Abstract
Eimeria spp. multiply within the intestinal tract causing severe inflammatory responses. Chitosan (CS), meanwhile, has been shown to exhibit anti-inflammatory activities in different experimental models. Here, we investigated the effect of CS on the outcome of inflammation caused by Eimeria papillata in the mouse intestine. Investigations were undertaken into the oocyst output in feces and developmental stages and goblet cells in intestinal tissue. Assays for lipid peroxidation, nitric oxide (NO), and myeloperoxidase (MPO) were also performed. T cells in intestinal tissue were counted using immunohistochemistry while total IgA in serum or intestinal wash was assayed using ELISA. In addition, mRNA expression of tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), interleukin (IL)-10, and IL-4 were detected using real-time PCR. The data indicated a reduction in both oocyst output and in the number of parasite developmental stages following CS treatment, while the goblet cell hypoplasia in infected mice was also inhibited. CS decreased lipid peroxidation, NO, and MPO but did not alter the T cell count or IgA levels in comparison to the infected group. The expression of TNF-α and TGF-β decreased but IL-10 and IL-4 increased after CS treatment in comparison to the non-treated infected group. In conclusion, CS showed anti-inflammatory and protective effects against E. papillata infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is a worldwide protozoan disease caused by parasites of the genus Eimeria. Eimerians can develop and multiply within the intestinal tract causing destruction of intestinal mucosa that can induce a severe inflammatory response (Metwaly et al. 2012; Dkhil et al. 2013). This tissue destruction is also associated with diminished food intake and nutrient absorption, reduced body-weight gain, dehydration, blood loss, and enhanced susceptibility to other diseases (Amer et al. 2015). Coccidiosis therefore causes massive economic losses in the meat and milk production industries (McDougald 2003). Eimeria papillata infecting mice represents an appropriate model to study animal coccidiosis through its development within cells of the murine small intestine (Al-Quraishy et al. 2011). Intestinal coccidiosis in mice leads to serious inflammatory responses manifested by increased production of nitric oxide (NO), increased catalase activity, and increased lipid peroxidation, as indicated by the elevated malondialdehyde level (Dkhil 2013; Wunderlich et al. 2014). Also, during the course of the infection, intraepithelial cells increase the production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) but decrease transforming growth factor β (TGF-β) production (Inagaki-Ohara et al. 2006).
Chitosan (CS) exhibits anti-inflammatory activities in different experimental models (Qiao et al. 2011; Choi et al. 2012; Li et al. 2014). CS has in addition antitumor (Fernandes et al. 2012), antifungal (Hussain et al. 2012), antimicrobial (Tavaria et al. 2012), and free radical scavenging activities (Kim et al. 2012). CS has in recent years been recommended as a healthy food supplement in Asian countries due to these properties (Nam et al. 2007). CS has well-defined properties, including good bioavailability and biocompatibility, low cost, and an ability to open intracellular tight junctions in intestinal epithelial cells (Van der Lubben et al. 2003). Moreover, functionalized forms of CS have attracted considerable interest due to improved mucoadhesivity, permeability, and stability (Islam et al. 2012). CS elicits the release of interleukin (IL)-10, IL-4, and TGF-β at mucosal tissues and the activation of CD3+ T cells in spleen (Porporatto et al. 2005).
Due to all the previously mentioned properties, the present study aimed to investigate the anticoccidial and anti-inflammatory effects of CS in mice following oral infection with E. papillata.
Materials and methods
Animals
Adult male white albino mice weighed 20–25 g and aged 10 weeks were used for the experiments. Animals were housed under controlled temperature (21 °C) and lighting, with 12 h of light and 12 h of dark, and had free access to water and a standard mouse chow diet. Mice were handled in accordance with the protocols approved by the Zoology Department, Faculty of Science, Beni-Suef University.
Infection by E. papillata
E. papillata oocysts in PBS were kindly provided by Professor Al-Quraishy, Zoology Department, College of Science, King Saud University, Saudi Arabia. Prior to infection, the initial stock of oocysts was used to infect some mice in order to activate their virulence. The feces of these mice were collected and the new oocysts were sporulated to be ready for used in the experiment.
The number of the newly collected oocysts was adjusted and each mouse was given 1000 sporulated oocysts in 100 μl of water by oral gavage. To follow up the oocyst shedding numbers, fecal samples were collected and oocysts per gram were estimated using the modified McMaster technique (Schito et al. 1996).
