Abstract
We investigated the immunomodulatory and parasiticidal effects of garlic extract on coccidiosis caused by Eimeria vermiformis infection in male ICR mice. One group received garlic extract daily until the end of the experiment by the oral route from 10 days prior to oral infection with 300 sporulated E. vermiformis oocysts (infected-garlic+). The other group served as a control positive with E. vermiformis infection alone (infected-garlic−). In the infected-garlic+ group, garlic extract treatment induced a significant reduction in fecal oocyst output when compared with the infected-garlic− group. Histopathological, immunohistochemical, and gene expression analysis for inflammatory cytokines in ileal tissues showed that the garlic extract treatment impaired intracellular development of E. vermiformis during the early stages by increasing the number of intraepithelial CD8+ T cells and decreasing IL-10 expression. This induced cell cytotoxicity which was reflected by a decrease in oocyst numbers in the intestinal villi and the feces, indicating anticoccidial effects of the garlic extract. However, further studies to explore the precise mechanism of the observed effects of garlic treatment during Eimeria infection are needed to verify our results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Garlic (Allium sativum) has acquired a reputation in many different traditions as a prophylactic as well as therapeutic medicinal plant. In addition to its proclaimed nutritional effects, garlic possesses several pharmacological properties such as antimicrobial (Ankri and Mirelman 1999), antioxidant (Chung 2006), and anticancer (Cao et al. 2014) activities. Also, garlic and its components have potent antiparasitic activities against many human and animal parasites (Anthony et al. 2005; Feng et al. 2012) such as Leishmania (Wabwoba et al. 2010), Schistosoma (Mantawy et al. 2011), Trypanosoma, Giardia, Entamoeba (Ankri et al. 1997; Lun et al. 1994), and Plasmodium (Coppi et al. 2006). There is also evidence supporting the immunomodulatory potential of garlic (Kyo et al. 2001) or selected garlic components which includes increased T lymphocyte blastogenesis, natural killer (NK) cell activity, phagocytosis (Ishikawa et al. 2006), and modulation of cytokine production during several pathogenic infections (Ghazanfari et al. 2000).
Allicin (diallylthiosulfinate) is the main active compound in garlic and has been implicated in antibacterial activity against a wide range of Gram-positive and Gram-negative bacteria, and has antiviral, antifungal as well as antiprotozoal activities (Ankri and Mirelman 1999; Shadkchan et al. 2004; Soffar and Mokhtar 1991). Allicin is produced by an enzymatic reaction after raw garlic is crushed or injured and the enzyme alliinase, which is stored in a separate compartment in garlic, combines with a compound called alliin in raw garlic (Waag et al. 2010). The latter is the most abundant compound representing about 70 % of the overall thiosulfinates generated when garlic is crushed and is responsible for its flavor and aroma as well as its potential health benefits (Han et al. 1995). In regard to parasitic infections, allicin has inhibitory effects on the cysteine proteases of some parasites (Waag et al. 2010). Treatment with garlic extract also promoted the shift towards a Th1 response in Leishmania major-infected susceptible mice (Ghazanfari et al. 2000) and enhanced the phagocytic activity of peritoneal macrophages (Ghazanfari et al. 2006), which significantly improved the disease outcome. Moreover, treatment with allicin enhances host innate and adaptive immunity against Plasmodium yoelii 17XL infection in a murine model which was evidenced by elevated numbers of macrophages, CD4+ T cells, and cytokines as well as the expansion and maturation of dendritic cells which play an essential role in initiating adaptive immunity (Feng et al. 2012).
Coccidiosis is a widespread disease affecting many vertebrates worldwide. It is caused by the unicellular eukaryote Eimeria spp., of which there are approximately 800 different species (Jenkins 2001). Eimeria spp. are intracellular protozoan parasites (phylum: Apicomplexa) closely related to infectious pathogens of humans such as Cryptosporidium spp. (Shields and Olson 2003). Coccidiosis is a disease complex of poultry and animals which causes prominent signs of mortality, morbidity, diarrhea, or bloody feces. Subclinical coccidiosis is manifested mainly by poor weight gain and reduced efficiency of feed conversion and is the biggest factor in the total economic losses caused by this disease (Williams 1999). Eimeria vermiformis, a murine pathogen, has been widely used as a coccidial model in the laboratory to elucidate the mechanisms of host protection against primary and secondary infections.
