Abstract
Sceloporus bicanthalis inhabits the Nevado de Toluca, México, and the males exhibit continuous spermatogenesis with mature sperm available year round. Females were collected monthly to evaluate the morphology of the oviducts and the presence of sperm storage. Histological examination revealed that the oviductal structure in this lizard is similar to that described for other lacertilian species: anterior infundibulum, glandular uterus, and a posterior non-glandular uterus. The oviduct wall consists of a superficial visceral pleuroperitoneum, a middle layer of smooth muscle with outer longitudinal and inner circular fibers, and a deep mucosa or lamina propria deeply lined by an epithelium containing ciliated and non-ciliated secretory cells. Spermatozoa are stored at the base of the mucosal folds, in crypts and in sperm storage tubules at the transition between the glandular uterus and non-glandular uterus, as well as in the anterior non-glandular uterus of previtellogenic, vitellogenic, pregnant, and postpartum females. The sperm in the oviductal cavity or in retention sites is in contact with secretory products derived from non-ciliated epithelial cells. Spermatozoa usually assume an orderly distribution with their heads aligned and oriented toward the base of the folds or crypts. This study shows that females of S. bicanthalis exhibit prolonged sperm storage that corresponds with the continuous reproductive strategy employed by males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In reptiles, oviducts are paired structures that are suspended by mesentery in the posterior pleuroperitoneal coelom, dorsally situated on each side of the body (Girling 2002). Generally in lizards, the oviduct is divided into three regions which differ in structure and function: anterior infundibulum, middle uterus, and posterior vagina (Cuellar 1966; Fox 1963, 1977; Blackburn 1998). These complex structures serve multiple functions such as fertilization, egg transport, albumin production, shell membrane formation, egg and/or embryo upkeep until hatching or birth, placentation in viviparous species, and sperm retention (Cuellar 1966; Blackburn 1998; Girling 2002).
Siegel et al. (2015) offered a recent review that incorporates much of Blackburn (1998) and Girling (2002) recent work in lizards. In this review Siegel et al. (2015) employed terminology provided by Siegel et al. (2011) in snakes in order to maintain consistency among lepidosaurs. This terminology includes the infundibulum at the cranial-most portion of the oviduct, glandular uterus for the middle region, and non-glandular uterus at the caudal-most region. Histologically, three distinct tissue layers are observed in the oviduct: (1) an outer pleuroperitoneal cover composed of a thin serosa membrane, (2) a middle myometrial layer composed of outer longitudinal and inner circular smooth muscle, and (3) an internal endometrial layer or mucosa which consists of the lamina propria and a luminal epithelium with ciliated and non-ciliated cells (Cuellar 1966; Guillette and Jones 1985; Blackburn 1998; Girling 2002).
Oviductal sperm storage is a common phenomenon known in females from all reptile taxa except Amphisbaenia (see Blackburn 1998; Girling 2002; Sever and Hamlett 2002). It is a mechanism that allows females the temporal separation of mating from fertilization, therefore optimizing the reproductive cycles of males and females to ensure fertile eggs regardless of the time ovulation, mating, and/or spermatogenic developmental strategy. This strategy is advantageous because it extends the available reproductive period of females, promotes fertilization in situations where males are few or less active, and contributes to sperm competition and/or multiple paternity (Girling 2002; Olsson and Madsen 1998).
Within reptiles, sperm storage duration is variable, depending on the species. Spermatozoa can be stored a few months (3 months in lizards, Cuellar 1966; Villagrán-Santa Cruz et al. 1992; Girling 2002; Siegel et al. 2015) or years (four years in turtles or seven in snakes; Sever and Hamlett 2002; Girling 2002). The specific location where sperm storage occurs is also variable within given taxa. In crocodiles two regions can contain sperm: one is the transition between glandular uterus and non-glandular uterus, and the other is the posterior infundibulum (Gist et al. 2008; Bagwill et al. 2009). In turtles, sperm storage is localized in specialized tubules located in the region of the posterior infundibulum and the glandular uterus (see Sever and Hamlett 2002). In squamates (lizards and snakes) it occurs in the posterior region of the infundibulum, and specifically in iguanids it is located at the transition between glandular uterus and non-glandular uterus (Cuellar 1966; Blackburn 1998; Girling 2002; Sever and Hamlett 2002; Siegel et al. 2015).