Experimental design
The mice were divided into three groups with eight animals in each group. The first group, with uninfected mice and gavaged only with saline, served as a control group. The second and third groups were infected with E. papillata. The second group was treated with saline and served as the control infected group while the third group was treated with 0.5 ml of 1 % CS (250 mg/kg) in saline once a day for 5 days.
On day 5 p. i., mice were euthanized and blood was collected. Sera were separated and kept at −20 °C. Parts of jejunum were fixed in 10 % neutral buffered formalin for histological and immunohistochemical analysis. Others were weighed, kept in phosphate-buffered saline, and stored at −80 °C for homogenate preparation or trizol for RNA extraction.
Parasitic developmental stages and goblet cells
Sections stained with hematoxylin–eosin were used for parasite detection or with Alcian blue for determination of the goblet cells (Dkhil 2013). For each animal, the number of parasitic developmental stages and goblet cells in the jejunum was counted in at least ten well-orientated villous crypt units (VCUs) and results were expressed as the mean number.
Biochemical analyses
Parts of the jejunum were homogenized to give 50 % (w/v) homogenate in ice-cold medium containing 50 mM Tris–HCl and 300 mM sucrose (Tsakiris et al. 2004). The homogenate was centrifuged at 500×g for 10 min at 4 °C. The supernatant was used for the biochemical determinations. Lipid peroxidation in jejunum homogenate was determined (Ohkawa et al. 1979) by using 1 ml of 10 % trichloroacetic acid and 1 ml of 0.67 % thiobarbituric acid followed by heating in a boiling water bath for 30 min. Thiobarbituric acid-reactive substances were determined by the absorbance at 535 nm and expressed as malondialdehyde (MDA) equivalents formed. The assay of NO in jejunum homogenate was done (Berkels et al. 2004).
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was measured according to the established method (Sun et al. 2007; Kim et al. 2012). Briefly, tissue was homogenized in 0.5 ml of 50 mM potassium phosphate buffer (pH 7.4) and centrifuged at 10,000×g at 4 °C for 30 min. The remaining pellet was resuspended in 0.5 ml of 50 mM potassium buffer at pH 6.0 with 0.5 % hexadecyltrimethylammonium bromide, sonicated on ice, and then centrifuged at 12,000×g at 4 °C for 10 min. Supernatants were then assayed at a 1:20 dilution in reaction buffer containing 50 mM phosphate buffer, 530 mM ortho-dianisidine, and 20 mM H2O2 solution. One unit of enzyme activity was defined as the amount of MPO that caused a change in absorbance measured at 460 nm for 3 min. MPO activity was expressed as unit/milligram tissue.
Immunohistochemistry
Paraffin-embedded intestinal tissues were cut at 5 μm sections. The slides were then incubated in 3 % H2O2 for 10 min at room temperature to block the endogenous peroxidase activity. Slides were treated with 1.5 % normal serum obtained from the same species in which the secondary antibody was developed for 30 min to block non-specific staining. Subsequently, slides were incubated with monoclonal antibodies against mouse CD3 (1:100; AbD Serotec, New York, USA) for overnight at 4 °C. Then slides were treated with a biotin-conjugated secondary antibody for 10 min followed by incubation with peroxidase-conjugated streptavidin for 10 min at room temperature according to the instructions of IHC Detection Kit (Dako, Glostrup, Denmark). All the above steps were followed by washing in Tris buffer (pH 7.4) for three times. Immunolabeling was detected by incubation with 0.06 % diaminobenzidine (Sigma) dissolved in tap water containing 0.01 % H2O2 for 3–5 min, followed by washing and staining with Mayer’s hematoxylin.
Anti-mouse IgA α chain-specific ELISA
Total IgA concentration was determined by sandwich ELISA (Lüllau et al. 1996). Goat anti-mouse IgA α chain-specific antiserum (Sigma) diluted 1:500 in 50 mM bicarbonate buffer (pH 9.6) was used to coat wells (100 μl/well) of Immulon (Dynatech) 96-well plates overnight at 4 °C, which were subsequently blocked (250 μl/well) with 1 % BSA (Sigma) in PBS, 0.1 % Tween 20 (Sigma) at 37 °C for 30 min. Between all antibody incubation steps except the last one, the plates were washed three times with PBS, 0.01 % Tween 20. IgA samples and mouse IgA standards (Sigma; range 10–200 ng) were diluted in blocking buffer and 100 μl was applied, in duplicate, to the wells. After incubation for 2 h at 37 °C, IgA was detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgA antibodies (Amersham) followed by development with ortho-phenyldiamine/H2O2. The reaction was stopped by the addition of 0.01 % sodium azide in citrate buffer (pH 5.0). The absorbance was read at 492 and 629 nm, with the latter serving as reference reading.