We have previously found that the number of intraepithelial lymphocytes (IELs) markedly increased when oocyst production reached a peak during E. vermiformis infection. IELs produce interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) with decreased transforming growth factor β (TGF-β) production, which may protect the host against E. vermiformis infection (Inagaki-Ohara et al. 2006). Although prophylactic chemotherapy at present is the most widely used method for control of coccidiosis, its use is constantly challenged by the emergence of drug-resistant parasites (Chapman 1997). The emergence of drug resistance and the societal pressure against the use of antibiotics is driving coccidiosis research towards the use of unconventional strategies. Therefore, plant oils and extract can be used as alternatives or adjuncts to the current antiparasitic drugs (Anthony et al. 2005; Metwaly et al. 2012). Thus, in the present study, we sought to determine the immunomodulatory and antiparasitic effects of garlic extract as a candidate for an herbal prophylactic regime in murine E. vermiformis infection.
Materials and methods
Experimental animals
Male ICR mice 6–8 weeks of age and weighing 29–32 g were purchased from Charles River Japan, Inc., Yokohama, Japan. All animals were housed in clean cages and given standard diet and clean water ad libitum in an air-conditioned room (23 ± 3 °C) with 12:12 h light:dark cycle. Animals were kept for 1 week before the experiment started for acclimatization. All protocols were approved by the institutional review board for animal experiments of the University of Miyazaki, Japan.
Parasite and infection
E. vermiformis was maintained in our laboratory by passage in mice and oocysts were purified and sporulated (Rose et al. 1984). After microscopically scoring stocks for sporulation, the experimental mice were given 300 sporulated oocysts at 4 weeks of age/mouse in 100 μl of distilled water by oral gavage. During the infection, feces were collected every 3 days. Oocysts were counted as oocysts per day (OPD) on McMaster chambers after salt flotation.
Garlic extract preparation
Dried garlic powder was mixed in distilled water at a concentration of 80 mg/ml and incubated overnight (Balasenthil et al. 1999). The mixture was centrifuged at 3500 rpm for 10 min, and the supernatant was kept at 4 °C until given orally to mice at a dose of 500 mg/kg by stomach tube. The extract preparation was done on a daily basis.
Analysis of mRNA expression of ileum IFN-γ, IL-10, IL-13, and TNF-α genes using real-time PCR
To better understand the effects of E. vermiformis infection on ileal cytokine production (IFN-γ, IL-10, IL-13, and TNF-α) and the effects of garlic extract administration on their synthesis in the ileum, the expression of ileal IFN-γ, IL-10, IL-13, and TNF-α genes was analyzed by real-time PCR using sense and antisense primers on days 3 and 6 postinfection (p.i.).
Total cellular RNA was extracted from ileal tissue using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Real-time PCR was performed using a Power SYBR Green RNA-to-Ct 1-step kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol.
PCR was performed in a 10-μl reaction volume containing 0.08 μl of RT enzyme mix (125×), 5 μl of RT-PCR mix (2×), 3 μl of primers (sense and antisense) (33.3 nM), 1 μl of each RNA template (0.1 pg/μl), and 0.92 μl of nuclease-free water. Primer sets were as follows: IFN-γ, sense (5-CGG CAC AGT CAT TGA AAG CCT-3′) and antisense (5-TGT CAC CAT CCT TTT GCC AGT-3′); IL-10, sense (5′-CGC TGT CAT CGA TTT CTC CCC-3′) and antisense (5′-TCA TGG CCT TGT AGA CAC CTT-3′); IL-13, sense (5′-TGT AGC CCT GGA TTC CCT GA-3′) and antisense (5′-GGT TAC AGA GGC CAT GCA AT-3′); TNF-α, sense (5′-TAT GGC CCA GCC CTC ACA-3′) and antisense (5′-GGA GTA GAC AAG GTA CC AT-3′); and β-actin as housekeeping gene, sense (5′-ATG GAG CCA CCA TCC ACA-3′) and antisense (5′-CAT CCG TAA AGA CCT CTA TGC CA-3′).