Sceloporus bicanthalis is a viviparous lizard (Guillette 1981) that is nested within the scalaris group (Leache 2010) in the family Phrynosomatidae. This species has been heavily studied in regard to reproductive biology. Studies on S. bicanthalis include investigations on the breeding strategies and breeding cycles by Guillette (1981), Guillette and Jones (1985), Manríquez-Morán (1995), Hernández-Gallegos (1995), Hernández-Gallegos et al. (2002), Mendoza-Cruz and Villagrán-Santa Cruz (2007). Recently, demography studies in a high-mountain population have been carried out by Rodríguez-Romero et al. (2011), and finally Gribbins et al. (2011) and Rheubert et al. (2012) have focused on the study of continuous spermatogenesis and spermiogenic development, respectively.
Sceloporus bicanthalis females reach reproductive maturity at 42 mm in SVL. They present a continuous reproductive cycle at the population level, which is characterized by long periods at the different gonadal activity stages (Ambriz-Rosales 2010), and by the birth of offspring throughout the year (Rodríguez-Romero et al. 2011). In S. bicanthalis, sperm storage has been reported in populations from high elevations (Guillette 1981; Guillette and Jones 1985), but its length along the reproductive cycle is unknown Therefore, the aim of this research was to determine in which region of the oviduct and in which breeding condition throughout the reproductive cycle sperm storage occurs in an interesting population from high elevation where the males shows continuous spermatogenesis (Gribbins et al. 2011; Rheubert et al. 2012).
Materials and methods
During the months of September 1994 to August 1995 and March to August 2001, 39 adult females (≥42 mm SVL) of S. bicanthalis were collected, under the scientific collector permit SEMARNAT-FAUT 0074, in The Nevado de Toluca location, Estado de México (19°07′30″N, 49°46′15″W) at an elevation of 4200 m. The study met the relevant legal regulations as well as institutional procedures for research of the Universidad Nacional Autónoma de México, and Ley de protección a los animales del Distrito Federal. The collected specimens were killed with an overdose of sodium pentobarbital given intraperitoneal, dissected, and their ovaries and oviducts removed and fixed in 10 % neutral-buffered formalin. Depending on their reproductive characteristics, based on the condition of the ovary (follicular development) and presence of developing embryos in utero, the females were divided into five categories: (1) previtellogenic—females with small follicles less than 2 mm without yolk; (2) vitellogenic—females with vitellogenic follicles larger than 2 mm; (3) early pregnancy—females with large corpora lutea and embryos in utero with early embryonic development (developmental stages 1–30); (4) late pregnancy—females with reduced corpora lutea and embryos in utero with late embryonic development (developmental stages 31–40); and (5) postpartum—females without developing embryos, but with distended and edematous oviducts. Also, monthly the percentage of females in each category was calculated. Developing embryos from the left oviduct were used for their categorization based on the development table proposed by Duffaure and Hubbert (1961). The right oviducts of the five reproductive groups of females were processed for histological analysis, for which they were dehydrated using a graded ethanol series (30–100 %), cleared with xylene, and embedded in Paraplast. Serial sections (7 μm thick) were obtained, and the tissues were stained alternately with different staining techniques including: hematoxylin–eosin, Mallory's tricrome, alcian blue—nuclear fast red, and alcian blue—PAS (Estrada et al. 1982; Kiernan 1981). Tissue samples were analyzed using a light microscope Olympus CX31. Photographs were taken using an Olympus C-5050 digital camera adapted to the microscope, and the plates were prepared using the Adobe Photoshop CS3 software.
