coding genes into mRNA is an intricate biological process that consists of multiple steps, from the formation of the preinitiation complex to transcript elongation (Espinoza et al. 2004; Yakovchuk et al. 2009). Each step in the transcription reaction has the potential to be regulated by trans-acting activators and repressors (Espinoza et al. 2004; Mariner et al. 2008; Yakovchuk et al. 2009, 2011). It is presently becoming clear that ncRNAs also play key roles in regulating the process of transcription. Diverse lncRNAs have been identified as regulators of nearly every step in the process of transcription and act through different mechanisms (Yakovchuk et al. 2009).
In eukaryotes, RNAP II transcribes protein-coding genes into mRNA in collaboration with general transcription factors (Espinoza et al. 2004; Mariner et al. 2008; Yakovchuk et al. 2009, 2011). Some lncRNAs have been reported to influence the general output of mRNAs by directly affecting the loading and activity of RNAP II or general transcription factors (shown in Fig. 2b). As an example, Alu RNA in human (Mariner et al. 2008; Yakovchuk et al. 2009) and B2 RNA in mouse (Espinoza et al. 2004; Yakovchuk et al. 2009) have been shown to function as general repressors of mRNA transcription and are transcribed by RNAP III from short interspersed elements (SINEs) (Yakovchuk et al. 2009). The levels of Alu and B2 RNA increase in response to heat shock, at which point they bind directly to RNAP II to assemble into preinitiation complexes at the promoter. This interaction disrupts important contacts between RNAP II and the promoter DNA, blocking RNAP II-dependent transcription (Espinoza et al. 2004; Mariner et al. 2008; Yakovchuk et al. 2009, 2011). Another lncRNA, 7SK, is transcribed by RNAP III and has been shown to regulate RNAP II-dependent transcription elongation (Yik et al. 2003; Chen et al. 2004; Peterlin et al. 2012). 7SK lncRNA binds to phosphorylated PTEFb (Chen et al. 2004) and serves as a scaffold to mediate the interaction of HEXIM1 and PTEFb. In this interaction, HEXIM1 inhibits the CTD-kinase activity of PTEFb (Yik et al. 2003; Chen et al. 2004; Peterlin et al. 2012), thereby preventing the phosphorylation of RNAP II and blocking transcription elongation (Yik et al. 2003; Chen et al. 2004; Peterlin et al. 2012). In contrast, stress-inducing agents, such as ultraviolet irradiation and actinomycin D, can dissociate HEXIM1 and 7SK from PTEFb, leading to an increase in nuclear levels of active PTEFb, an effect that may activate stress-induced gene expression (Yik et al. 2003; Chen et al. 2004; Peterlin et al. 2012).
LncRNAs also act as co-factors or inhibitors to regulate the activity of a particular transcription factor. The lncRNA Evf2 is transcribed from the Ei region, one of the two DLX-5/6 ultraconserved intergenic enhancer regions (Feng et al. 2006). Evf2 specifically binds to the homeodomain protein DLX-2 and recruits DLX-2 to this same enhancer, resulting in an induction of both DLX-5 and -6 expression. This result indicates that Evf2 lncRNA acts as a co-factor of DLX-2 (Feng et al. 2006) (shown in Fig. 2b). Unlike Evf2, NRON lncRNA, as mentioned earlier, binds to the NFAT transcription factor and renders it inactive by preventing its nuclear accumulation, inhibiting the expression of target genes (Sharma et al. 2011).
Post-transcriptional regulation
Another class of ncRNAs, referred to as NATs, are derived from the anti-sense strand of many protein-coding genes (Beltran et al. 2008). The ubiquitous NATs in the human transcriptome and their ability to bind with complementary sequences suggest that they may play a key role in gene expression regulation, especially in the post-transcriptional processing of mRNA, including mRNA editing, splicing, transport, translation and degradation (Beltran et al. 2008; Annilo et al. 2009).
Antisense lncRNAs can mediate alternative splicing of mRNA by forming RNA duplexes. One of these ncRNAs, Zeb2 (zinc finger E-box binding homeobox 2) NAT, overlaps the 5′ splice site in an intron located in the 5′-untranslated region (UTR) of Zeb2 mRNA. Zeb2 NAT can bind Zeb2 mRNA through complementary base-pairing and prevent the splicing processing of the intron. This event results in the up-regulation of Zeb2 protein without significantly altering the level of Zeb2 mRNA (Beltran et al. 2008) (shown in Fig. 2c). Another study has shown that the natriuretic peptide precursor A (NPPA) also has an antisense partner, NPPA-AS. The NPPA gene codes for a precursor of atrial natriuretic peptide (ANP), which protects the cardiovascular system from volume and pressure overload. NPPA-AS lncRNA can bind to NPPA, generating RNA duplexes and displaying a complex pattern of alternative splicing in vivo. This process reduces the levels of the intron-retained NPPA mRNA isoform (Annilo et al. 2009).
lncRNAs in imprinting and X chromosome inactivation (XCI)
Genomic imprinting is an epigenetic phenomenon resulting in a monoallelic, parental-specific expression pattern and an excellent model for understanding how lncRNAs regulate transcriptional gene silencing in cis. Although imprinted genes accounts for only a few of the human genome (perhaps less than 1 % of known genes), they paly important roles in growth and development, particularly in higher-order brain processes, such as learning and behavior (Davies et al. 2005; Wilkinson et al. 2007; Bartolomei and Ferguson-Smith 2011). Imprinting is established in cis primarily by DNA methylation marks on imprinting control regions (ICRs) in the gametes (Abramowitz and Bartolomei 2012). It has been demonstrated that most imprinted genes are located in clusters and all the well-characterized imprinted clusters contain at least one lncRNA as their partners (Sleutels and Barlow 2002; Mohammad et al. 2009). For the most part, the imprinted genes in each cluster are expressed from the same parental chromosome, whereas the lncRNA is expressed from the other parental chromosome (shown in Fig. 3a) (Barlow and Bartolomei 2014). Generally, lncRNAs functionally implicated in the parental-specific expression of genes can be classified into antisense lncRNAs (Kcnq1ot1, Airn, Nespas, Ube3a-ATS), intergenic lncRNAs (H19, IPW and MEG3 lncRNA) and enhancer RNAs (IG-DMR eRNA) (Barlow and Bartolomei 2014). These imprinted lncRNAs employ diverse molecular mechanisms to control epigenetically regulated transcription across imprinted clusters. Antisense lncRNAs epigenetically regulate expression of multiple genes in imprinted clusters by interacting with and recruiting chromatin-remodeling complexes in a sequence-specific fashion (Kanduri 2015). Enhancer RNAs can regulate genomic imprinting by promoting higher-order chromatin establishment, thereby enabling early replication and inner subnuclear positioning of target locus (Darrow and Chadwick 2013). As for intergenic lncRNAs, it seems that there is no common mechanism being employed by them. For example, IPW, a lncRNA in the critical region of the Prader-Willi syndrome (PWS) locus, may promote a cross-talk between two imprinted clusters through regulating the chromatin structure of an imprinting control region (Stelzer et al. 2014; Kanduri 2015). However, the methylation status of H19 promoter can regulate IGF2 expression through affecting the chromatin insulator CTCF binding to the ICR of the cluster (Delaval and Feil 2004).
LncRNAs are involved in genomic imprinting and X chromosome inactivation (XCI). a Most imprinted genes (IG) are located in clusters and contain multiple protein-coding genes and at least one lncRNA (IG-lncRNA). Nonimprinted genes (NG) can also be present. Imprinting is established in cis primarily by DNA methylation (Me) marks on imprinting control regions (ICRs) in the gametes. One pair of diploid chromosomes is shown: the pink is of maternal origin and the gray of paternal origin. b In female mammalian cells, one of the two X chromosomes is randomly silenced. At around the implantation stage of early embryogenesis, both X chromosomes express the Tsix lncRNA, which negatively regulates its antisense transcript, Xist. On the future active X chromosome (Xa), TSIX continue being expressed, and the other allele on the future inactive X chromosome (Xi) is silenced. As a result, XIST is de-repressed on the Xi. The Xist lncRNA coats the X chromosome in cis and recruits chromatin-remodeling complexes PRC2, which trimethylates lysine 27 on histone H3 (H3k27me3) and induces the formation of heterochromatin, ultimately leading to X chromosome inactivation. X represents most genes on the X chromosome outside of the XIC (color figure online)
In mammals, females possess two X chromosomes, however, males have one X and one Y. Thus, a dosage equalization mechanism is necessary for most X-linked genes. XCI occurs randomly in female post-implantation embryonic somatic cells (Autuoro et al. 2014). The molecular mechanisms of XCI are still obscure, but several lncRNAs have been identified from a 500 kb stretch of DNA at Xq13 known as the X-inactivation center (XIC) (Kanduri 2011). LncRNA X-inactive specific transcript (Xist) and its antisense transcript Tsix are of key importance. Xist lncRNA is expressed exclusively from the XIST gene on the inactive X chromosome and is spliced and polyadenylated (Goto and Monk 1998; Pontier and Gribnau 2011). The Xist lncRNA coats the X chromosome in cis and recruits chromatin-remodeling complexes PRC2, which trimethylates lysine 27 on histone H3 (H3k27me3) and induces the formation of heterochromatin, ultimately leading to X chromosome inactivation (Brockdorff 2011; Pontier and Gribnau 2011). Meanwhile, the function of Xist is antagonized by the antisense transcript Tsix lncRNA, which is transcribed from the active X chromosome (Pontier and Gribnau 2011). Tsix may inhibit XIST expression by transcriptional interference (Sado et al. 2006) or by recruiting chromatin-remodeling complexes to the XIST promoter (Sun et al. 2006). However, another study suggests that Xist and Tsix can form a duplex RNA that is then processed by the RNA interference pathway to generate siRNAs required for depositing chromatin-remodeling complexes (Ogawa et al. 2008; Pontier and Gribnau 2011).