The real-time PCR cycling program consisted of reverse transcription at 48 °C for 30 min and initial PCR activation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, and a dissociation curve was added to the protocol whenever necessary. A real-time PCR assay was performed using a 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA).
Changes in gene expression were calculated from the obtained cycle threshold (C t) values provided by real-time PCR instrumentation using the \( {2}^{-\varDelta \varDelta {C}_{\mathrm{t}}} \) calculation, where ∆C t indicates the C t changes in target genes in comparison to a reference (housekeeping) gene (β-actin) (Schmittgen and Livak 2008).
Histopathological and immunohistochemical analysis
Ileum tissues were collected from infected-garlic− and infected-garlic+ groups and were fixed in neutral buffered formalin (pH 7.2) (Nacalai Tesque Inc., Kyoto, Japan). Tissues were scored for the degree of inflammation by examining sections stained with hematoxylin and eosin from each mouse at a magnification of ×400. Inflammatory lesions (hemorrhagic zones, lymphocyte infiltration, plasma cell infiltration, and eosinophil infiltration) and tissue destruction (enterocyte loss and mucosal atrophy) were scored as 3 (no change from normal tissue), 5 (mild lesions involving few areas of the intestinal section), or 7 (severe lesions involving most areas of the intestinal section including mucosa and submucosa). The tissue destruction score was used to represent the total histological injury score (HIS) for each intestinal section (Dommels et al. 2007).
For immunohistochemical analysis, ileal tissues at days 0, 3, 6, and 9 p.i. were frozen in Optimal Cutting Temperature compound (OCT) at −80 °C. Tissues were sectioned using a Cryostat (Leica Biosystems) and stained with CD8α monoclonal antibody (Bio-Rad Laboratories Inc., USA) using an indirect immunoperoxidase technique according to Yasuda et al. (1998).
Experimental design
Twenty-four male ICR mice were randomly allocated into two groups (n = 12/group). The groups were as follows: (1) infected-garlic− group, infected with 300 sporulated oocysts of E. vermiformis orally without garlic treatment, and (2) infected-garlic+ group, infected with 300 sporulated oocysts of E. vermiformis orally and given garlic extract (500 mg/kg body weight) orally for 10 days before infection and until the end of the experiment. Ileum samples were collected on days 0, 3, 6, and 9 p.i.
Statistical analysis
Statistical analysis was performed with the statistical software package SPSS for Windows (version 20.0; SPSS Inc., Chicago, IL). Student’s t test was used to compare data from the infected-garlic− and the infected-garlic+ groups. Results are expressed as mean ± standard error of the mean (SEM). A P value of less than 0.05 was considered significant.
Results
Effect of garlic extract treatment on fecal oocyst output (OPD)
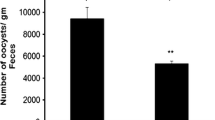
Fecal OPD after infection with 300 sporulated E. vermiformis oocysts was monitored as shown in Fig. 1. Peak OPD occurred on day 9 p.i. in both groups, but treatment of infected mice with garlic extract (infected-garlic+) significantly reduced the OPD by approximately 53 % in comparison to the infected-garlic− group.
Effect of garlic extract treatment on oocyst output per day (OPD) of E. vermiformis-infected male ICR mice (infective dose, 300 sporulated oocysts). Garlic treatment (500 mg/kg given orally) induced a significant decrease in OPD of infected treated mice (infected-garlic+) compared to infected mice (infected-garlic−). **P < 0.01, compared with infected-garlic− values. Bars represent means ± SEM (n = 3)
Effects of garlic extract on ileum pro-inflammatory cytokine gene expression
Treatment of infected mice with garlic extract showed a slight reduction in IL-10 gene expression on day 3 p.i. when compared with the infected group; there was no statistically significant difference in cytokine gene expression (Fig. 2a). However, on day 6 p.i., garlic extract treatment induced a significant decrease in the expression of IL-10. There were no significant changes in IFN-γ, TNF-α, and IL-13 expression levels (Fig. 2b).
