Abstract
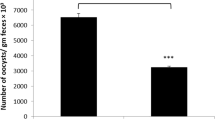
Coccidiosis in poultry is caused by protozoan parasites of the genus Eimeria, which is responsible for worldwide economic losses. The methanolic extract of Azadirachta indica (neem) leaves was used in vivo for its pharmacological, antioxidant, and anticoccidial properties. Four groups of mice were investigated. The first group was inoculated only with sterile saline and served as the control group. The second group was treated by oral gavage with neem extract (500 mg/kg) daily for 4 days. The third and fourth groups were infected with 103 sporulated oocysts of Eimeria papillata. The fourth group was also treated once daily with neem extract for 4 days. Paraffin sections from the jejunum as well as jejunal homogenate were prepared for the histopathological and biochemical investigations, respectively. The data showed that mice infected with E. papillata revealed an output of 6.5 × 105 ± 29,753 oocysts per gram feces on day 4 postinoculation. This output is significantly decreased to 2.7 × 105 ± 37,341 oocysts in neem-treated mice. Infection with E. papillata induced marked histopathological alterations in the jejunum in the form of inflammation, vacuolation of the epithelium, and destruction of some villi. Also, the neem extract greatly diminished body weight loss of infected mice. Moreover, the number of goblet cells stained with Alcian blue within the infected villi was significantly lowered (P ≤ 0.05). In addition, E. papillata enhanced lipid peroxidation and nitric oxide production in both serum and jejunum with concomitant reduction in glutathione. Neem induced marked improvements in all of the studied parameters as well as the histopathological features of the jejunum. Our study revealed that neem as a natural product has protective effects against E. papillata-induced coccidiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is an enteric parasitic disease caused by multiple protozoan parasite species of the genus Eimeria and is one of the most common and expensive diseases in the poultry industry (Mehlhorn 2008; Khalafalla et al. 2011). It has a major economic impact on both growers and the poultry industry worldwide (Al-Quraishy et al. 2011; Metwaly et al. 2012).
In recent years, evidence has accumulated for a role of reactive oxygen metabolites as a mediator of tissue injury in several animal models (Özdemir et al. 2010; Fukui et al. 2011; Sato et al. 2011). Although the exact mechanisms of free-radical generation are still not completely understood, it is postulated that the antioxidant glutathione (GSH) depletion by the intestinal parasites may be a trigger for the production of reactive oxygen species (ROS) (Cam et al. 2008). Generation of ROS in the cytoplasm of cells may increase the mitochondrial hydrogen peroxide production and lipid peroxidation of cell and mitochondrial membranes, resulting in loss of membrane integrity and, ultimately, cell necrosis or apoptosis (Valko et al. 2007).
Attention has been focused on the protective effect of naturally occurring antioxidants in biological systems against parasites (Schmahl et al. 2010; Al-Quraishy et al. 2011; Dkhil et al. 2011; Kayano et al. 2011). The present study uses the leaves of Azadirachta indica that is commonly known as the neem tree. It has attracted worldwide prominence in recent years, owing to its wide range of medicinal properties (Abdel-Ghaffar et al. 2010). Alkaloids, tannins, proteins, coumarin, flavonoids, polyphenols, saponins, and sugars have been isolated from the neem tree (Anyaehie 2009). The leaves of the neem tree are traditionally used as medicinal preparations for their immunomodulatory, anti-inflammatory, antihyperglycemic, antiulcer, antimalarial, antifungal, antibacterial, antiviral, antimutagenic and anticarcinogenic properties (Anyaehie 2009), antioxidant, hepatoprotective (Yanpallewar et al. 2003), and cardioprotective effects (Peer et al. 2008). The present study has been designed to investigate the ameliorative effect of neem leaf extract on the histopathology and oxidative stress mediated by the intestinal coccidian parasite Eimeria papillata.
Materials and methods
Animals
Swiss albino mice were bred under specified pathogen-free conditions and fed a standard diet and water ad libitum. The experiments were performed only with male mice at an age of 9–11 weeks, approved by state authorities, and followed Saudi Arabian rules for animal protection.