The roles of lncRNAs in complex human diseases
There is increasing interest in the potential role of lncRNAs in the pathogenesis of many human diseases. Moreover, lncRNAs also act as diagnostic or prognostic biomarkers and attractive therapeutic targets for a variety of human conditions, such as cancers, cardiovascular diseases, autoimmune diseases and neurodegenerative disorders.
lncRNAs involved in cancers
A larger number of literatures find that aberrant expressed lncRNAs were involved in the etiology of cancers and tumor metastasis (Serviss et al. 2014). Besides H19, HOTAIR, ANRIL, MALAT1, MEG3,PCGEM1 and PCAT-1, which have been extensively reviewed previously (Maruyama and Suzuki 2012), many newer lncRNAs were found to play a critical regulatory role in the pathogenesis of cancer in recent years (Poliseno et al. 2010; Chen et al. 2015a, b; Nie et al. 2015; Yang et al. 2015a) (Summarized in Table 1).
More recently, the lncRNA PVT1 has been shown to be dysregulated in several cancers, such as hepatocellular carcinoma (HCC) (Wang et al. 2014a), non-small cell lung cancer (NSCLC) (Yang et al. 2014), pancreatic cancer (Huang et al. 2015), gastric cancer (Ding et al. 2014) and acute promyelocytic leukemia (APL) (Zeng et al. 2015). Wang et al. (Wang et al. 2014a) have found that PVT1 was upregulated in HCC and promoted cell proliferation, cell cycling, and stem cell-like properties of HCC cells by enhancing the stability of NOP2, a RNA-binding protein that binds to PVT1. Another study reported that elevated PVT1 expression in APL may result from gain of supernumerary copies of the 8q24 chromosomal region and MYC protein activation in APL cells, which correlated with leukemic cell proliferation (Zeng et al. 2015). The novel lncRNA prostate cancer non-coding RNA 1 (PRNCR1), which has been demonstrated to be associated with prostate cancer susceptibility (Chung et al. 2011; Yang et al. 2013a), is also located in the susceptible genomic area of colorectal cancer (CRC). A latest study has suggested that PRNCR1 was significantly overexpressed in CRC tissues compared with adjacent tissues, which associated with large tumor volume (Yang et al. 2016), Further study found that knockdown of PRNCR1 by a specific antisense oligonucleotide (ASO) induced cell cycle arrest in the G0/G1 phase and a significant decrease in the proportion of cells in the S phases, but did not affect cell apoptosis or invasive ability.
LINC00152 was documented as an important lncRNA participated in cell cycle arrest, apoptosis, epithelial-mesenchymal transition (EMT), cell migration and invasion in gastric cancer (Zhao et al. 2015). Further exploring found that LINC00152 can also exist in blood stably because of its protected by exosomes, indicating that it may act as a circulating biomarker for gastric cancer (Li et al. 2015b). More recently, Zhou et al. (2015) have confirmed that cytoplasmic LINC00152 could directly bind with epidermal growth factor receptor (EGFR) which caused a constitutive expression of EGFR and an activation of PI3K/AKT signaling, then promoting tumor growth. Another study has demonstrated that hypomethylated LINC00152 was upregulated in HCC and induced cell proliferation as well as tumor growth by binding to the promoter of epithelial cell adhesion molecule (EpCAM) through a cis-regulation and then activating the mechanistic target of rapamycin (mTOR) pathway (Ji et al. 2015).
Taurine-up-regulated gene 1 (TUG1), a 7.1-kb lncRNA, is generally down-regulated in NSCLC. Decreased TUG1 lncRNA expression was also found to be associated with a higher tumor (T), lymph node (N), metastasis (M) stage and tumor size, as well as poorer overall survival (Zhang et al. 2014). Further experiments revealed that TUG1, as a direct transcriptional target of P53, could epigenetically represses the expression of HOXB7 (a known oncogene) by binding to PRC2, thus inhibiting cell proliferation in NSCLC. Together, these results suggested that P53-regulated TUG1 lncRNA is a growth regulator that acts partly by controlling HOXB7. In addition, the P53/TUG1/PRC2/HOXB7 network might serve as targets for NSCLC diagnosis and therapy.
Recently, Wu et al. (2015) have demonstrated that a lncRNA TCF7 was induced by IL-6 in a time- and dose-dependent manner through activating STAT3, which was essential for IL-6 induced epithelial-mesenchymal transition (EMT) process, invasion and mobility of HCC cells. LncRNA urothelial cancer-associated 1 (UCA1) was reported to be involved in several type of tumors, including bladder cancer (Li et al. 2014c), tongue squamous cell carcinoma (TSCC) (Fang et al. 2014), melanoma (Tian et al. 2014), esophageal squamous cell carcinoma (ESCC) (Li et al. 2014a), gastric cancer (Zheng et al. 2015), HCC (Wang et al. 2015a), NSCLC (Wang et al. 2015c) and ovarian cancer (Wang et al. 2015b), mostly by regulating cellular migration and invasion. Recently Xue et al. (2015) have discovered that UCA1 induced EMT of bladder cancer cells by increasing the expression of ZEB1/2, and regulated bladder cancer cell migration and invasion by hsa-miR-145 and its target gene the FSCN1.
It has been suggested that interactions of tumor cells in microenvironment are recognized as a critical determinant of the carcinogenesis and promote the invasion and metastasis of tumors (Bissell et al. 2002). Exosomes, as intercellular communication tools originating from endosomal compartments called multivesicular bodies (MVBs), have recently been reported to be important carriers for some lncRNAs that play roles in tumor progression (Wang et al. 2014b; Isin et al. 2015). For example, a recent study has found that intercellular transfer of lncRNA TUC399, which was functionally implicated in modulating tumor cell growth and adhesion, can be mediated by HCC cells-derived exosomes (Kogure et al. 2013). The exosomes-mediated intercellular transfer of TUC399 may be a mechanism by which tumor cells can modulate their local cellular environment. Taken together, functionally active lncRNA molecules can be transferred by exosomes, enabling cells to exert genetic influences on other cells within the microenvironment.
lncRNAs involved in cardiovascular diseases
It is becoming more evident that lncRNAs play important roles in cardiovascular development and diseases. Two recent reports have demonstrated that the two lncRNAs Fendrr and Braveheart (Bvht) are involved in heart development (Grote et al. 2013; Klattenhoff et al. 2013). With the application of high-throughput sequencing techniques, some lncRNAs were found to be master regulators in the pathogenesis of human heart diseases, including myocardial infarction, cardiomyopathy, heart failure, and atherosclerosis (summarized in Table 2).
lncRNAs in myocardial infarction
Ishii et al. (2006) identified and named the lncRNA myocardial infarction associated transcript (MIAT), which might confer a genetic risk of myocardial infarction (MI). The MIAT gene has six SNPs, and the minor variant of one SNP (A11741G) caused a 1.3-fold increase in MIAT lncRNA transcriptional level in vitro, with the normal A allele exhibiting tighter binding to an unidentified nuclear factor (Ishii et al. 2006; Schonrock et al. 2012). According to the results of this study, it is possible that the altered expression of MIAT lncRNA due to this SNP may play a role in MI pathogenesis.