Expression of IFN-γ, IL-10, IL-13, and TNF-α mRNA in the ileum. Total RNA was prepared from ileum tissues of mice infected with E. vermiformis with and without garlic treatment on days 3 and 6 p.i. The expression levels were evaluated by real-time PCR. **P < 0.01, compared with control values. Bars represent means ± SEM (n = 3)
Effect of garlic extract on histopathological damage during infection
E. vermiformis infection-induced histopathological changes are observed mainly in the parasitized distal part of the small intestine known as the ileum. In our experiment, E. vermiformis infection induced destructive changes and necrosis in the crypt epithelium of the ileum. Subsequently, villous atrophy was observed followed by mild infiltration of lymphocytes and plasma cells and a few eosinophils in the lamina propria and intestinal crypts. The parasitic oocysts were mainly observed in the crypts of the ileum of infected mice (Fig. 3a). Treatment of mice with garlic extract largely ameliorated E. vermiformis-induced histopathological changes in the ileum which was indicated by a decrease in the incidence of necrosis and villous destruction associated with an obvious decrease in the number of inflammatory cells (lymphocytes, plasma cells, and eosinophils). Parasite load was also reduced in the intestinal crypts as shown in Fig. 3b. Moreover, the HIS for garlic extract treatment showed a significant reduction in tissue inflammation and destructive changes as indicated in Fig. 3c
Histological changes, inflammatory cell infiltration (a and b) and histology index (c) in hematoxylin and eosin-stained sections of the ileum following infection with E. vermiformis without [infected-garlic−] (a) and with [infected-garlic+] garlic extract treatment (b) on day 9 p.i. The infected ileum shows necrosis and villous atrophy associated with inflammatory cell infiltration (head arrow) as well as a high parasitic oocyst population (arrow) in the intestinal crypts (a). Garlic extract treatment shows less histological damage evidenced by infiltrating inflammatory cells and villous destruction (head arrow) as well as decreased oocyst numbers (arrow) (b). Histology index score on day 9 p.i with E. vermiformis shows a significant reduction in the index score as a result of garlic treatment (c). **P < 0.01, infected-garlic+ value compared with infected-garlic− value. Bars represent means ± SEM (n = 3)
CD8+ T cell population in the epithelial layer
Immunohistochemical (IHC) examination revealed that treatment of infected mice with garlic extract induced a significant increase in the CD8+ population which was widely distributed in the epithelial layer of the ileum in comparison to the infected-garlic− group (Fig. 4). CD8+ T cell numbers increased from day 0 and became significantly higher at days 3 and 6 p.i. before decreasing to lower values by day 9 p.i. in both groups (Fig. 5).
Immunohistochemical examination shows CD8+ T cell population (arrow) in ten random VCU of the ileum on days 0, 3, 6, and 9 p.i., infected-garlic− (a, c, e, and g) and infected-garlic+ (b, d, f, and h), respectively. Garlic extract treatment induced an increased number of CD8+ cells which started to increase from day 0 (b) and reached a peak at day 3 (d) and then declined by day 6 (f) to the lower level by day 9 (h) p.i., in comparison to the infected-garlic− group (a, c, g, and d). VCU means villous crypt units
Kinetics of CD8+ T cell expression in ten random VCU of the ileum infected with E. vermiformis oocysts. After garlic extract (infected-garlic+) treatment, the CD8+ T cell population increased gradually from day 0 and became significantly higher at days 3 and 6 p.i. before decreasing by day 9 p.i., compared to the infected-garlic− group. **P < 0.01, infected-garlic+ value compared with infected-garlic− value. Bars represent means ± SEM (n = 3). VCU means villous crypt units
Discussion
In this study, the immunomodulating effects of garlic extract on the cellular immune response at local sites of Eimeria parasitization were evaluated in vivo using an E. vermiformis-infected mice model. This study clearly demonstrated that garlic extract treatment enhanced immunity against E. vermiformis infection to protect the host by increasing the intraepithelial CD8+ T lymphocyte population.