Quantitative RT-PCR of mRNA
On day 5 p.i., pieces of jejunum were aseptically removed, rapidly frozen, and stored in liquid nitrogen until use. Total RNA was isolated using SV Total RNA Isolation system (Promega, Madison, WI, USA). Contaminating genomic DNA was digested with the DNA-free™ kit (Applied Biosystems, Darmstadt, Germany), before cDNA was synthesized using Reverse Transcription kit (Stratagene, USA). Real-time PCR (RT-PCR) was performed in a TaqMan7500 (Applied Biosystems) using the QuantiTect™ SYBR® Green PCR kit (Qiagen) according to the manufacturer’s instructions. Qiagen (Hilden, Germany) delivered the primers for TNF-α, TGF-β, IL-10 and IL-4, and 18S rRNA. Initial incubation was done at 50 °C for 2 min, followed by Taq polymerase activated at 95 °C for 10 min, 1 cycle followed at 95 °C for 10 min, at 60 °C for 35 s, and for 30 s at 72 °C. All PCR reactions yielded only a single product of the expected size as detected by melting point analysis and gel electrophoresis. Quantitative evaluation was performed with Taqman7500 system software (Applied Biosystems). Expression of genes was normalized to that of 18S rRNA (Delic et al. 2010).
Statistical analysis
SPSS (version 20) statistical program (SPSS Inc., Chicago, IL) was used to carry out a one-way analysis of variance (ANOVA) on our data. When significant differences by ANOVA were detected, analysis of differences between the means of the treated and control groups were performed by using Dunnett’s t test.
Results
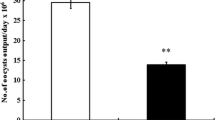
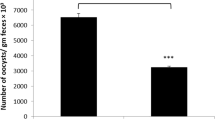
The oocyst output was calculated per gram of feces on day 5 p.i. (Fig. 1). CS treatment reduced (p < 0.01) the oocyst output by 43.5 % compared to the infected group. Inspection of hematoxylin and eosin-stained sections using light microscopy revealed that the epithelial cells of the jejunum were infected with different developmental stages of E. papillata parasites (meront, microgamont, macrogamont, and developing oocysts). The number of parasites per 10 villous-crypt units was significantly reduced (p < 0.01) by 47 % when the mice were treated with CS (Fig. 2). In addition, while there was a significant reduction (p < 0.001) in the number of goblet cells seen at the site of the E. papillata infection in the jejunum (Fig. 3), CS treatment significantly reversed this effect (p < 0.001).
The developmental stages of E. papillata in infected and infected + CS groups at day 5 p.i. a Non-infected group shows no parasitic infections. b Infected group shows the different developmental stages, c CS treatment shows decreased occurrence of developmental stages; d number of developmental stages per 10 villous-crypt units shows a significant (p < 0.01) reduction by 47 % of stages in infected + CS compared to infected. Double asterisks indicates a statistical significance at p < 0.01. Scale bar represents 10 μm
Goblet cells stained with Alcian blue in non-infected, infected, and infected + CS groups at day 5 p.i. a Non-infected group shows high numbers of goblet cells. b Infected group shows decreased number of goblet cells compared to non-infected group; c CS treatment shows an increased occurrence of goblet cells compared to infected group; d statistical analysis shows a significant (p < 0.001) reduction in infected compared to non-infected but CS increased (p < 0.001) the number of goblet cells compared to infected. Triple asterisks indicates a statistical significance at p < 0.001. Scale bar = 10 μm
E. papillata infections also induced a highly significant increase (p < 0.001) in jejunal NO and MDA (Fig. 4) but CS treatment significantly (p < 0.001) lowered the E. papillata-induced increase in both NO and MDA. MPO, which is a marker for neutrophil infiltration into the intestinal tissue was significantly (p < 0.001) increased in infection and decreased (p < 0.01) after CS treatment.