Furthermore, a microarray-based study identified 20 up-regulated lncRNAs and ten down-regulated lncRNAs following MI in mice, among which two specific lncRNAs, MIRT1 and MIRT2, were significantly up-regulated by fivefold and 13-fold, respectively. MIRT1 and MIRT2 were associated with genes involved in left ventricular remodeling (Zangrando et al. 2014).
lncRNAs in ischemia/reperfusion injury
LncRNAs were also studied in the pathology of cardiac ischemia/reperfusion (I/R). Liu et al. (2014) analyzed the expression changes of lncRNAs in the early stage of reperfusion in the mouse infarct region. After ischemia, 64 lncRNAs were up-regulated and 87 lncRNAs were down-regulated among total 31,423 lncRNAs. Specifically, further analysis by the co-expression modules indicated that a down-regulated lncRNA uc007prv.1 was connected to a tumor necrosis factor-alpha-inducible gene, encoding A20 (TNFAIP3) which was reported to be involved in I/R (Yu et al. 2011). PHB2, as a subunit of the prohibition complex, has an important role in inhibiting mitochondrial fission and apoptosis and is abundantly expressed in cardiomyocytes under physiological conditions. Recently, a study reported that the lncRNA cardiac apoptosis-related lncRNA (CARL) can act as an endogenous sponge of miR-539 that targets PHB2 (Wang et al. 2014d). CARL can impair miR-539-dependent PHB2 down-regulation by directly binding to miR-539 and inhibiting its expression upon I/R. The ultimate result in a reduction in mitochondrial fission, apoptosis and infract sizes.
lncRNAs in cardiac hypertrophy and heart failure
Clusters of lncRNAs have been associated with cardiac hypertrophy. Recently, Pei et al. (Han et al. 2014) identified a cluster of lncRNA transcripts from the myosin heavychain 7 (MYH7) loci, which they termed myosin heavy-chain-associated RNA transcripts (Myheart, or Mhrt). These authors demonstrated a reciprocal Mhrt-Brg1 inhibition feedback circuit that is crucial for heart function. Brg1, a chromatin-remodeling factor that is activated by pathological stress, can repress MYH6 and MHRT in sense and antisense directions in the heart by occupying their promoter regions. This repression causes a pathological switch of MYH6/7 expression, contributing to cardiomyopathy. Interestingly, this stress/Brg1-dependent MYH switch can be largely eliminated by restoring Mhrt lncRNA to pre-stress levels. In this context, Mhrt lncRNA sequesters Brg1 from its genomic DNA targets by competitive binding to the helicase domain of Brg1, a domain with a dual-binding feature and which is crucial for Brg1′s tethering to chromatinized DNA targets. The studies of these authors thereby identified a cardioprotective lncRNA and established a new paradigm for lncRNA-chromatin interactions. Another research has identified a lncRNA termed cardiac hypertrophy related factor (CHRF), which regulates myeloid differentiation primary response gene 88 (MYD88) expression and consequent cardiac hypertrophy by repressing miR-489 expression (Wang et al. 2014c).
lncRNAs in coronary heart diseases
Apolipoprotein A-1 (APOA-1), which plays an important role in cholesterol efflux, is the major protein component of high-density lipoprotein (HDL) in plasma (Fatemi et al. 2014). Halley et al. (2014) have identified an endogenously expressed lncRNA, APOA1-AS, that can negatively regulate APOA1 through the recruitment of chromatin-modifying complexe. These authors found that knockdown of APOA1-AS up-regulated APOA1 both in vitro and in vivo, with increased active H3K4met3 marks and decreased repressive H3K27met3 in the promoter and enhancer regions of the APOA1 gene. The knockdown of APOA1-AS caused a reduction in the chromatin binding of lysine (K)-specific demethylase 1 (LSD1) and suppressor of zeste 12 (SUZ12) protein, a key component of the PRC2 complex. This study suggested that targeting APOA1-AS may have therapeutic potential in protecting against coronary heart disease. Similarly, a mouse study found that oxidized low-density lipoproteins (LDLs) stimulated an lncRNA called DYN-LRB2-2, which promoted cholesterol efflux mediated by a transmembrane transport protein functions to transport cellular cholesterols to its corresponding apolipoproteins (ABCA1) and up-regulated G protein-coupled receptor 119 (GPR119), which regulated atherosclerotic plaque formation by decreasing cellular cholesterol levels and inflammation (Hu et al. 2014).
Another lncRNA associated with coronary heart disease is lincRNA-p21, which was observed to be down-regulated in patients with coronary artery disease. In a recent study, lincRNA-p21 was identified as a key regulator of cell proliferation and apoptosis during atherosclerosis (Wu et al. 2014). The authors showed that lincRNA-p21 repressed cell proliferation, neointima formation and induced apoptosis by directly binding to mouse double minute 2 (Mdm2). This binding leads to P53 release from Mdm2 and its binding to P300, thereby enhancing P53 activity.
lncRNAs and autoimmune diseases
In recent years, a few of lncRNAs have been identified in the context of autoimmune diseases, suggesting that lncRNAs may be involved in the pathogenesis of these conditions (Table 3).
lncRNAs in systemic lupus erythematosus (SLE)
SLE is a chronic systemic autoimmune disease characterized by the production of multiple autoantibodies, complement activation and immune-complex deposition, resulting in widespread tissue damage (Zhao et al. 2010). Recently, a lncRNA GAS5 has been linked with increased susceptibility of SLE in mouse models, presumably as a result of its effect on the immunosuppressant role of glucocorticoids (Kino et al. 2010). Since suppressed GAS5 may inhibit cell cycle and apoptosis, it is implicated in autoimmune diseases by leading promotion antigen exposure and production of autoantibodies (Coccia et al. 1992; Haywood et al. 2006).
lncRNAs in rheumatoid arthritis (RA)
RA is a systemic autoimmune disorder characterized by chronic inflammation of synovial tissue (ST) that results in irreversible destruction of small to medium size joints (Smolen et al. 2007). LncRNA H19, as discussed above, may act as both a tumor suppressor and oncofetal gene. More interestingly, a previous study has reported that H19 lncRNA may also play an important role in RA (Stuhlmuller et al. 2003). Stuhlmuller et al. found that H19 lncRNA was significantly overexpressed in ST of RA patients relative to normal/joint trauma controls. Moreover, H19 lncRNA exhibited increased sensitivity to starvation/cytokine regulation in RA (Stuhlmuller et al. 2003).
Recently, Muller et al. (2014) have found that lincRNA (used total number, 7.419 lincRNA) was regulated by TNFα and superior to IL-6 in CD14 monocytes in human subjects with RA. These cytokines have a specific correlation with lincRNA transcription. Therefore, the interregulation of lincRNA may be important intracellular molecular effectors of different cytokines in cells of innate immune system in the context of RA.
lncRNAs in psoriasis
A previous study reported that the lncRNA Psoriasis susceptibility-related RNA Gene induced by stress (PRINS) is essential in the survival of keratinocytes under stress conditions and may contribute to psoriasis susceptibility (Sonkoly et al. 2005). Recently, Tsoi et al. (2015) identified and characterized the expressed lncRNAs in lesional psoriatic, uninvolved psoriatic and normal skin biopsies by RNA-seq. These authors detected 2942 previously annotated and 1080 novel lncRNAs that are expected to be skin specific. Notably, over 40 % of the novel lncRNAs, such as lncRNAs G2608, G25746, and G36220 (a differentially expressed lncRNA (ENSG00000237499) in psoriatic skin and located within a psoriasis susceptibility locus TNFAIP3), are differentially expressed in psoriatic lesions versus uninvolved or normal skin. This study implicates many lncRNAs in the immunopathogenesis of psoriasis and provides a resource for lncRNA studies in other autoimmune diseases.
lncRNAs in autoimmune thyroid disease (AITD)
AITD, including Graves disease (GD) and Hashimoto’s thyroiditis (HT), is caused by an autoimmune response to selfthyroid antigens and has a significant genetic component. LncRNA SAS-ZFAT (small antisense transcript of ZFAT) is a CD19+B cell-specific antisense transcript. Shirasawa et al. (2004) discovered that the T allele of SNP Ex9b-SNP10, located in intron 9 of ZFAT and the promoter region of SAS-ZFAT, is associated with an increased risk for AITD. Further studies demonstrated that an unknown nuclear factor binds more tightly to the Ex9b-SNP10-T-associated ZFAT-allele. This binding results in the repression of SAS-ZFAT expression, which inversely affects the expression level of its sense counterpart, truncated ZFAT.
lncRNAs in other autoimmune diseases
Li et al. (2014b) have identified a lncRNA named TNFα and hnRNPL related immunoregulatory LincRNA (THRIL), which bound specifically to heterogenous nuclear ribonucleoprotein L (hnRNPL) and formed a functional THRIL–hnRNPL complex that regulated basal and stimulated transcription of TNFα gene by binding to its promoter. In activated cells, high levels of TNFα secretion initiated a negative feedback loop in which THRIL and, in turn, TNFα expression is down-regulated. Interestingly, they found that THRIL expression was clearly lower during the acute phase of Kawasaki disease during which TNFα levels were elevated. This effect mirrored the negative feedback loop of THRIL regulation demonstrated in previous vitro experiments and suggested that THRIL could be a novel biomarker for immune activation.
lncRNAs involved in neurological diseases
The human brain is one of the most complex biological systems, and recent studies have emphasized an important role for lncRNAs in the development and function of the nervous system, highlighting the functional importance of this subclass of non-coding RNAs in neurological diseases (Table 4).