It is well known that during primary E. vermiformis infection CD4+ cells are important in the induction of protective immunity and that Th1-type cytokines, particularly IFN-γ, are essential for mediating the immune mechanisms underlying resistance to E. vermiformis in susceptible mice (Rose et al. 1992; Smith and Hayday 1998). However, CD8+ T lymphocytes are likely to be significant in resistance to re-infection with the parasite (Ford et al. 2001; Rose et al. 1992). We observed that CD8+ T lymphocytes were increased in the E. vermiformis-infected group treated with garlic extract even before infection (day 0) and became significant at days 3 and 6 of infection, indicating garlic targets CD8+ T lymphocytes directly and, following parasite infection, synergistically accelerated the responses. An increase in the number of cytotoxic CD8+ T lymphocytes suggests initiation of specific effector-cell-mediated immune activities against parasitic stages in intestinal tissue. Effector CD8+ T cells produce IFN-γ and TNF-α which enhance cell-mediated immune responses. In addition, cytotoxic components such as perforin and granzymes secreted by these cells ultimately cause a direct or indirect cytotoxic effect on infected cells (Chien et al. 2014). This in turn impairs the intracellular development of parasites in the ileal epithelium of mice before oocysts are formed and released. This accounts for the observed significant decrease in fecal output of E. vermiformis oocysts as well as inflammatory cell infiltration in mice treated with garlic extract. Importantly, the duration of patency and fecal oocyst production are used as measures of the level of infection with E. vermiformis (Yun et al. 2003). Although garlic extract treatment did not affect the duration of patency, it significantly decreased the fecal oocyst production, indicating enhancement of the host immune defense against E. vermiformis infection by garlic extract treatment. It is worth mentioning that garlic produces its effects through allicin, a sulfur compound produced in garlic with antibacterial, antifungal, and antiparasitic activities. The main mechanism and mode of action of allicin is generally via its reaction with the SH group on cysteine residues of the pathogen’s enzymes, resulting in their inactivation (Kyung 2012). Although garlic may have effects on immune mechanisms, it has surely no immune mechanism on its own.
The cellular characterization of intestinal immune response during parasitic infections could not be interpreted far from that of intestinal epithelial cells (IECs). Increasing evidence suggests an active role for IECs as non-professional antigen-presenting cells in sampling luminal antigens, regulating associated T cell responses and preferentially activating CD8+ T cells (Hershberg and Mayer 2000; Ibarra-Velarde and Alcala-Canto 2007). This would, in part, explain the activation of CD8+ T cell function by stimulated IECs due to direct parasitic effects and their role during E. vermiformis infection in our experiment.
An objective of this study was to investigate the gene expression of some cytokines in the infected ileum in the presence and absence of garlic extract treatment. IL-10 is produced by T cells and macrophages, and its major biological function is the control of innate immune responses and cell-mediated immunity. IL-10 is known to regulate immune responses in Eimeria by inhibiting the production of pro-inflammatory cytokines, major histocompatibility complex expression, and nitric oxide production, by direct effects on T cells, B cells, NK cells, and antigen-presenting cells (Rothwell et al. 2004). Major sources of IL-10 include activated T cells, B cells, macrophages, mast cells, and epithelial cells. In the current study, IL-10 expression was found to be significantly suppressed in the ileum following garlic extract treatment. IL-10 diminishes the capacity of innate immune cells to kill pathogens and generate and maintain responsive antigen-specific T cells. This occurs through downregulation of major histocompatibility complex class II proteins (Bogdan et al. 1991) and suppression of the activated macrophage function, which are strongly required to assist CD8+ T cell cytotoxicity (Gazzinelli et al. 1992). IL-10 has also been reported to have immunostimulatory effects by increasing IFN-γ production in mice and humans (Shibata et al. 1998). This might explain the absence of a significant change in IFN-γ during garlic extract treatment as a result of a significant downregulation of intestinal IL-10 gene expression.