Infection of mice
A self-healing strain of E. papillata was kindly provided by Prof. Mehlhorn (Heinrich Heine University, Duesseldorf, Germany). We maintained E. papillata in naturally infected mice, collected oocysts (Fig. S1) from feces, surface sterilized the oocysts with sodium hypochlorite, and washed at least four times with sterile saline before oral inoculation as described by Schito et al. (1996). Each mouse was orally inoculated with 103 sporulated oocysts of E. papillata suspended in 100 μl sterile saline. Subsequently, fresh fecal pellets were collected every 24 h. The collected pellets from each mouse were weighed, and the bedding was changed to eliminate reinfection. Oocyst output was measured as previously described (Schito et al. 1996). Fecal pellets were suspended in 2.5 % (wt/vol) potassium dichromate and diluted in saturated sodium chloride for oocyst flotation. Oocysts were counted in a McMaster chamber and expressed as number of oocysts per gram of wet feces.
Preparation of the neem leaf extract
Fresh matured leaves of A. indica (neem tree) were collected in August from the garden in Obour City, Cairo, Egypt. The samples were authenticated by Dr. Jacob Thomas (Botany Department, College of Science, King Saud University, Saudi Arabia) on the basis of taxonomic characters and by direct comparison with the herbarium specimens with voucher number KSU-10804 available at the herbarium of the Botany Department (King Saud University, Saudi Arabia). Neem leaf extract was prepared according to the method described by Manikandan et al. (2008) with some modification. Air-dried powder (100 g) of neem leaves was extracted by percolation at room temperature with 70 % methanol and kept at 4 °C for 24 h. The obtained extract was concentrated under reduced pressure (bath temperature, 50 °C) and dried in a vacuum evaporator. The residue was dissolved in distilled water, filtered, and used in our experiment.
Experimental design
Four groups of mice, with six animals per group, were investigated. The first group was inoculated only with sterile saline and served as the control group. The second group was treated by oral gavage with 100 μl neem leaf extract (500 mg/kg) daily over 4 days. The dose and the route of injection were selected on the basis of the previous studies (Bhanwra et al. 2000; Ezz-Din et al. 2011). The third and fourth groups were infected with 103 sporulated oocysts of E. papillata and treated either with sterile saline or neem leaf extract over 4 days, respectively.
Histological analysis
Pieces of jejunum were freshly prepared, fixed in 10 % neutral buffered formalin, and then embedded in paraffin. Sections were cut and then stained with hematoxylin and eosin. According to Dommels et al. (2007), tissue sections were scored for inflammatory lesions (infiltrations by mononuclear cells, neutrophils, eosinophils, and plasmacytes, for fibrin exudation and lymphangiectasis, for tissue destruction (enterocyte loss, ballooning degeneration, edema, and mucosal atrophy), and for tissue repair (hyperplasia, angiogenesis, granulomas, and fibrosis). A rating score between 0 (no change from normal tissue) and 3 (lesions involved most areas and all the layers of the intestinal section including the mucosa, muscle, and omental fat) was given for each aspect of inflammatory lesion, tissue destruction, and tissue repair. The sum of inflammatory lesions, tissue destruction, and tissue repair scores was used to represent the total histological injury score (HIS) for each intestinal section. The sum of the inflammatory lesions was multiplied by 2 to give more weight to this value since the tissue changes were mainly characterized by inflammatory lesions (Dommels et al. 2007).
Parasite number and goblet cells
Sections stained with hematoxylin–eosin were used for parasite detection, and those stained with Alcian Blue were used for the determination of goblet cells. For each animal, the number of goblet cells in the jejunum was counted on at least ten well-orientated villous-crypt units (VCU). Results were expressed as the mean number of goblet cells per ten VCU (Allen et al. 1986). The number of parasitic infections in ten VCU (mainly found within the crypt) was counted.