lncRNAs and neurodegeneration
In a study of Alzheimer’s disease (AD), Faghihi et al. (2008) identified a conserved noncoding antisense transcript for β-secretase-1 (BACE1), a crucial enzyme for the accumulation of amyloid-β 1–42 (Aβ 1–42) that plays a key role in the pathogenesis of Alzheimer’s disease. The antisense transcript (lncRNA BACE1-AS) rapidly and reversibly up-regulates BACE1 levels by increasing BACE1 mRNA stability in response to a variety of cell stresses, including Aβ1–42 exposure; this effect results in additional Aβ1–42 production. Subsequently, increased Aβ1–42 levels further increase BACE1-AS expression through a post-transcriptional feed-forward mechanism (Faghihi et al. 2008). In addition, Faghihi et al. also found that treatment with BACE1-AS siRNA can abolish stress-induced increases in BACE1 expression without disturbing physiologically essential expression levels. Consequently, the authors proposed that BACE1-AS may be a perfect biomarker and therapeutic target in Alzheimer’s disease (Faghihi et al. 2008). Another lncRNA that was found to be dysregulated in AD was brain cytoplasmic 200 (BC200) RNA, a translational regulator that is selectively targeted to the somatodendritic domains of neurons (Mus et al. 2007). BC200 levels were found to be up-regulated in brain areas that are affected in AD, such as Brodmann’s area 9 and the hippocampus. Relative BC200 levels in those areas increased in parallel with disease severity. Moreover, in more advanced stages of AD, BC200 was mislocalized and clustered in the perikaryon. These observations suggested a potential role of this dendritic lncRNA in the synaptodendritic deterioration that occurs in AD.
Spinocerebellar ataxia type 8 (SCA8) is characterized by bidirectional transcription of the CTG/GAG expansion repeat, which produces both (1) a lncRNA transcript containing a CUG expansion (CUGexp), ATXN8OS; and (2) a polyglutamine expansion protein encoded by the ATXN8 CAG expansion (CAGexp) transcript, which is transcribed in the opposite direction (Moseley et al. 2006). A recent study found that the expanded ATXN8OS transcripts accumulated as ribonuclear inclusions that co-localized with the splicing factor MBNL1 in a subset of neurons in SCA8 patients and mice (Daughters et al. 2009). In addition, ATXN8OS transcripts induced splicing changes and increased the expression of a CUGBP1-MBNL1-regulated CNS target, GABA-A transporter 4 (Gabt4). This transporter is associated with the loss of GAB Aergic inhibition within the granular cell layer (Daughters et al. 2009). Together, these results suggested that the expanded ATXN8OS transcripts can alter the activities of alternative splicing factors through an RNA gain-of-function mechanism, contributing to SCA8 pathogenesis.
To elucidate the lncRNA network changes in Huntington’s disease (HD), Johnson (2012) have mined existing microarray data (Hodges et al. 2006) to identify seven new lncRNAs that were dysregulated in HD brains. Three of them were novel, among which LINC00341 and RPS20P22 were elevated in HD compared to controls, whereas LINC00342 was decreased. The four known lncRNAs changed significantly in the brains of HD patients including TUG1 and NEAT1 (which were upregulated in HD) and MEG3 and DGCR5 (which were downregulated). Interestingly, several of these lncRNAs contained genomic binding sites for RE1 Silencing transcription factor (REST), a key mediator of transcriptional changes in HD, including the known REST target lncRNA, DGCR5. Similarly, a separate study showed that the HAR1 lncRNA locus (including both HAR1F and HAR1R transcripts) was a direct target of REST, and that the expression of HAR1 lncRNA was significantly reduced in the brains of HD patients (Johnson et al. 2010).
lncRNAs and neurodevelopmental/neuropsychiatric disorders
Fragile X syndrome (FXS) is caused by the expansion of CGG trinucleotide repeats in the 5′UTR of the fragile X mental retardation 1 (FMR1) gene, which encodes the crucial neuronal development protein FMRP. The expansion of CGG repeats to above 200 leads to promoter methylation and consequently to the repression or silencing of the FMR1 gene (Garber et al. 2006; Khalil et al. 2008). A recent study identified the lncRNA FMR4, the transcription of which is initiated upstream and likely shares a bidirectional promoter with FMR1 (Khalil et al. 2008). The researchers found that FMR4 lncRNA was silenced in Fragile X patients. In addition, FMR4 lncRNA was determined to markedly affect cell survival given that its overexpression increased cell proliferation while its down-regulation induced apoptosis. Thus, the silencing of FMR4 lncRNA in the neurons of Fragile X patients might contribute to the pathogenesis of this disease.
Schizophrenia (SZ) is a complex disease that has been associated with alternative splicing (Morikawa and Manabe 2010). Recently, Barry et al. (2014) demonstrated that the lncRNA Gomafu (also known as MIAT and RNCR2), which localized to a novel nuclear compartment consisting of pre-mRNA splicing factors, was involved in alternative splicing of the SZ pathology-related genes DISC1 (disrupted in schizophrenia 1) and 4ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog). These studies functionally link activity-regulated lncRNAs and alternative splicing in neuronal function and open up new avenues for potential SZ therapies that target lncRNA (Barry et al. 2014).
Autism spectrum disorder (ASD), whose pathophysiology is poorly understood, refers to a heterogeneous group of neurodevelopmental disorders characterized by defects in social interactions, language, and behavioral flexibility (Geschwind 2008). A GWAS conducted by Kerin et al. (2012) identified a SNP associated with ASD in a gene-poor region of chromosome 5p14.1. This locus was found to encode a 3.9 kb lncRNA (named MSNP1AS) antisense to moesin pseudogene 1 (MSNP1), which shows no evidence of being transcribed in the sense orientation. The SNP-containing MSNP1AS transcript was highly overexpressed in post-mortem cerebral cortex of patients with ASD and regulated expression of moesin protein (a known regulator of nuclear architecture (Paglini et al. 1998) in human cells lines, suggesting a possible role in ASD pathophysiology.
The potential of lncRNAs as biomarkers and therapeutic targets
As introduced previously, lncRNAs show higher tissue specificity compared with mRNAs and miRNAs and are significantly dysregulated expression in a wide range of diseases. In addition, they are detectable in tissue and body fluids steady. These features make lncRNAs very suitable as biomarkers. In recent years, most of lncRNAs biomarkers have been identified in many kinds of cancers and cardiovascular diseases.
As aberrant regulation of lncRNAs promotes tumor formation, progression and metastasis, they may be attractive novel biomarkers and therapeutic targets for cancers (Ge et al. 2013; Ishibashi et al. 2013; Konishi et al. 2015; Shi et al. 2015; Tian and Xu 2015; Walsh et al. 2014; Wei and Niu 2015; Yan et al. 2015). On the one hand, lncRNAs with differential expressions in tumor tissues can be used as biomarkers (Chen et al. 2015c; Guo et al. 2015; Ellinger et al. 2015; Liu et al. 2015b). For example, lncRNA PVT1, which was up-regulated in human gastric cancer, was identified as a new biomarker for gastric cancer and may indicate lymph node invasion (Ding et al. 2014). LncRNA ABHD11-AS1 (ABHD11 Antisense RNA 1) expression was significantly increased in gastric cancer compared with adjacent non-tumor tissues, indicating its potential to be a biomarker for the diagnosis of gastric cancer (Lin et al. 2014). Prensner et al. (2014) have identified and validated high expression of lncRNA SChLAP1 as significantly prognostic for metastatic disease progression of prostate cancer, suggesting the potential of SChLAP1 as a biomarker for treatment intensification in aggressive prostate cancer.
On the other hand, circulating lncRNAs may be extremely suitable as noninvasive biomarkers for cancers. For example, HULC and LINC00152 are novel plasma biomarkers in predicting diagnosis of HCC (Li et al. 2015a). GAS5 may be a plasma biomarker in breast cancer for assessing the surgical effects (Han et al. 2015). PTENP1 are serum diagnostic markers for gastric cancer (Dong et al. 2015). PCA3 (DD3) (Nilsson et al. 2009) and TRPM2-AS (Orfanelli et al. 2015) are urine biomarkers for prostate cancer. Recently, Tong et al. (2015) have identified lncRNA POU3F3 as a novel plasma biomarker for diagnosis of ESCC, and the combination of POU3F3 and serum squamous cell carcinoma antigen (SCCA) was more efficient for ESCC detection, in particular for early tumor screening. Another research has reported three lncRNAs including RP11-397D12.4, AC007403.1, and ERICH1-AS1 as potential biomarkers for predicting the tumorigenesis of NSCLC in the future (Tang et al. 2015). More biomarkers for various tumors were summarized in Table 5.