IL-13 is a cytokine produced mainly by Th2 cells (Kidd 2003) which causes marked goblet cell differentiation (Kondo et al. 2002). Because E. vermiformis infection was associated mainly with Th1 responses (Smith and Hayday 1998) and reduced numbers of goblet cells in our previous study (Linh et al. 2009), we did not expect to find significant changes in the expression of IL-13 in the infected ileum treated with garlic extract in the current study. Further studies exploring the effects of garlic treatment on cytokine synthesis during Eimeria infections would be helpful to resolve this issue.
In conclusion, garlic extract treatment enhanced the host protective immunity against a primary infection with E. vermiformis in ICR mice by increasing the intraepithelial CD8+ T lymphocyte population without increasing IFN-γ production. Further studies are needed to elucidate the signals responsible for CD8+ T cell recruitment or activation, and how this is linked to gene expression of functional molecules in epithelial cells and IELs following garlic extract treatment.
References
Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1(2):125–129
Ankri S, Miron T, Rabinkov A, Wilchek M, Mirelman D (1997) Allicin from garlic strongly inhibits cysteine proteinases and cytopathic effects of Entamoeba histolytica. Antimicrob Agents Chemother 41(10):2286–2288
Anthony JP, Fyfe L, Smith H (2005) Plant active components—a resource for antiparasitic agents? Trends Parasitol 21(10):462–468
Balasenthil S, Arivazhagan S, Ramachandran CR, Nagini S (1999) Effects of garlic on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Cancer Detect Prev 23(6):534–538
Bogdan C, Vodovotz Y, Nathan C (1991) Macrophage deactivation by interleukin 10. J Exp Med 174(6):1549–1555
Cao HX, Zhu KX, Fan JG, Qiao L (2014) Garlic-derived allyl sulfides in cancer therapy. Anti Cancer Agents Med Chem 14(6):793–799
Chapman HD (1997) Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 26(2):221–244
Chien YH, Meyer C, Bonneville M (2014) γδ T cells: first line of defense and beyond. Annu Rev Immunol 32:121–155
Chung LY (2006) The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food 9(2):205–213
Coppi A, Cabinian M, Mirelman D, Sinnis P (2006) Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents Chemother 50(5):1731–1737
Dommels YE, Butts CA, Zhu S, Davy M, Martell S, Hedderley D, Barnett MP, McNabb WC, Roy NC (2007) Characterization of intestinal inflammation and identification of related gene expression changes in mdr1a(−/−) mice. Genes Nutr 2(2):209–223
Feng Y, Zhu X, Wang Q, Jiang Y, Shang H, Cui L, Cao Y (2012) Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar J 11:268
Ford JT, Wong CW, Colditz IG (2001) Effects of dietary protein types on immune responses and levels of infection with Eimeria vermiformis in mice. Immunol Cell Biol 79(1):23–28
Gazzinelli RT, Oswald IP, James SL, Sher A (1992) IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol 148(6):1792–1796
Ghazanfari T, Hassan ZM, Ebtekar M, Ahmadiani A, Naderi G, Azar A (2000) Garlic induces a shift in cytokine pattern in Leishmania major-infected BALB/c mice. Scand J Immunol 52(5):491–495
Ghazanfari T, Hassan ZM, Khamesipour A (2006) Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment. J Ethnopharmacol 103(3):333–337
Han J, Lawson L, Han G, Han P (1995) A spectrophotometric method for quantitative determination of allicin and total garlic thiosulfinates. Anal Biochem 225(1):157–160
Hershberg RM, Mayer LF (2000) Antigen processing and presentation by intestinal epithelial cells—polarity and complexity. Immunol Today 21(3):123–128
Ibarra-Velarde F, Alcala-Canto Y (2007) Downregulation of the goat beta-defensin-2 gene by IL-4 in caprine intestinal epithelial cells infected with Eimeria spp. Parasitol Res 101(3):613–618
Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith AL, Jimi F, Miyahira M, Abdel-Aleem AS, Horii Y, Nawa Y (2006) Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun 74(9):5292–5301
Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K (2006) Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr 136(3 Suppl):816S–820S