Effect of CS on the levels of malondialdehyde (a), nitric oxide (b), and myeloperoxidase (c) in jejunum of infected mice with E. papillata at day 5 p.i. All had increased (p < 0.001) in infected compared to non-infected group. The levels were decreased (p < 0.001) in CS-treated when compared to infected group. Triple asterisks indicates a statistical significance at p < 0.001
The immunohistochemically detected CD3+ cells per 10 villous-crypt units were decreased significantly (p < 0.05) during infection (Fig. 5). Treatment with CS did not show a remarkable change from the infected group. The total IgA in sera and intestinal wash samples was significantly reduced (p < 0.001) after infection in comparison to the non-infected group (Fig. 6) but again CS treatment did not reverse this reduction in IgA levels.
Effect of CS treatment on the number of CD3+ T cells in the mouse villi at day 5 p.i. a Immunohistochemical staining for T cells (arrows denotes to the stained cells). b The mean number of CD3+ T cells ± SEM in 10 villi. The number was decreased in both of infected and infected + CS compared to the non-infected group. Asterisk indicates a statistical significance at p < 0.05
Comparing the infected group to the non-infected one, the expression of TNF-α and TGF-β was increased (p < 0.001), while IL-10 and IL-4 was decreased (p < 0.001). The levels of expressed mRNA for TNF-α and TGF-β in the jejunum were significantly decreased (p < 0.001; Fig. 7), while the expression of both IL-10 and IL-4 increased (p < 0.001) after CS treatment compared to the infected group.
RT-PCR analysis of TNF-α, TGF-β, IL-10, and IL-4 in the mouse jejunum at day 5 p.i. Fold induction of mRNA expression relative to non-infected group was given. All values are means ± SME. a indicates a significance (p < 0.001) for infected versus non-infected, while b indicates a statistical significance (p < 0.001) for infected + CS versus infected group
Discussion
The current study indicates that CS exhibits anti-Eimeria activity in mice infected with E. papillata, perhaps due to its anti-inflammatory and anti-oxidant activity. The anti-Eimeria activity of CS is indicated by the reduction in the number of fecal oocyst shedding. This denotes that CS treatment impairs intracellular development and replication of E. papillata in the jejunal epithelium of mice. In addition, our results show that CS decreases oxidative stress in the infected jejunum, proven here as an impaired E. papillata-induced increase in both NO and MDA. These results are in line with Dkhil et al. (2015) and Khalil et al. (2015) that described similar effects for berberine and garlic treatments of Eimeria infections in mice. In the same time, CS also attenuates the inflammatory response, since it suppresses the release of the infection-induced MPO and decreases mRNA expression of TNF-α and TGF-β in the mouse jejunum infected with E. papillata (Kim et al. 2004).
The decrease in MDA, NO, and MPO in intestinal tissue confirms the anti-inflammatory effect of CS (Hierholzer et al. 2004). NO was found to play a role in intestinal injury while MPO was measured as an indicator for neutrophil infiltration (Ohtsuka et al. 2001; Barocelli et al. 2006). The increased NO and MPO in the infected group in this study may be related to the increased expression of TNF-α and TGF-β as suggested by Prabhu and Guruvayoorappan (2014).
A single dose of CS at 500 mg/kg was found to have anti-inflammatory properties against carrageenan-induced paw edema (Fernandes et al. 2010). In this study, a dose of 250 mg/kg over 5 days was found to be enough to induce an effect against Eimeria infection.
The ability of CS to restore the number of goblet cells in the infected intestine is important because of the role of goblet cells in protection against intestinal infection with Eimeria spp. (Yunus et al. 2005; Linh et al. 2009; Dkhil et al. 2015). The increased number of goblet cells after CS treatment has also been associated with increased expression of occludin which has been in turn linked to the increased expression of IL-4 and IL-10 which are markers of T helper cell type 2 (Th2) responses (Xiao et al. 2013). Furthermore, CS was found to be protective against Toxoplasma infections due to increased production of IFN-γ which is a Th1 cytokine (Gaafar et al. 2014). IL-4 was found to induce goblet cell hyperplasia in the intestine, which is related to increased secretion of mucin (Blanchard et al. 2004). The production of mucin maintains the integrity of mucosal surfaces and is considered to be one of the mechanisms of innate immunity against invading pathogens (Dharmani et al. 2009).
The number of T cells in intestinal tissue was decreased in infected groups compared to the non-infected, while CS treatment did not induce any changes, as was also the case in respect to decreased total IgA levels in both serum and intestinal wash in infected groups. This indicated that T and B cells did not play a role in protection after CS treatment because there was no stimulus or vaccine for their activation as effector arms in acquired immunity. It seems that T cells mediate their effects on the parasite in primary infections through secretion of cytokines while CD8+ T cells play the greater role in secondary infections (Ovington et al. 1995). CD8+ T cells, however, were found to be essential in garlic extract-induced protection in primary infection with Eimeria vermiformis (Khalil et al. 2015), while CS was found to be effective as an IgA inducer when coupled with the pathogen protein as an adjuvant or delivery system in immunization (Xu et al. 2015).