Since tissues of heart and vascular are difficult to obtain from patients of cardiovascular diseases, the identified biomarkers are mainly circulating lncRNAs (Van Roosbroeck et al. 2013). For instance, the mitochondrial lncRNA LIPCAR, which showed decreased expression early after myocardial infarction but increased expression during later stages, was identified as a novel plasma biomarker for cardiac remodeling and predicts future death in patients with heart failure (Kumarswamy et al. 2014). LncRNA Coromaker was increased in the plasma of coronary artery disease (CAD) patients with a 92 % specificity and was very stable in plasma samples due to their contained in the plasma extracellular vesicles (Evs), making it a stable, sensitive and specific biomarker for CAD (Yang et al. 2015b).
LncRNAs also have widespread application prospects in the targeted therapies. Chemically synthetic oligonucleotides that have been proven to be effective for targeting endogenous miRNAs in mice also have potential applications in lncRNAs-related therapies (Wang et al. 2012; Cai et al. 2015). For example, systemically administered ASOs in mice effectively knocked down the lncRNA MALAT1 in muscles, correcting myotonic dystrophy in vivo (Wheeler et al. 2012). Recently, Meng et al. (2015) developed a potential therapeutic intervention for AS by reducing UBE3A-ATS with ASOs and inducing a re-expression of UBE3A at both the RNA and protein level. In addition, preventing the interaction of lncRNAs with their target proteins may represent another therapeutic avenue. For example, the administration of an “antagolinc” against HOTAIR would lead to competitive inhibition of a chromatin-remodeling complex, such as PRC2, by binding to the lncRNA. This inhibition would normalize the chromatin state to inhibit cancer cell growth and metastasis (Tsai et al. 2011). Recently, lncRNAs related to tumor chemotherapy resistance were reported. Reversing the expression of these lncRNAs in tumors may improve tumor chemoresistance. For example, both HOTAIR (Hutzelmann et al. 2000) and AK126698 (Yang et al. 2013b) lncRNAs participate in resistance to cisplatin in NSCLC. Effective inhibitors that down-regulate HOTAIR expression or sensitize AK126698 may be efficient therapeutic interventions for alleviating cisplatin resistance in NSCLC patients. Taken together, these recent advances indicate a significant potential in developing lncRNA-mediated diagnostics and therapies.
Techniques of identifying lncRNAs deregulated in human diseases
As lncRNAs are highly abundant in mammals and have been demonstrated to play important roles in many biological processes and diseases, there has been an ever increasing need to develop accurate detecting methods for deregulated lncRNAs in human diseases. So far, lncRNA detection technologies are mainly devided into two categories: high throughput technologies mainly including lncRNA microarray and next-generation RNA-seq, and gene-specific detection methods such as RT-qPCR and Northern blot.
LncRNA microarray technique is designed for the global profiling of human lncRNAs and mRNAs, which can identify the lncRNAs involved in known signaling pathways according to the relevant mRNA expression changes in different diseases and control samples (Wang et al. 2015d). Next-generation RNA-seq enables non-biased, high throughput, probe-independent inspection of expression data and high coverage and quantification of global transcript levels as well as the detection of expressed exons and junctions given a sufficient sequencing depth (Soreq et al. 2015). All RNA-seq experiments follow a similar protocol, during which total RNA is isolated from a sample of interest prior to preparing an lncRNA library, and ultimately produce one read in a single-end sequencing reaction or two ends separated by an unsequenced fragment in paired-end reactions (Mortazavi et al. 2008). Recently, researchers proposed a target RNA-seq technology named RNA capture sequencing (RNA CaptureSeq), which integrated current gene capture techniques and the latest deep sequencing technology (Mercer et al. 2012; Clark et al. 2015). This technology can identify and characterize unannotated transcripts whose rare or transient expression is below the detection limits of conventional sequencing approaches (Mercer et al. 2012).
Besides custom RT-qPCR and in situ hybridization (ISH), there are several more advanced technologies for deregulated lncRNAs in human diseases. For example, Yue et al. (2014) have reported a new technique combining RNA fluorescent in situ hybridization (FISH) with immunofluorescence (immuno-FISH), that can be employed at the single cell level to detect the spatial dynamics of specific lncRNA localization with simultaneous insight into the localization of proteins, epigenetic modifications and other details which can be highlighted by immunofluorescence. Eissa et al. (2015) have developed a PCR-free nanoparticle-based specific hybridization assay for direct detection of lncRNA urothelial carcinoma associated-1 (UCA1). Very recently, Liu et al. (2015a) also proposed a novel strategy based on Pt–Pd bimetallic nanodendrites/nanoflower-like clusters on graphene oxide/Au/horseradish peroxidase (PtPd BND/BNF@GO/Au/HRP) to enhance the catalytic efficiency and sensitivity. These strategies may provide more advanced selections for identifying lncRNAs in clinical tissues.
Perspectives
Due to the diversity of lncRNAs function and their important roles in a wide range of diseases, lncRNAs have become the focuses of scientists. Correspondingly, the field of lncRNAs is rapidly evolving with numerous novel lncRNA molecules discovered covering a variety of biological and cellular processes. Although several breakthroughs in understanding the mechanisms and roles of lncRNAs have occurred, researchers are still striving to identify novel lncRNAs and their associated molecules using bioinformatics and high-throughput sequencing technologies. However, there remain significant gaps in our understanding of this RNA species. First, we have only hit the tip of the iceberg as only a handful of mammal lncRNAs involved in diseases have been identified in robust genetic models, and their roles in diseases are obscure. So, it’s urgent to identify more diseases-related lncRNAs and clarify their acting mechanisms. Development of unbiased genetic screens, systematic identification of lncRNA expression patterns, as well as a characterization of the lncRNAs themselves and their associated proteins, should pave the way towards this issue. Second, lncRNAs are fast becoming novel targets for gene therapy because of their large number and broad means of gene expression regulation. For instance, ASOs, siRNA and ‘antagolinc’ have been used to target diseases-associated lncRNAs for therapeutic purpose. However, there are still a lot of challenges to overcome: there is no mature gene therapy means based on lncRNAs as yet, and many technologies have their defects, with delivery and stability issues being the most common difficulties. So, it’s necessary to overcome these challenges and develop better therapeutic technologies for exploring lncRNAs-based therapies to a greater degree, which ultimately unveil their utility as novel diagnostic and therapeutic strategies in human complex diseases. Third, given the fact that lncRNAs can mediate epigenetic regulation of gene expression by recruiting chromatin-remodeling complexes to specific locations in the genome, they may have potentials to be novel epigenetic intervention tools for specific sites within the genome. Exploring lncRNAs-based epigenetic intervention tools will provide a new landscape for research and therapy of human complex diseases which involve epigenetic mechanisms. Taken together, further comprehensive study of lncRNAs will provide new answers to old questions in evolution, development as well as disease diagnostics and therapeutics.