Jenkins MC (2001) Advances and prospects for subunit vaccines against protozoa of veterinary importance. Vet Parasitol 101(3–4):291–310
Kidd P (2003) Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 8(3):223–246
Kondo M, Tamaoki J, Takeyama K, Nakata J, Nagai A (2002) Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 27(5):536–541
Kyo E, Uda N, Kasuga S, Itakura Y (2001) Immunomodulatory effects of aged garlic extract. J Nutr 131(3s):1075S–1079S
Kyung KH (2012) Antimicrobial properties of allium species. Curr Opin Biotechnol 23(2):142–147
Linh BK, Hayashi T, Horii Y (2009) Eimeria vermiformis infection reduces goblet cells by multiplication in the crypt cells of the small intestine of C57BL/6 mice. Parasitol Res 104(4):789–794
Lun ZR, Burri C, Menzinger M, Kaminsky R (1994) Antiparasitic activity of diallyl trisulfide (Dasuansu) on human and animal pathogenic protozoa (Trypanosoma sp., Entamoeba histolytica and Giardia lamblia) in vitro. Ann Soc Belg Med Trop 74(1):51–59
Mantawy MM, Ali HF, Rizk MZ (2011) Therapeutic effects of Allium sativum and Allium cepa in Schistosoma mansoni experimental infection. Rev Inst Med Trop Sao Paulo 53(3):155–163
Metwaly MS, Dkhil MA, Al-Quraishy S (2012) The potential role of Phoenix dactylifera on Eimeria papillata-induced infection in mice. Parasitol Res 111(2):681–687
Rose ME, Owen DG, Hesketh P (1984) Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology 88(Pt 1):45–54
Rose ME, Hesketh P, Wakelin D (1992) Immune control of murine coccidiosis: CD4+ and CD8+ T lymphocytes contribute differentially in resistance to primary and secondary infections. Parasitology 105(Pt 3):349–354
Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, Smith AL, Kaiser P (2004) Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol 173(4):2675–2682
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Shadkchan Y, Shemesh E, Mirelman D, Miron T, Rabinkov A, Wilchek M, Osherov N (2004) Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J Antimicrob Chemother 53(5):832–836
Shibata Y, Foster LA, Kurimoto M, Okamura H, Nakamura RM, Kawajiri K, Justice JP, Van Scott MR, Myrvik QN, Metzger WJ (1998) Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol 161(8):4283–4288
Shields JM, Olson BH (2003) Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int J Parasitol 33(4):371–391
Smith AL, Hayday AC (1998) Genetic analysis of the essential components of the immunoprotective response to infection with Eimeria vermiformis. Int J Parasitol 28(7):1061–1069
Soffar SA, Mokhtar GM (1991) Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasis. J Egypt Soc Parasitol 21(2):497–502
Waag T, Gelhaus C, Rath J, Stich A, Leippe M, Schirmeister T (2010) Allicin and derivates are cysteine protease inhibitors with antiparasitic activity. Bioorg Med Chem Lett 20(18):5541–5543
Wabwoba BW, Anjili CO, Ngeiywa MM, Ngure PK, Kigondu EM, Ingonga J, Makwali J (2010) Experimental chemotherapy with Allium sativum (Liliaceae) methanolic extract in rodents infected with Leishmania major and Leishmania donovani. J Vector Borne Dis 47(3):160–167
Williams RB (1999) A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 29(8):1209–1229
Yasuda M, Taura Y, Yokomizo Y, Ekino S (1998) A comparative study of germinal center: fowls and mammals. Comp Immunol Microbiol Infect Dis 21(3):179–189
Yun CH, Estrada A, Van Kessel A, Park BC, Laarveld B (2003) Beta-glucan, extracted from oat, enhances disease resistance against bacterial and parasitic infections. FEMS Immunol Med Microbiol 35(1):67–75
Acknowledgments
One of the authors, A.M. Khalil, received financial support from the Egyptian government for Channel System Program towards a PhD. This work was supported by the Project for Zoonoses Education and Research, University of Miyazaki, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalil, A.M., Yasuda, M., Farid, A.S. et al. Immunomodulatory and antiparasitic effects of garlic extract on Eimeria vermiformis-infected mice. Parasitol Res 114, 2735–2742 (2015). https://doi.org/10.1007/s00436-015-4480-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4480-5