Results
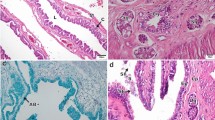
The oviduct in S. bicanthalis is differentiated into three regions: infundibulum, glandular uterus, and non-glandular uterus. The more cranial region, the infundibulum, terminates at the ostium and opens into the coelom; the mucosa is limited by a cuboidal epithelium with ciliated and non-ciliated epithelial cells (Fig. 1A). The thin lamina propria is composed of fibers of connective tissue in which blood vessels are observed, and superficially the muscular is very much reduced to a point that determining the layers becomes difficult; only are evident some fibers, surrounded by the visceral pleuroperitoneum (Fig. 1A). In S. bicanthalis the caudal region of infundibulum shows differences with the cranial region. The mucosa of posterior infundibulum is thicker and presents small and uniform folds (Fig. 1B) and a columnar epithelium with ciliated and non-ciliated secretory cells, the latter of which stain positively with alcian blue (Fig. 1B), indicating the presence of acid glycosaminoglycans. The lamina propria is thin, with the typical elements of connective tissue and blood vessels, and some glands are observed in the most posterior region (Fig. 1B), and the muscular layer is thin (Fig. 1B). Subsequently, the glandular uterus is located caudal to the infundibulum and is the longest region of the oviduct. The glandular uterus is characterized by the presence of glands in the lamina propria (Fig. 1C) and a limiting cuboidal epithelium presenting ciliated and non-ciliated secretory cells, whose secretion is positive for alcian blue staining. Externally, both smooth muscle layers are defined and structured by deep circular and superficial longitudinal fibers (Fig. 1C). The most posterior region of the oviduct is the non-glandular uterus, which opens into the cloaca. The non-glandular uterus presents a folded mucosa (Fig. 1D) whose folds increase in size toward the near region as they approach the cloaca; the luminal epithelium is low columnar with ciliated and non-ciliated secretory cells (Fig. 1D) which stained positive for acid glycosaminoglycans; the muscle layer is thick, and the internal circular and external longitudinal muscle fiber layers are evident (Fig. 1D).
Photomicrographs of longitudinal sections through the oviduct of Sceloporus bicanthalis. A Infundibulum showing the characteristic thin wall and cubic simple epithelium (e) limiting toward the lumen (l) with ciliated cells (*) and non-ciliated cells, fibers of connective tissue and blood vessels (bv), some muscular fibers and superficially visceral pleuroperitoneum (p) are evident. B Posterior infundibulum with thicker mucosa with small and uniform folds (f) limited by a columnar simple epithelium with ciliated cells (*) and non-ciliated secretory cells (stained positively for alcian blue), posterior glands (g) and thin muscle layers were outer longitudinal (ml) and inner circular fibers (mc) are evident. C Glandular uterus showing the cubic simple epithelium (e), glands (g) and blood vessels (bv) in the lamina propria (lp), a thick musculature of outer longitudinal (ml) and inner circular (mc) smooth muscle and visceral pleuroperitoneum (p) superficially. D Non-glandular uterus with the mucosa arranged with longitudinal folds (f) that extended toward the lumen (l), note sperm (sp) aggregations within them lumen and between the folds; externally the outer longitudinal (ml) and inner circular (mc) smooth muscle layers are evident. A, C Hematoxylin–eosin stain, B Alcian blue—nuclear fast red stain, and D Alcian blue—PAS stain. Bar 25 μm
Oviductal sperm storage was recorded in previtellogenic, vitellogenic, pregnant (early and late females), and postpartum females throughout the year (Fig. 2). The sperm storage sites were observed only in the transition between glandular uterus and non-glandular uterus and in the anterior non-glandular uterus (Fig. 3A); besides being evident in these regions sperm were also noted in the lumina of previtellogenic (Fig. 3A) and vitellogenic (Fig. 3B) females. The spermatozoa are retained between the mucosa folds (Fig. 3B), in deep crypts between the folds, which are irregular invaginations of the epithelium (Fig. 3A–C), and in the sperm storage tubules, which are formed by the closure of the base of the crypts between the folds and by the invagination of the oviduct wall toward the lamina propria (Fig. 3A–D). Spermatozoa between the folds, in the crypts, and in the sperm storage tubules are arranged with their heads toward the limiting epithelium of the crypt, and with their tails extending parallel toward the lumen. Within some tubules, the sperm have no defined arrangement and form a mass in contact with the luminal secretion (Fig. 3D).