Biochemical analysis
Animals were cervically dislocated on day 4 postinoculation (p.i.); blood was collected into heparinized tubes, and plasma was separated and kept at −20 °C until use. Parts of the jejunum were weighed and homogenized immediately to give 50 % (w/v) homogenate in ice-cold medium containing 50 mM Tris–HCl and 300 mM sucrose (Tsakiris et al. 2004). The homogenate was centrifuged at 500×g for 10 min at 4 °C. The supernatant (10 %) was used for the various biochemical determinations.
GSH was determined chemically in jejunum homogenate using Ellman's reagent (Ellman 1959). The method is based on the reduction of Ellman's reagent (5,5′ dithiobis (2-nitrobenzoic acid) with GSH to produce a yellow compound. The chromogen is directly proportional to GSH concentration, and its absorbance was measured at 405 nm.
Lipid peroxidation in plasma and jejunum homogenate was determined according to the method of Ohkawa et al. (1979) by using 1 ml of trichloroacetic acid 10 % and 1 ml of thiobarbituric acid 0.67 %, followed by heating in a boiling water bath for 30 min. Thiobarbituric acid-reactive substances were determined by the absorbance at 535 nm and expressed as malondialdehyde (MDA) equivalents formed.
The assay of nitric oxide (NO) in plasma and jejunum homogenate was done according to the method of Berkels et al. (2004). In acid medium and in the presence of nitrite, the formed nitrous acid diazotizes sulfanilamide, which is coupled with N-(1-naphthyl) ethylenediamine. The resulting azo dye has a bright reddish-purple color which was measured at 540 nm.
Statistical analysis
One-way ANOVA was carried out, and the statistical comparisons among the groups were performed with Duncan's test using a statistical package program (SPSS version 17.0). All P values are two tailed, and P < 0.05 was considered as significant for all statistical analyses in this study.
Results
The effect of neem leaf extract on the outcome of E. papillata infections was investigated. During the first 3 days of infection, there was no fecal output of oocysts. On day 4 p.i., the output differed between neem-treated and non-treated mice. In the latter, the number of excreted oocysts reached approximately 6.5 × 105 per gram feces (Table. 1). However, the neem treatment significantly lowered the shedding of oocysts to about 60 % on day 4 p.i. (Table 1). Concomitantly, the average weight of mice was significantly decreased (P < 0.01). This weight loss was associated with watery mucoid diarrhea and decreased uptake of water and food. Interestingly, the treatment of infected mice with neem leaf extract resulted in lower weight loss induced by infection (Table 1).
Light microscopic inspection of hematoxylin and eosin-stained sections revealed that the epithelial cells of the jejunum were infected by E. papillata (Fig. S2). Concomitantly, we observed some histological changes which were semiquantified by applying the scoring according to Dommels et al. (2007). Histological analysis revealed that mice infected with sporulated oocysts of E. papillata suffered a moderate inflammatory injury in the jejunum (Fig. 1). This injury was diminished when mice were treated with neem leaf extract.
The developmental stages of E. papillata parasites were located within the cytoplasm of the epithelial cells of the jejunum. The number of parasites per ten villous-crypt units was reduced to about 50 % when mice were treated with neem leaf extract (Table 1). In addition, there was a significant reduction of the goblet cell numbers seen at the site of the E. papillata infection in the jejunum (Figs. 2, S3). Again, neem leaf extract treatment was associated with a significant increase in the E. papillata-induced decreases in goblet cells (Figs. 2, S3). Treatment of uninfected mice with neem for 4 days had no effect on goblet cell numbers in the jejunum (Figs. 2, S3).
E. papillata infections also induced a highly significant increase in jejunum NO and MDA by approximately 85.7 and 67.5 %, respectively (Table 2). In blood plasma, increases in NO and MDA were less pronounced (Table 2). Again, neem treatment significantly lowered the E. papillata-induced increase in both NO and MDA, respectively (Table 2). Finally, we also determined GSH which is the major component involved in the downregulation of substances formed during oxidative stress (Fig. 3). Conspicuously, GSH was significantly downregulated by E. papillata infections, and these effects were largely prevented by neem treatment (Fig. 3).