References
Abramowitz LK, Bartolomei MS (2012) Genomic imprinting: recognition and marking of imprinted loci. Curr Opin Genet Dev 22:72–78
Annilo T, Kepp K, Laan M (2009) Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol Biol 10:81
Archer K, Broskova Z, Bayoumi AS, Teoh JP, Davila A, Tang Y, Su H, Kim IM (2015) Long non-coding RNAs as master regulators in cardiovascular diseases. Int J Mol Sci 16:23651–23667
Autuoro JM, Pirnie SP, Carmichael GG (2014) Long noncoding RNAs in imprinting and X chromosome inactivation. Biomolecules 4:76–100
Barlow DP, Bartolomei MS (2014) Genomic imprinting in mammals. Cold Spring Harb Perspect Biol 6:a018382
Barry G, Briggs JA, Vanichkina DP, Poth EM, Beveridge NJ, Ratnu VS, Nayler SP, Nones K, Hu J, Bredy TW, Nakagawa S, Rigo F, Taft RJ, Cairns MJ, Blackshaw S, Wolvetang EJ, Mattick JS (2014) The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry 19:486–494
Bartolomei MS, Ferguson-Smith AC (2011) Mammalian genomic imprinting. Cold Spring Harb Perspect Biol 3
Beltran M, Puig I, Pena C, Garcia JM, Alvarez AB, Pena R, Bonilla F, de Herreros AG (2008) A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev 22:756–769
Bierhoff H, Schmitz K, Maass F, Ye J, Grummt I (2010) Noncoding transcripts in sense and antisense orientation regulate the epigenetic state of ribosomal RNA genes. Cold Spring Harb Symp Quant Biol 75:357–364
Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ (2007) Identification and analysis of functional elements in 1 % of the human genome by the ENCODE pilot project. Nature 447:799–816
Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW (2002) The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation 70:537–546
Brockdorff N (2011) Chromosome silencing mechanisms in X-chromosome inactivation: unknown unknowns. Development 138:5057–5065
Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang S, Liu X (2015) Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res
Chen R, Yang Z, Zhou Q (2004) Phosphorylated positive transcription elongation factor b (P-TEFb) is tagged for inhibition through association with 7SK snRNA. J Biol Chem 279:4153–4160
Chen CL, Tseng YW, Wu JC, Chen GY, Lin KC, Hwang SM, Hu YC (2015a) Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials 44:71–81
Chen SX, Yin JF, Lin BC, Su HF, Zheng Z, Xie CY, Fei ZH (2015b) Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumour Biol
Chen X, Kong J, Ma Z, Gao S, Feng X (2015c) Up regulation of the long non-coding RNA NEAT1 promotes esophageal squamous cell carcinoma cell progression and correlates with poor prognosis. Am J Cancer Res 5:2808–2815
Chung S, Nakagawa H, Uemura M, Piao L, Ashikawa K, Hosono N, Takata R, Akamatsu S, Kawaguchi T, Morizono T, Tsunoda T, Daigo Y, Matsuda K, Kamatani N, Nakamura Y, Kubo M (2011) Association of a novel long non-coding RNA in 8q24 with prostate cancer susceptibility. Cancer Sci 102:245–252
Clark MB, Mercer TR, Bussotti G, Leonardi T, Haynes KR, Crawford J, Brunck ME, Cao KA, Thomas GP, Chen WY, Taft RJ, Nielsen LK, Enright AJ, Mattick JS, Dinger ME (2015) Quantitative gene profiling of long noncoding RNAs with targeted RNA sequencing. Nat Methods 12:339–342
Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, Lawrence JB (2009) An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell 33:717–726
Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L, Sorrentino V (1992) Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol 12:3514–3521
Darrow EM, Chadwick BP (2013) Boosting transcription by transcription: enhancer-associated transcripts. Chromosome Res 21:713–724
Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LP (2009) RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet 5:e1000600
Davies W, Isles AR, Wilkinson LS (2005) Imprinted gene expression in the brain. Neurosci Biobehav Rev 29:421–430
Delaval K, Feil R (2004) Epigenetic regulation of mammalian genomic imprinting. Curr Opin Genet Dev 14:188–195
Di Gesualdo F, Capaccioli S, Lulli M (2014) A pathophysiological view of the long non-coding RNA world. Oncotarget
Ding J, Li D, Gong M, Wang J, Huang X, Wu T, Wang C (2014) Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther 7:1625–1630
Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY, Du X (2015) Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer
Eissa S, Matboli M, Essawy NO, Shehta M, Kotb YM (2015) Rapid detection of urinary long non-coding RNA urothelial carcinoma associated one using a PCR-free nanoparticle-based assay. Biomarkers 20:212–217
Ellinger J, Alam J, Rothenburg J, Deng M, Schmidt D, Syring I, Miersch H, Perner S, Muller SC (2015) The long non-coding RNA lnc-ZNF180-2 is a prognostic biomarker in patients with clear cell renal cell carcinoma. Am J Cancer Res 5:2799–2807
Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA (2004) B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol 11:822–829
Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St LGR, Kenny PJ, Wahlestedt C (2008) Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 14:723–730
Fang Z, Wu L, Wang L, Yang Y, Meng Y, Yang H (2014) Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: a possible correlation with cancer metastasis. Oral Surg Oral Med Oral Pathol Oral Radiol 117:89–95
Fatemi RP, Velmeshev D, Faghihi MA (2014) De-repressing LncRNA-targeted genes to upregulate gene expression: focus on small molecule therapeutics. Mol Ther Nucl Acids 3:e196
Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, Et A (1995) The RNA component of human telomerase. Science 269:1236–1241
Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD (2006) The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev 20:1470–1484
Fox AH, Lamond AI (2010) Paraspeckles. Cold Spring Harb Perspect Biol 2:a687
Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI (2002) Paraspeckles: a novel nuclear domain. Curr Biol 12:13–25
Garber K, Smith KT, Reines D, Warren ST (2006) Transcription, translation and fragile X syndrome. Curr Opin Genet Dev 16:270–275
Ge X, Chen Y, Liao X, Liu D, Li F, Ruan H, Jia W (2013) Overexpression of long noncoding RNA PCAT-1 is a novel biomarker of poor prognosis in patients with colorectal cancer. Med Oncol 30:588
Geschwind DH (2008) Autism: many genes, common pathways? Cell 135:391–395
Goto T, Monk M (1998) Regulation of X-chromosome inactivation in development in mice and humans. Microbiol Mol Biol Rev 62:362–378
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413
Grote P, Wittler L, Hendrix D, Koch F, Wahrisch S, Beisaw A, Macura K, Blass G, Kellis M, Werber M, Herrmann BG (2013) The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24:206–214
Guo XB, Hua Z, Li C, Peng LP, Wang JS, Wang B, Zhi QM (2015) Biological significance of long non-coding RNA FTX expression in human colorectal cancer. Int J Clin Exp Med 8:15591–15600
Gutschner T, Diederichs S (2012) The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 9:703–719
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482:339–346
Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES (2011) Lincrnas act in the circuitry controlling pluripotency and differentiation. Nature 477:295–300
Halley P, Kadakkuzha BM, Faghihi MA, Magistri M, Zeier Z, Khorkova O, Coito C, Hsiao J, Lawrence M, Wahlestedt C (2014) Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep 6:222–230
Han P, Li W, Lin CH, Yang J, Shang C, Nurnberg ST, Jin KK, Xu W, Lin CY, Lin CJ, Xiong Y, Chien HC, Zhou B, Ashley E, Bernstein D, Chen PS, Chen HS, Quertermous T, Chang CP (2014) A long noncoding RNA protects the heart from pathological hypertrophy. Nature 514:102–106
Han L, Ma P, Liu SM, Zhou X (2015) Circulating long noncoding RNA GAS5 as a potential biomarker in breast cancer for assessing the surgical effects. Tumour Biol
Haywood ME, Rose SJ, Horswell S, Lees MJ, Fu G, Walport MJ, Morley BJ (2006) Overlapping BXSB congenic intervals, in combination with microarray gene expression, reveal novel lupus candidate genes. Genes Immun 7:250–263
Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, Elliston LA, Hartog C, Goldstein DR, Thu D, Hollingsworth ZR, Collin F, Synek B, Holmans PA, Young AB, Wexler NS, Delorenzi M, Kooperberg C, Augood SJ, Faull RL, Olson JM, Jones L, Luthi-Carter R (2006) Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet 15:965–977
Hu YW, Yang JY, Ma X, Chen ZP, Hu YR, Zhao JY, Li SF, Qiu YR, Lu JB, Wang YC, Gao JJ, Sha YH, Zheng L, Wang Q (2014) A lincRNA-DYNLRB2-2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J Lipid Res 55:681–697
Huang C, Yu W, Wang Q, Cui H, Wang Y, Zhang L, Han F, Huang T (2015) Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med 106:143–149
Hutzelmann A, Tetzlaff K, Reuter M, Muller-Hulsbeck S, Heller M (2000) Does diving damage the brain? MR control study of divers’ central nervous system. Acta Radiol 41:18–21
Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, Sugimachi K, Mimori K, Wakabayashi G, Mori M (2013) Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep 29:946–950
Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, Takahashi A, Miyamoto Y, Ikegawa S, Kamatani N, Hori M, Saito S, Nakamura Y, Tanaka T (2006) Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet 51:1087–1099
Isin M, Uysaler E, Ozgur E, Koseoglu H, Sanli O, Yucel OB, Gezer U, Dalay N (2015) Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet 6:168