Photomicrographs of locations of sperm retention in Sceloporus bicanthalis. A, B Longitudinal section through the transition between glandular uterus and non-glandular uterus of previtellogenic (A) and vitellogenic (B) female, respectively, both probably had a recent copulation as evident by large masses sperm (sp) occupying the lumen (l), between the folds (f), crypts (c) and sperm storage tubules (sst). Note in A the presence of uterine glands (g) at the margin of the glandular uterus. C Transverse folds (f) and crypts (c) with sperm (sp) in the anterior non-glandular uterus of an early pregnancy female. D Longitudinal section through non-glandular uterus of a postpartum female with sperm (sp) in the sperm storage tubules (sst) formed by invaginations of the epithelium or by fusion of folds (f). A Alcian blue—PAS stain, B Mallory’s trichrome stain, C Alcian blue—nuclear fast red stain, and D Hematoxylin–eosin stain. Bar 125 μm
In previtellogenic females, a large quantity of spermatozoa is observed in the troughs of the folds in the mucosa and in the lumen of the transition between glandular and non-glandular uterus. Simple tubular glands are a typical feature of the posterior uterus (Fig. 3A). The epithelium that limits the mucosa of this zone of transition was cuboidal with ciliated and non-ciliated secretion cells positive for alcian blue staining. The orientation of the spermatozoa in the lumen was disorganized (Fig. 3A), and in areas of sperm storage, the spermatozoa were arranged with their heads facing the epithelium (Fig. 4A, B).
Photomicrographs of sections of the anterior non-glandular uterus showing sperm retention of females in different reproductive conditions. A, B Previtellogenic. Transverse folds (f) and sperm storage tubules (sst) having stored sperm (sp), some containing dark staining sperm bundles. C, D Vitellogenic. The spermatozoa (sp) generally assume an orderly distribution with the heads oriented toward the base of the crypts (c) also sperm storage tubules are evident. E, F Early and late pregnancy. Sperm (sp) oriented in crypts (c), and transverse folds (f) of a female having many stored sperm, respectively. G, H Postpartum. Sperm (sp) aggregations residing in the sperm storage tubules (sst) or are visible in the crypt (c) with heads oriented toward epithelium. In B, D, and E note the secretion (*) of the non-ciliated cells stained positively for alcian blue showing the presence of acid glycosaminoglycans. A Alcian blue—PAS stain, B, C, F Mallory’s trichrome stain, G, H Hematoxylin–eosin stain, and D, E Alcian blue—nuclear fast red stain. Bar 25 μm
The anterior non-glandular uterus of females in the vitellogenic stage presented a thick mucosa with an increase in the number and height of the wall folds. The spermatozoa were found in the lumen, between the depressions of the folds of the uterine mucosa, in the crypts, and in the sperm storage tubules (Figs. 3B, 4C, D). The large amount of spermatozoa in the lumen was mixed with secretion forming an irregular mass, which occupied a large volume, but in the folds and crypts their heads usually appeared aligned and oriented toward the epithelium (Fig. 4C, D). The limiting mucosal epithelium was low columnar, where ciliated cells were more prevalent and possessed long cilia. It is important to note that during vitellogenesis a greater secretory activity was seen in non-ciliated epithelial cells, a secretion that stained positively for acid glycosaminoglycans, which was evident by its affinity to alcian blue (Fig. 4D).