Discussion
The prolonged use of synthetic anticoccidials such as toltrazuril, a common anticoccidial product considered as reference drug in controlling coccidiosis (Mehlhon et al. 1984), often causes development of resistance to these drugs (Toulah et al. 2010). The initial hope underlying the present study was to find a natural product with anticoccidial properties that could be used as a feed additive with minimal processing. Neem acts like toltrazuril (Toulah et al. 2010) by exhibiting anticoccidial activity, evidenced as a significant lowering in the output of E. papillata oocysts in the feces of the infected mice. This diminished output reflects that neem impairs the development of parasites in the host before the relatively inert oocysts are formed and finally released. The fact that neem possesses anticoccidial activity has been also reported in chicken coccidiosis (Toulah et al. 2010). Also, Toulah et al. (2010) reported that toltrazuril has apparent toxic histopathological effects on ceca of infected chickens while neem could improve the induced histopathological alterations induced by E. papillata (Fig. 1).
Our study shows that neem does not only target Eimeria parasites in hosts but also exhibits anti-inflammatory activity, thus protecting host tissues. Indeed, E. papillata infections cause an inflammatory response in the jejunum of mice. Kaur et al. (2004) reported that certain fractions and extracts of neem may possess anti-inflammatory properties.
Manikandan et al. (2008) have reported that one of the major protective functions of neem is to decrease the oxidative damage in mice. Indeed, neem prevents the infection-induced loss of GSH and increased production of NO and MDA. These components are normally lowered during oxidative damage induced by infection as it has been also described by Georgieva et al. (2006) who showed an increase in MDA levels of Eimeria tenella-induced coccidiosis. In accordance, Balasenthil et al. (1999) have found that neem significantly decreased lipid peroxidation and increased GSH. The antioxidative property of neem has been previously ascribed mainly to its major chemical component, azadirachtin (Manikandan et al. 2008). Azadirachtin in neem leaves has been reported to produce antiprotozoan, antibacterial, and antifungal effects (Morgan 2009).
The intestinal tract is a composite of immune tissues and is important as a first barrier of host defense against pathogen invasion (MacDonald and Monteleone 2005). The effectiveness of the mucosal immune system in the intestine to prevent pathogen invasion is mainly dependent on the collaboration of immunocompetent cells as well as other factors (MacDonald and Monteleone 2005). It is well known that mast cells and goblet cells are the major intestinal immunocompetent cells. Some studies have provided evidence that the mucus released by goblet cells can function as a defensive barrier (Deplancke and Gaskins 2001). In short, these immunocompetent cells are very important to the normal physiological functions of the intestine (MacDonald and Monteleone 2005). The depletion of goblet cells was most evident in the infected crypt and much less so in the neighboring and other uninfected areas of the intestine. In addition, the parasites were mostly discovered at the bottom of the intracrypt epithelium. However, there was no evidence of an E. papillata infection within the goblet cells. These findings suggest that the decrease in the goblet cell numbers resulted directly or indirectly from infections of the crypt. Since E. papillata parasites develop in the crypt region that contains the multipotential stem cells (Cheng 1974), the reduction in goblet cells may reflect damage to the stem cell population. Indeed, goblet cells arise by mitosis from multipotential stem cells at the base of the crypt (Cheng 1974). Changes in goblet cell numbers may affect the susceptibility of the parasite-infected host to limit the capacity of opportunistic pathogens from increasing or penetrating the local epithelium (Yunus et al. 2005). Neem was able to increase the number of goblet cells of the mouse jejunum after infection with E. papillata sporulated oocysts. The mechanism of goblet cell upregulation should be studied.
Collectively, our data indicate that neem exhibits a significant anticoccidial activity and, coincidently, a significant improvement in histopathological picture of the jejunum as well as the antioxidant status, and protects the host tissue from injuries induced by parasites.