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, Poliakov A, Cao X, Dhanasekaran SM, Wu YM, Robinson DR, Beer DG, Feng FY, Iyer HK, Chinnaiyan AM (2015) The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 47:199–208
Ji J, Tang J, Deng L, Xie Y, Jiang R, Li G, Sun B (2015) LINC00152 promotes proliferation in hepatocellular carcinoma by targeting EpCAM via the mTOR signaling pathway. Oncotarget
Johnson R (2012) Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiol Dis 46:245–254
Johnson R, Richter N, Jauch R, Gaughwin PM, Zuccato C, Cattaneo E, Stanton LW (2010) Human accelerated region 1 noncoding RNA is repressed by REST in Huntington’s disease. Physiol Genom 41:269–274
Kanduri C (2011) Long noncoding RNA and epigenomics. Adv Exp Med Biol 722:174–195
Kanduri C (2015) Long noncoding RNAs: lessons from genomic imprinting. Biochim Biophys Acta
Kerin T, Ramanathan A, Rivas K, Grepo N, Coetzee GA, Campbell DB (2012) A noncoding RNA antisense to moesin at 5p14.1 in autism. Sci Transl Med 4:128r–140r
Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C (2008) A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS One 3:e1486
Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP (2010) Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 3:a8
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, Ding H, Butty VL, Torrey L, Haas S, Abo R, Tabebordbar M, Lee RT, Burge CB, Boyer LA (2013) Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell 152:570–583
Kogure T, Yan IK, Lin WL, Patel T (2013) Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer 4:261–272
Konishi H, Ichikawa D, Yamamoto Y, Arita T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, Shiozaki A, Ikoma H, Ochiai T, Otsuji E (2015) Plasma MALAT1 level is associated with liver damage and predicts development of hepatocellular carcinoma. Cancer Sci
Kornienko AE, Guenzl PM, Barlow DP, Pauler FM (2013) Gene regulation by the act of long non-coding RNA transcription. BMC Biol 11:59
Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T (2014) Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 114:1569–1575
Li JY, Ma X, Zhang CB (2014a) Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol 7:7938–7944
Li Z, Chao TC, Chang KY, Lin N, Patil VS, Shimizu C, Head SR, Burns JC, Rana TM (2014b) The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci USA 111:1002–1007
Li Z, Li X, Wu S, Xue M, Chen W (2014c) Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci 105:951–955
Li J, Wang X, Tang J, Jiang R, Zhang W, Ji J, Sun B (2015a) HULC and Linc00152 act as novel biomarkers in predicting diagnosis of hepatocellular carcinoma. Cell Physiol Biochem 37:687–696
Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J (2015b) Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol 36:2007–2012
Lin X, Yang M, Xia T, Guo J (2014) Increased expression of long noncoding RNA ABHD11-AS1 in gastric cancer and its clinical significance. Med Oncol 31:42
Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR (1997) Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276:561–567
Liu J, Jung C, Xu J, Wang H, Deng S, Bernad L, Arenas-Huertero C, Chua NH (2012) Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 24:4333–4345
Liu Y, Li G, Lu H, Li W, Li X, Liu H, Li X, Li T, Yu B (2014) Expression profiling and ontology analysis of long noncoding RNAs in post-ischemic heart and their implied roles in ischemia/reperfusion injury. Gene 543:15–21
Liu F, Xiang G, Jiang D, Zhang L, Chen X, Liu L, Luo F, Li Y, Liu C, Pu X (2015a) Ultrasensitive strategy based on PtPd nanodendrite/nano-flower-like@GO signal amplification for the detection of long non-coding RNA. Biosens Bioelectron 74:214–221
Liu Y, Zhang M, Liang L, Li J, Chen YX (2015b) Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol 8:11480–11484
Ma H, Hao Y, Dong X, Gong Q, Chen J, Zhang J, Tian W (2012) Molecular mechanisms and function prediction of long noncoding RNA. Sci World J 2012:541786
Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA (2008) Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell 29:499–509
Maruyama R, Suzuki H (2012) Long noncoding RNA involvement in cancer. BMB Rep 45:604–611
Meng L, Ward AJ, Chun S, Bennett CF, Beaudet AL, Rigo F (2015) Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 518:409–412
Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10:155–159
Mercer TR, Gerhardt DJ, Dinger ME, Crawford J, Trapnell C, Jeddeloh JA, Mattick JS, Rinn JL (2012) Targeted RNA sequencing reveals the deep complexity of the human transcriptome. Nat Biotechnol 30:99–104
Mohammad F, Mondal T, Kanduri C (2009) Epigenetics of imprinted long noncoding RNAs. Epigenetics-US 4:277–286
Mondal T, Subhash S, Vaid R, Enroth S, Uday S, Reinius B, Mitra S, Mohammed A, James AR, Hoberg E, Moustakas A, Gyllensten U, Jones SJ, Gustafsson CM, Sims AH, Westerlund F, Gorab E, Kanduri C (2015) MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun 6:7743
Morikawa T, Manabe T (2010) Aberrant regulation of alternative pre-mRNA splicing in schizophrenia. Neurochem Int 57:691–704
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, Chen G, Weatherspoon MR, Clark HB, Ebner TJ, Day JW, Ranum LP (2006) Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet 38:758–769
Muller N, Doring F, Klapper M, Neumann K, Schulte DM, Turk K, Schroder JO, Zeuner RA, Freitag-Wolf S, Schreiber S, Laudes M (2014) Interleukin-6 and tumour necrosis factor-alpha differentially regulate lincRNA transcripts in cells of the innate immune system in vivo in human subjects with rheumatoid arthritis. Cytokine 68:65–68
Mus E, Hof PR, Tiedge H (2007) Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci USA 104:10679–10684
Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P (2008) The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322:1717–1720
Nie FQ, Ma S, Xie M, Liu YW, De W, Liu XH (2015) Decreased long noncoding RNA MIR31HG is correlated with poor prognosis and contributes to cell proliferation in gastric cancer. Tumour Biol
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A (2009) Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer 100:1603–1607
Ogawa Y, Sun BK, Lee JT (2008) Intersection of the RNA interference and X-inactivation pathways. Science 320:1336–1341
O’Leary VB, Ovsepian SV, Carrascosa LG, Buske FA, Radulovic V, Niyazi M, Moertl S, Trau M, Atkinson MJ, Anastasov N (2015) PARTICLE, a triplex-forming long ncRNA, regulates locus-specific methylation in response to low-dose irradiation. Cell Rep 11:474–485
Orfanelli U, Jachetti E, Chiacchiera F, Grioni M, Brambilla P, Briganti A, Freschi M, Martinelli-Boneschi F, Doglioni C, Montorsi F, Bellone M, Casari G, Pasini D, Lavorgna G (2015) Antisense transcription at the TRPM2 locus as a novel prognostic marker and therapeutic target in prostate cancer. Oncogene 34:2094–2102
Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A (1998) Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol 143:443–455
Peterlin BM, Brogie JE, Price DH (2012) 7SK snRNA: a noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscip Rev RNA 3:92–103
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Pontier DB, Gribnau J (2011) Xist regulation and function explored. Hum Genet 130:223–236
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136:629–641
Prensner JR, Zhao S, Erho N, Schipper M, Iyer MK, Dhanasekaran SM, Magi-Galluzzi C, Mehra R, Sahu A, Siddiqui J, Davicioni E, Den RB, Dicker AP, Karnes RJ, Wei JT, Klein EA, Jenkins RB, Chinnaiyan AM, Feng FY (2014) RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol 15:1469–1480
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129:1311–1323
Sado T, Hoki Y, Sasaki H (2006) Tsix defective in splicing is competent to establish Xist silencing. Development 133:4925–4931
Schonrock N, Harvey RP, Mattick JS (2012) Long noncoding RNAs in cardiac development and pathophysiology. Circ Res 111:1349–1362
Serviss JT, Johnsson P, Grander D (2014) An emerging role for long non-coding RNAs in cancer metastasis. Front Genet 5:234
Sharma S, Findlay GM, Bandukwala HS, Oberdoerffer S, Baust B, Li Z, Schmidt V, Hogan PG, Sacks DB, Rao A (2011) Dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor is regulated by an RNA-protein scaffold complex. Proc Natl Acad Sci USA 108:11381–11386
Shi WH, Wu QQ, Li SQ, Yang TX, Liu ZH, Tong YS, Tuo L, Wang S, Cao XF (2015) Upregulation of the long noncoding RNA PCAT-1 correlates with advanced clinical stage and poor prognosis in esophageal squamous carcinoma. Tumour Biol 36:2501–2507
Shirasawa S, Harada H, Furugaki K, Akamizu T, Ishikawa N, Ito K, Ito K, Tamai H, Kuma K, Kubota S, Hiratani H, Tsuchiya T, Baba I, Ishikawa M, Tanaka M, Sakai K, Aoki M, Yamamoto K, Sasazuki T (2004) SNPs in the promoter of a B cell-specific antisense transcript, SAS-ZFAT, determine susceptibility to autoimmune thyroid disease. Hum Mol Genet 13:2221–2231
Sleutels F, Barlow DP (2002) The origins of genomic imprinting in mammals. Adv Genet 46:119–163
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P (2007) New therapies for treatment of rheumatoid arthritis. Lancet 370:1861–1874
Sonkoly E, Bata-Csorgo Z, Pivarcsi A, Polyanka H, Kenderessy-Szabo A, Molnar G, Szentpali K, Bari L, Megyeri K, Mandi Y, Dobozy A, Kemeny L, Szell M (2005) Identification and characterization of a novel, psoriasis susceptibility-related noncoding RNA gene, PRINS. J Biol Chem 280:24159–24167