In pregnant females in early gestation the characteristics of sperm retention areas were similar to those described for the previous breeding conditions; the mucosa was folded and limited by a columnar epithelium with ciliated and non-ciliated cells, which secrete acid glycosaminoglycans (Fig. 3C). The disposition of the spermatozoa matched the previous description of previtellogenic and vitellogenic females. In pregnant females, it was observed that the sperm were oriented with their heads toward the epithelium in contact with the secretion (Figs. 3C, 4E, F).
The wall of the anterior non-glandular region in postpartum females was thick with large folds (Fig. 3D) with a low columnar ciliated and non-ciliated secretory epithelium. The lamina propria presented a great quantity of blood vessels and the muscular layer was thick. There were no free spermatozoa in the lumen of the transition between glandular uterus and non-glandular uterus zone, and the spermatozoa were only evident in the crypts and in the sperm storage tubules from the anterior non-glandular uterus, which were located between the mucosa and the muscular wall (Figs. 3D, 4G, H). Some females had sperm retention sites only within depressions formed by folds in the mucosa where the spermatozoa were oriented with their heads toward the epithelium (Fig. 4H).
Discussion
The morphology and regionalization in the oviduct of S. bicanthalis are similar to those described in other lacertilian species (Cuellar 1966; Fox 1963, 1977; Blackburn 1998; Girling 2002; Siegel et al. 2015). The examined oviducts showed an activity pattern, which reflected the reproductive condition of the females: previtellogenic, vitellogenic, pregnant, and postpartum; i.e., the oviducts showed morphological changes associated with the reproductive condition. These changes most likely correspond to seasonal hormonal changes during the reproductive cycle (Blackburn 1998; Girling 2002). In S. bicanthalis, the changes in hypertrophy of the oviduct, increase in the number and size of folds, changes in the endometrial layer, uterine gland synthesis and secretion, and increase in the number of ciliated and not ciliated cells, were more evident during vitellogenesis. These data suggest similarities among other lizards have been studied (Blackburn 1998; Girling 2002) and potentially a retained ancestral state. The region that we denominated as posterior infundibulum in S. bicanthalis shows differences with the anterior infundibulum and glandular uterus especially in vitellogenic females: the mucosa is thicker, with small and uniform folds, and non-ciliated cells stained positively with alcian blue indicating the presence of acid glycosaminoglycans. This zone has been described as tuba, tube, or uterine tube (Blackburn 1998; Girling 2002; Sever and Hopkins 2004), and in some species of snakes and scleroglossid lizards has the important function of sperm storage, a characteristic that has been proposed as the ancestral condition (see Sever and Hamlett 2002). In S. bicanthalis sperm storage does not occur in this zone.
The uterine wall showed an increase in the myometrial and endometrial layers and in the endometrial layer hypertrophic simple alveolar glands, and the connective tissue is vascularized. The synthesis and gland recrudescence occur during ovarian vitellogenesis, and the glands secrete the collagen fibers of the eggshell membrane during the early gestational stage (Blackburn 1998; Mendoza-Cruz 2006). The increase in the number and size of folds in the vaginal mucosa depends on regionalization toward the posterior region and also on reproductive condition, which is consistent with observations obtained in other lizards, such as Sceloporus tristichus (Cuellar 1966), S. grammicus (Villagrán-Santa Cruz et al. 1992), S. woodi (Palmer et al. 1993), S. mucronatus (Villagrán-Santa Cruz et al. 2009), and Hemidactylus mabouia (Nogueira et al. 2011). The changes in the non-glandular uterus that are correlated with the reproductive cycle include an increase in musculature, in mucosa fold height, in epithelial and cilia height, and in epithelial secretory activity (Blackburn 1998).