Although the secretion of TGF-β in mouse intestine has been found to be protective against Eimeria infection (Inagaki-Ohara et al. 2006), the current study has failed to demonstrate such a protective role. This refers to its significant decrease in treated-infected compared to the infected group and nearly normalized when compared to the non-infected group. Its expression coincided with the expression of TNF-α in mouse intestinal tissue. In fact, the production of TGF-β has been found to be combined with production of both IFN-γ and TNF-α following Eimeria infection (Lillehoj 1998). TGF-β4, in particular, has been found to increase in chickens after coccidian infection (Jakowlew et al. 1997).
In conclusion, CS appears to protect mice against E. papillata primary infection by reducing developmental stages and oocyst output. This protective effect was due to the anti-inflammatory action of CS and thus a reduction in epithelial cell damage which facilitates the parasite invasion into the host cell. The protective effect was also a result of increased production of IL-4 which serves to normalize goblet cell numbers. In addition, the development of Th2 response is protective against infection more than Th1 response. CS can be used as a food additive for poultry and other animals.
References
Al-Quraishy S, Delic D, Sies H, Wunderlich F, Abdel-Baki AS, Dkhil MA (2011) Differential miRNA expression in the mouse jejunum during garlic treatment of Eimeria papillata infections. Parasitol Res 109:387–394
Amer OS, Dkhil MA, Hikal WM, Al-Quraishy S (2015) Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. Biomed Res Int 2015:219670
Barocelli E, Ballabeni V, Ghizzardi P, Cattaruzza F, Bertoni S, Lagrasta CA, Impicciatore M (2006) The selective inhibition of inducible nitric oxide synthase prevents intestinal ischemia-reperfusion injury in mice. Nitric Oxide 14:212–218
Berkels R, Purol-Schnabel S, Roesen R (2004) Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol Biol 279:1–8
Blanchard C, Durual S, Estienne M, Bouzakri K, Heim MH, Blin N, Cuber JC (2004) IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J Immunol 172:3775–3783
Choi EH, Yang HP, Chun HS (2012) Chitooligosaccharide ameliorates diet-induced obesity in mice and affects adipose gene expression involved in adipogenesis and inflammation. Nutr Res 32:218–228
Delic D, Gailus N, Vohr HV, Dkhil M, Al-Quraishy S, Wunderlich F (2010) Testosterone-induced permanent changes of hepatic gene expression in female mice sustained during Plasmodium chabaudi malaria infection. J Mol Endocrinol 45:379–390
Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K (2009) Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1:123–135
Dkhil MA (2013) Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol Res 112:2639–2646
Dkhil MA, Al-Quraishy S, Abdel Moneim AE, Delic D (2013) Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 112:101–106
Dkhil MA, Metwaly MS, Al-Quraishy S, Sherif NE, Delic D, Al Omar SY, Wunderlich F (2015) Anti-Eimeria activity of berberine and identification of associated gene expression changes in the mouse jejunum infected with Eimeria papillata. Parasitol Res 114:1581–1593
Fernandes J, Sereno J, Garrido P, Parada B, Cunha MF, Reis F, Pintado ME, Santos-Silva A (2012) Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Mar Drugs 10:2661–2675
Fernandes JC, Spindola H, de Sousa V, Park PJ, Shin ES, Sohn JH, Seo DB, Lim KM, Kim WG, Lee SJ (2010) Antiinflammatory activity of chitooligosaccharides in vivo. Mar Drugs 8:1763–1768
Gaafar MR, Mady RF, Diab RG, Shalaby TI (2014) Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp Parasitol 143:30–38
Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG (2004) Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 286:225–233
Hussain I, Singh T, Chittenden C (2012) Preparation of chitosan oligomers and characterization: their antifungal activities and decay resistance. Holzforschung 66:119–125
Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith AL, Jimi F, Miyahira M, Abdel-Aleem AS, Horii Y, Nawa Y (2006) Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun 74:5292–5301
Islam MA, Firdous J, Choi YJ, Yun CH, Cho CS (2012) Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review. Int J Nanomedicine 7:6077–6093