Soreq L, Salomonis N, Guffanti A, Bergman H, Israel Z, Soreq H (2015) Whole transcriptome RNA sequencing data from blood leukocytes derived from Parkinson’s disease patients prior to and following deep brain stimulation treatment. Genom Data 3:57–60
Stelzer Y, Sagi I, Yanuka O, Eiges R, Benvenisty N (2014) The noncoding RNA IPW regulates the imprinted DLK1-DIO3 locus in an induced pluripotent stem cell model of Prader-Willi syndrome. Nat Genet 46:551–557
Struhl K (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol 14:103–105
Stuhlmuller B, Kunisch E, Franz J, Martinez-Gamboa L, Hernandez MM, Pruss A, Ulbrich N, Erdmann VA, Burmester GR, Kinne RW (2003) Detection of oncofetal h19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol 163:901–911
Sun BK, Deaton AM, Lee JT (2006) A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell 21:617–628
Tang Q, Ni Z, Cheng Z, Xu J, Yu H, Yin P (2015) Three circulating long non-coding RNAs act as biomarkers for predicting NSCLC. Cell Physiol Biochem 37:1002–1009
Tian X, Xu G (2015) Clinical value of lncRNA MALAT1 as a prognostic marker in human cancer: systematic review and meta-analysis. BMJ Open 5:e8653
Tian Y, Zhang X, Hao Y, Fang Z, He Y (2014) Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res 24:335–341
Tong YS, Wang XW, Zhou XL, Liu ZH, Yang TX, Shi WH, Xie HW, Lv J, Wu QQ, Cao XF (2015) Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer 14:3
Tsai MC, Spitale RC, Chang HY (2011) Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res 71:3–7
Tsoi LC, Iyer MK, Stuart PE, Swindell WR, Gudjonsson JE, Tejasvi T, Sarkar MK, Li B, Ding J, Voorhees JJ, Kang HM, Nair RP, Chinnaiyan AM, Abecasis GR, Elder JT (2015) Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol 16:24
Van Roosbroeck K, Pollet J, Calin GA (2013) miRNAs and long noncoding RNAs as biomarkers in human diseases. Expert Rev Mol Diagn 13:183–204
Walsh AL, Tuzova AV, Bolton EM, Lynch TH, Perry AS (2014) Long noncoding RNAs and prostate carcinogenesis: the missing ‘linc’? Trends Mol Med 20:428–436
Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454:126–130
Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta RA, Wysocka J, Lei M, Dekker J, Helms JA, Chang HY (2011a) A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472:120–124
Wang X, Song X, Glass CK, Rosenfeld MG (2011b) The long arm of long noncoding RNAs: roles as sensors regulating gene transcriptional programs. Cold Spring Harb Perspect Biol 3:a3756
Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi C, Wang J, Li R, Ran X, Su Y, Zou Z (2012) miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol 181:1911–1920
Wang F, Yuan JH, Wang SB, Yang F, Yuan SX, Ye C, Yang N, Zhou WP, Li WL, Li W, Sun SH (2014a) Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology 60:1278–1290
Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, Tian L (2014b) Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol 31:148
Wang K, Liu F, Zhou LY, Long B, Yuan SM, Wang Y, Liu CY, Sun T, Zhang XJ, Li PF (2014c) The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res 114:1377–1388
Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF (2014d) CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun 5:3596
Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, Chen J, Liu X, Wang SK (2015a) Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget 6:7899–7917
Wang F, Zhou J, Xie X, Hu J, Chen L, Hu Q, Guo H, Yu C (2015b) Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma 62:432–438
Wang HM, Lu JH, Chen WY, Gu AQ (2015c) Upregulated lncRNA-UCA1 contributes to progression of lung cancer and is closely related to clinical diagnosis as a predictive biomarker in plasma. Int J Clin Exp Med 8:11824–11830
Wang Y, Li Y, Yang Z, Liu K, Wang D (2015d) Genome-wide microarray analysis of long non-coding RNAs in eutopic secretory endometrium with endometriosis. Cell Physiol Biochem 37:2231–2245
Wei Y, Niu B (2015) Role of MALAT1 as a prognostic factor for survival in various cancers: a systematic review of the literature with meta-analysis. Dis Mark 2015:164635
Wheeler TM, Leger AJ, Pandey SK, MacLeod AR, Nakamori M, Cheng SH, Wentworth BM, Bennett CF, Thornton CA (2012) Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 488:111–115
Wierzbicki AT (2012) The role of long non-coding RNA in transcriptional gene silencing. Curr Opin Plant Biol 15:517–522
Wilkinson LS, Davies W, Isles AR (2007) Genomic imprinting effects on brain development and function. Nat Rev Neurosci 8:832–843
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C (2014) LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 130:1452–1465
Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W, Zhang L (2015) Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res 34:116
Xue M, Pang H, Li X, Li H, Pan J, Chen W (2015) Long noncoding RNA UCA1 promotes bladder cancer cell migration and invasion via hsa-miR-145/ZEB1/2/FSCN1 pathway. Cancer Sci
Yakovchuk P, Goodrich JA, Kugel JF (2009) B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci USA 106:5569–5574
Yakovchuk P, Goodrich JA, Kugel JF (2011) B2 RNA represses TFIIH phosphorylation of RNA polymerase II. Transcription 2:45–49
Yan TH, Yang H, Jiang JH, Lu SW, Peng CX, Que HX, Lu WL, Mao JF (2015) Prognostic significance of long non-coding RNA PCAT-1 expression in human hepatocellular carcinoma. Int J Clin Exp Pathol 8:4126–4131
Yang H, Huang ZZ, Wang J, Lu SC (2001) The role of c-Myb and Sp1 in the up-regulation of methionine adenosyltransferase 2A gene expression in human hepatocellular carcinoma. FASEB J 15:1507–1516
Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W, Merkurjev D, Ohgi KA, Meng D, Zhang J, Evans CP, Rosenfeld MG (2013a) lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature 500:598–602
Yang Y, Li H, Hou S, Hu B, Liu J, Wang J (2013b) The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One 8:e65309
Yang YR, Zang SZ, Zhong CL, Li YX, Zhao SS, Feng XJ (2014) Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol 7:6929–6935
Yang H, Liu P, Zhang J, Peng X, Lu Z, Yu S, Meng Y, Tong WM, Chen J (2015a) Long noncoding RNA MIR31HG exhibits oncogenic property in pancreatic ductal adenocarcinoma and is negatively regulated by miR-193b. Oncogene
Yang Y, Cai Y, Wu G, Chen X, Liu Y, Wang X, Yu J, Li C, Chen X, Jose PA, Zhou L, Zeng C (2015b) Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci (Lond) 129:675–685
Yang L, Qiu M, Xu Y, Wang J, Zheng Y, Li M, Xu L, Yin R (2016) Upregulation of long non-coding RNA PRNCR1 in colorectal cancer promotes cell proliferation and cell cycle progression. Oncol Rep 35:318–324
Yik JH, Chen R, Nishimura R, Jennings JL, Link AJ, Zhou Q (2003) Inhibition of P-TEFb (CDK9/Cyclin T) kinase and RNA polymerase II transcription by the coordinated actions of HEXIM1 and 7SK snRNA. Mol Cell 12:971–982
Yu J, Lee HS, Lee SM, Yu HC, Moon WS, Chung MJ, Park JW, Park BH (2011) Aggravation of post-ischemic liver injury by overexpression of A20, an NF-kappaB suppressor. J Hepatol 55:328–336
Yue M, Charles RJ, Yamada N, Ogawa A, Ogawa Y (2014) Quick fluorescent in situ hybridization protocol for Xist RNA combined with immunofluorescence of histone modification in X-chromosome inactivation. J Vis Exp e52053
Zangrando J, Zhang L, Vausort M, Maskali F, Marie PY, Wagner DR, Devaux Y (2014) Identification of candidate long non-coding RNAs in response to myocardial infarction. BMC Genom 15:460
Zeng C, Yu X, Lai J, Yang L, Chen S, Li Y (2015) Overexpression of the long non-coding RNA PVT1 is correlated with leukemic cell proliferation in acute promyelocytic leukemia. J Hematol Oncol 8:126
Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS, De W, Shu YQ, Wang ZX (2014) P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis 5:e1243
Zhao S, Long H, Lu Q (2010) Epigenetic perspectives in systemic lupus erythematosus: pathogenesis, biomarkers, and therapeutic potentials. Clin Rev Allergy Immunol 39:3–9
Zhao J, Liu Y, Zhang W, Zhou Z, Wu J, Cui P, Zhang Y, Huang G (2015) Long non-coding RNA Linc00152 is involved in cell cycle arrest, apoptosis, epithelial to mesenchymal transition, cell migration and invasion in gastric cancer. Cell Cycle 14:3112–3123
Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C, Ye H, Zhou B, Chen JJ, Chen P (2015) Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol 17:640–646
Zhou J, Zhi X, Wang L, Wang W, Li Z, Tang J, Wang J, Zhang Q, Xu Z (2015) Linc00152 promotes proliferation in gastric cancer through the EGFR-dependent pathway. J Exp Clin Cancer Res 34:135
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81522038, 81270024, 81220108017, 81371743, 81373205 and 81430074), the Hunan Provincial Natural Science Foundation of China (14JJ1009) and the New Century Talent Supporting Project by Education Ministry.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Communicated by J. Graw.
Rights and permissions
About this article
Cite this article
Wu, R., Su, Y., Wu, H. et al. Characters, functions and clinical perspectives of long non-coding RNAs. Mol Genet Genomics 291, 1013–1033 (2016). https://doi.org/10.1007/s00438-016-1179-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-016-1179-y