Sperm storage in S. bicanthalis from the study site occurs all year long and in all reproductive conditions. The sperm storage area is the transition between glandular and non-glandular uterus and the anterior non-glandular uterus, which is consistent with the observations obtained by Guillette and Jones (1985), who investigated a different population and found abundant sperm storage during the fall and winter, at the bottom of folds formed by the endometrium. In the present study we have also observed spermatozoa storage in the crypts and in sperm storage tubules. The deep crypts between the folds are practically a continuation of the oviduct layer and are commonly seen in other lizards (Cuellar 1966; Bou-Resli et al. 1981). The sperm storage tubules are the more common structures seen in sperm storage (Sever and Hamlett 2002), and in some species such as Anolis sagrei they are alveolar tubular glands in the transition between glandular and non-glandular area (Sever and Hopkins 2004). In the case of some vitellogenic S. bicanthalis females, a great mass of spermatozoa mixed with secretion and cellular debris in the lumen of the transition zone was evident. This highlights two possibilities: these females mated recently, and/or this sperm mass mixed with secretions, desquamated epithelial cells and membranous structures, called “carrier matrix,” promotes the mobilization of sperm to the anterior oviduct in vitellogenic females that are close to ovulation (Sever and Ryan 1999; Sever and Hamlett 2002). In the specific storage areas in S. bicanthalis, spermatozoa are bundled in packages with their heads oriented toward the crypt or folded epithelium as observed in other lizards (Cuellar 1966; Sever and Hopkins 2004).
Sperm storage has been observed in other species of viviparous sceloporines (Sceloporus jarrovi—Goldberg 1970, S. grammicus microlepidotus—Guillette and Casas-Andrew 1981 and, Villagrán-Santa Cruz et al. 1992; Sceloporus mucronatus—Ortega-León et al. 2009 and Villagrán-Santa Cruz et al. 2009); as well as in other iguanids (see Cuellar 1966; Blackburn 1998; Girling 2002; Sever and Hamlett 2002). In viviparous populations, sperm storage appears to be necessary to ensure fertilization (Guillette and Jones 1985). Sperm storage is essential in some species due to asynchronous reproductive cycles of males and females (Murphy et al. 2006; Ortega-León et al. 2009). Likewise, sperm retention allows the independence of copulation from fertilization processes (Girling 2002), which may explain the ability of females to have fertile eggs throughout the year (Vitt 1986; Anjos and Rocha 2008; Nogueira 2008), or the reproduction of females when males are absent (Cuellar 1966; Villagrán-Santa Cruz et al. 1992; Blackburn 1998).
The storage of sperm even after fertilization requires further investigation. It is possible that the sperm found in the sperm storage sites following fertilization in this semelparous species are due to left over sperm from future mating events. These sperm may be undergoing phagocytosis which has been noted in other species (Sever and Ryan 1999), and future ultrastructural analyses may help elucidate this. Furthermore, the retained sperm may be from subsequent matings as males constantly produce sperm in this population. This continuous mating may result in sperm selection, sperm competition, and/or multiple paternity (Olsson and Madsen 1998; Schuett 1992; Birkhead and Moller 1993; Gist and Fischer 1993). Future studies investigating female mating patterns and multiple paternity may help to elucidate these hypotheses.
In S. bicanthalis during the different reproductive conditions, the non-ciliated epithelial cells of the anterior non-glandular stain positively for alcian blue, indicating a secretory function as observed in other lacertilian species (Girling 2002). In some species this secretion has been associated with the transport and upkeep of sperm (see Girling 2002), or the protection and lubrication of the epithelial surface, which facilitates the passage of oocytes and eggs through the oviduct (Botte 1973; Girling 2002; Sever et al. 2000). Furthermore, it has been suggested that sperm storage sites provide protection to mechanical damage and that secretions have a nutritive function for the spermatozoa (Cuellar 1966; Saint-Girons 1973; Bou-Resli et al. 1981); also, the mucous secretion of the epithelium ensures prolonged survival (Saint-Girons 1962).