Jakowlew SB, Mathias A, Lillehoj HS (1997) Transforming growth factor-beta isoforms in the developing chicken intestine and spleen: increase in transforming growth factor-beta 4 with coccidia infection. Vet Immunol Immunopathol 55:321–339
Khalil AM, Yasuda M, Farid AS, Desouky MI, Mohi-Eldin MM, Haridy M, Horii Y (2015) Immunomodulatory and antiparasitic effects of garlic extract on Eimeria vermiformis-infected mice. Parasitol Res 114:2735–2742
Kim JA, Ahn BN, Kong CS, Kim SK (2012) Chitooligomers inhibit UV-A-induced photoaging of skin by regulating TGF-beta/Smad signaling cascade. Carbohydr Polym 88:490–495
Kim MS, You HJ, You MK, Kim NS, Shim BS, Kim HM (2004) Inhibitory effect of water-soluble chitosan on TNF-alpha and IL-8 secretion from HMC-1 cells. Immunopharmacol Immunotoxicol 26:401–409
Li Y, Liu H, Xu QS, Du YG, Xu J (2014) Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-κB and endothelial inflammatory response. Carbohydr Polym 99:568–578
Lillehoj HS (1998) Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 28:1071–1081
Linh BK, Hayashi T, Horii Y (2009) Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res 104:789–794
Lüllau E, Heyse S, Vogel H, Marison I, von Stockar U, Kraehenbuhl JP, Corthésy B (1996) Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J Biol Chem 271:16300–16309
McDougald LR (2003) Coccidiosis. In: Saif (ed) Diseases of poultry. Iowa State Press, Iowa, pp 1001–1010
Metwaly MS, Dkhil MA, Al-Quraishy S (2012) The potential role of Phoenix dactylifera on Eimeria papillata-induced infection in mice. Parasitol Res 111:681–687
Nam KS, Kim MK, Shon YH (2007) Chemopreventive effect of chitosan oligosaccharide against colon carcinogenesis. J Microbiol Biotechnol 17:1546–1549
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Ohtsuka Y, Lee J, Stamm DS, Sanderson IR (2001) MIP-2 secreted by epithelial cells increases neutrophil and lymphocyte recruitment in the mouse intestine. Gut 49:526–533
Ovington KS, Alleva LM, Kerr EA (1995) Cytokines and immunological control of Eimeria spp. Int J Parasitol 25:1331–1351
Porporatto C, Bianco ID, Correa SG (2005) Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J Leukocyte Biol 78:62–69
Qiao Y, Bai XF, Du YG (2011) Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int Immunopharmacol 11:121–127
Schito ML, Barta JR, Chobotar B (1996) Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 82:255–262
Sun BW, Chen ZY, Chen X, Liu C (2007) Attenuation of leukocytes sequestration by carbon monoxide-releasing molecules: liberated carbon monoxide in the liver of thermally injured mice. J Burn Care Res 28:173–181
Tavaria FK, Soares JC, Reis IL, Paulo MH, Malcata FX, Pintado ME (2012) Chitosan: antimicrobial action upon staphylococci after impregnation onto cotton fabric. J Appl Microbiol 112:1034–1041
Tsakiris S, Schulpis KH, Marinou M, Behrakis P (2004) Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+-ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 495:475–479
Prabhu VV, Guruvayoorappan C (2014) Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int Immunopharmacol 18:124–134
Van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Coos Verhoef J, Junginger HE (2003) Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine 21:1400–1408
Wunderlich F, Al-Quraishy S, Steinbrenner H, Sies H, Dkhil MA (2014) Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol Res 113:3547–3556
Xiao D, Tang Z, Yin Y, Zhang B, Hu X, Feng Z, Wang J (2013) Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occludin, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int Immunopharmacol 17:670–676
Xu JH, Dai WJ, Chen B, Fan XY (2015) Mucosal immunization with PsaA protein, using chitosan as a delivery system, increases protection against acute otitis media and invasive infection by Streptococcus pneumoniae. Scandinavian J Immunol 81:177–185
Yunus M, Horii Y, Makimura S, Smith AL (2005) Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J Vet Sci 67:311–315
Acknowledgments
We extend our appreciation to the Dean of Scientific Research, King Saud University, Riyadh, for funding the work through the research group project number RG -004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdel-Latif, M., Abdel-Haleem, H.M. & Abdel-Baki, AA.S. Anticoccidial activities of Chitosan on Eimeria papillata-infected mice. Parasitol Res 115, 2845–2852 (2016). https://doi.org/10.1007/s00436-016-5035-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5035-0