References
Abdel-Ghaffar F, Semmler M, Al-Rasheid KA, Strassen B, Fischer K, Aksu G, Klimpel S, Mehlhorn H (2010) The effects of different plant extracts on intestinal cestodes and on trematodes. Parasitol Res 108:979–984
Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA (1986) The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Supp 125:71–78
Al-Quraishy S, Delic D, Sies H, Wunderlich F, Abdel-Baki AA, Dkhil MA (2011) Differential miRNA expression in the mouse jejunum during garlic treatment of Eimeria papillata infections. Parasitol Res 109:387–394
Anyaehie UB (2009) Medicinal properties of fractionated acetone/water neem (Azadirachta indica) leaf extract from Nigeria: a review. Nig J Physiol Sci 24:157–169
Balasenthil S, Arivazhagan S, Ramachandran CR, Ramachandran V, Nagini S (1999) Chemopreventive potential of neem (Azadirachta indica) on 7,12-dimethylbenz[a]anthracene (DMBA) induced hamster buccal pouch carcinogenesis. J Ethnopharmacol 67:189–195
Berkels R, Purol-Schnabel S, Roesen R (2004) Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol Biol 279:1–8
Bhanwra S, Singh J, Khosla P (2000) Effect of Azadirachta indica (neem) leaf aqueous extract on paracetamol-induced liver damage in rats. Ind J Physiol Pharmacol 44:64–68
Cam Y, Atasever A, Eraslan G, Kibar M, Atalay O, Beyaz L, Inci A, Liman BC (2008) Eimeria stiedae: experimental infection in rabbits and the effect of treatment with toltrazuril and ivermectin. Exp Parasitol 119:164–172
Cheng H (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. II. Mucous cells. Am J Anat 141:481–501
Deplancke B, Gaskins HR (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73:1131S–1141S
Dkhil MA, Abdel-Baki AS, Wunderlich F, Sies H, Al-Quraishy S (2011) Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 175:66–72
Dommels YE, Butts CA, Zhu S, Davy M, Martell S, Hedderley D, Barnett MP, McNabb WC, Roy NC (2007) Characterization of intestinal inflammation and identification of related gene expression changes in mdr1a(−/−) mice. Genes Nutr 2:209–223
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ezz-Din D, Gabry MS, Farrag AH, Abdel Moneim AE (2011) Physiological and histological impact of Azadirachta indica (neem) leaves extract in a rat model of cisplatin-induced hepato and nephrotoxicity. J Med Plants Res 5:5499–5506
Fukui K, Takatsu H, Koike T, Urano S (2011) Hydrogen peroxide induces neurite degeneration: prevention by tocotrienols. Free Rad Res 45:681–691
Georgieva N, Koinarski V, Gadjeva V (2006) Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet J 172:488–492
Kaur G, Sarwar Alam M, Athar M (2004) Nimbidin suppresses functions of macrophages and neutrophils: relevance to its antiinflammatory mechanisms. Phytother Res 18:419–424
Kayano ACA, Lopes SCP, Bueno FG, Cabral EC, Souza-Neiras WC, Yamauchi LM, Foglio MA, Eberlin MN, Mello JCP, Costa FTM (2011) In vitro and in vivo assessment of the antimalarial activity of Caesalpinia pluviosa. Malaria J 10:112–122
Khalafalla RE, Daugschies A, Dyachenko V (2011) Cross-reactivity of anti-Eimeria tenella antibody fragments on merozoites and sporozoites of different chicken Eimeria species. Parasitol Res 108:745–749
MacDonald TT, Monteleone G (2005) Immunity, inflammation, and allergy in the gut. Science 307:1920–1925
Manikandan P, Letchoumy PV, Gopalakrishnan M, Nagini S (2008) Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and in vivo modulation of biomarkers of chemoprevention in the hamster buccal pouch carcinogenesis model. Food Chem Toxicol 46:2332–2343
Mehlhon H, Ortiman FG, Ha-berkorn A (1984) Effects of sym. Trizanones on developmental stages of Eimeria tenella, E. maxima and E. acervulina: a light and electron microscopy study. Parasitol Res 70:173–182
Mehlhorn H (ed) (2008) Encyclopedic reference of parasitology, vol 1, 3rd edn. Berlin, Springer
Metwaly MS, Dkhil MA, Al-Quraishy S (2012) The potential role of Phoenix dactylifera on Eimeria papillata-induced infection in mice. Parasitol Res 111:681–687
Morgan ED (2009) Azadirachtin, a scientific gold mine. Bioorg Med Chem 17:4096–4105
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem 95:351–358
Özdemir ÖM, Ergin H, Yenisey C, Türk NS, Şimşek NG (2010) Protective effects of clarithromycin in rats with hypoxia/reoxygenation-induced intestinal injury. J Pediatric Surg 45:2169–2174
Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD (2008) Cardioprotective effect of Azadirachta indica A. Juss. on isoprenaline induced myocardial infarction in rats. Int J Cardiol 126:123–126
Sato Y, Itagaki S, Oikawa S, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K (2011) Protective effect of soy isoflavone genistein on ischemia-reperfusion in the rat small intestine. Biol Pharmaceut Bullet 34:1448–1454
Schito ML, Barta JR, Chobotar B (1996) Comparison of four murine Eimeria species in immunocompetent and immunodeficient mice. J Parasitol 82:255–262
Schmahl G, Al-Rasheid KA, Abdel-Ghaffar F, Klimpel S, Mehlhorn H (2010) The efficacy of neem seed extracts (Tre-san, MiteStop) on a broad spectrum of pests and parasites. Parasitol Res 107:261–269
Toulah FH, Ismeel HA, Khan S (2010) Effect of treatment with neem (Azadirachta indica) compared with Baycox drug on the caecum of chicken experimentally infected with Eimeria tenella. J Egypt Soc Parasitol 40:93–106
Tsakiris S, Schulpis KH, Marinou M, Behrakis P (2004) Protective effect of L-cysteine and glutathione on the modulated suckling rat brain Na+, K+, -ATPase and Mg2+ -ATPase activities induced by the in vitro galactosaemia. Pharmacol Res 495:475–479
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Yanpallewar SU, Sen S, Tapas S, Kumar M, Raju SS, Acharya SB (2003) Effect of Azadirachta indica on paracetamol-induced hepatic damage in albino rats. Phytomedicine 10:391–396
Yunus M, Horii Y, Makimura S, Smith AL (2005) Murine goblet cell hypoplasia during Eimeria pragensis infection is ameliorated by clindamycin treatment. J Vet Med Sci 67:311–315
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project no. RGP-VPP-198.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Figure S1
Unsporulated (a) and sporulated (b) oocyst of E. papillata. OM outer membrane, IM inner membrane, SPW sporoblast wall, SP sporoblast, Sc sporocyst, Scw sporocyst wall, RB residual body. (JPEG 33 kb)

Figure S2
Sections of mouse jejunum infected with E. papillata on day 4 p.i. a Noninfected jejunum with normal architecture. b Noninfected treated mouse jejunum with normal structure. c Infected jejunum with some pathological changes and developmental stages appearing in the inner epithelium. d Infected treated mouse with less parasites. Sections are stained with hematoxylin and eosin. Bar = 25 μm (JPEG 595 kb)

Figure S3
Changes in goblet cell numbers in mouse jejunum infected with E. papillata on day 4 p.i. a Noninfected jejunum, b noninfected treated mouse jejunum, c infected jejunum with decreased number of goblet cells, d infected treated mouse. Sections are stained with Alcian blue. Bar = 25 μm (JPEG 472 kb)
Rights and permissions
About this article
Cite this article
Dkhil, M.A., Al-Quraishy, S., Abdel Moneim, A.E. et al. Protective effect of Azadirachta indica extract against Eimeria papillata-induced coccidiosis. Parasitol Res 112, 101–106 (2013). https://doi.org/10.1007/s00436-012-3109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3109-1