Sperm storage is an important aspect of the reproductive biology of S. bicanthalis as indicated by its presence throughout the reproductive cycle in different reproductive conditions. On the other hand, S. bicanthalis demographics from Nevado de Toluca study site (cold high-elevation mountains) give the population a semelparous life cycle, which gives an early sexual maturity and an 8-month survival rate (Rodríguez-Romero et al. 2011). Due to the fact that this population dissociates ovulation from breeding (Ambriz-Rosales 2010) and that males present continuous spermatogenesis (Gribbins et al. 2011), it is likely that sperm storage allows the independence of copulation from the fertilization processes, which is one of the advantages of sperm storage as denoted by Girling (2002).
We believe that sperm retention is an adaptive strategy to the life cycle of S. bicanthalis. This strategy allows females to ensure fertilization during their short lifetime as sperm are always present in their reproductive tracts. As in males, this strategy could suggest that there may be a sexual selection pressure to so that sperm is always available for females that are ready to breed (Gribbins et al. 2011). Sperm storage within the female reproductive tract is a prerequisite for sperm competition (Friesen et al. 2014), and this suggest that there may be selection on sperm, and females might gain substantial benefits by using only sperm from the most competitive male (Ortega-León et al. 2009). S bicanthalis from our study site is ideal model for the study of the origin of sperm retention and postcopulatory selection mechanism.
References
Ambriz-Rosales IA (2010) Actividad Reproductora de Sceloporus bicanthalis (Squamata: Phrynosomatidae) en el volcán Nevado de Toluca. Tesis de Licenciatura Universidad Autónoma del Estado de México, Estado de México
Anjos LA, Rocha CFD (2008) Reproductive ecology of the invader species gekkonid lizard Hemidactylus mabouia in an area of southeastern Brazil. Iheringia Ser Zool 98:205–209
Bagwill A, Sever DM, Elsey RM (2009) Seasonal variation of the oviduct of the American alligator, Alligator mississippiensis (Reptilia: Crocodylia). J Morphol 270:702–713
Birkhead TR, Moller AP (1993) Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Boil J Linnean Soc 50:295–311
Blackburn DG (1998) Structure, function and evolution of the oviducts of squamate reptiles, with special reference to viviparity and placentation. J Exp Zool 282:560–617
Botte V (1973) Some aspects of oviduct biochemistry in the lizard Lacerta sicula in relation to the annual cycle. Boll Zool 40:315–321
Bou-Resli MN, Bishhay LF, AL-Zaid NS (1981) Observations on the fine structure of the sperm storage crypts in the lizard Acanthodactylus scutellatus hardyi. Arch Biol 92:287–298
Cuellar O (1966) Oviductual anatomy and sperm structures in lizards. J Morphol 19:7–20
Duffaure JP, Hubbert J (1961) Table de development du lezad vivipare: Lacerta (Zootoca) vivípara Jacquin. Arch Anat Microsc Morphol Exp 50:309–328
Estrada FE, Peralta ZL, Rivas MP (1982) Manual de técnicas histológicas. AGT Editor S. A. México
Fox W (1963) Special tubules for sperm storage in female lizards. Nature 198:500–501
Fox W (1977) The urogenital system of reptiles. In: Gans C (ed) Biology of the Reptilia, vol 6. Academic Press, New York, pp 1–157
Friesen CR, Kerns AR, Mason RT (2014) Factors influencing paternity in multiply mated female red-sided garter snakes and the persistent use of sperm stored over winter. Behav Ecol Sociobiol 68:1419–1430
Girling JE (2002) The reptilian oviduct: a review of structure and function and directions for future research. J Exp Zool 293:141–170
Gist DH, Fischer EN (1993) Fine structure of the sperm storage tubules in the box turtle oviduct. J Reprod Fert 97:463–468
Gist DH, Bagwill A, Lance V, Sever DM, Elsey RM (2008) Sperm storage in the oviduct of the American Alligator. J Exp Zool 309A:1–7
Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous iguanid Sceloporus jarrovi Cope. J Morphol 132:265–276
Gribbins K, Anzalone M, Collier M, Granados-González G, Villagrán-SantaCruz M, Hernández-Gallegos O (2011) Temporal germ cell development strategy during continuous spermatogenesis within the montane lizard, Sceloporus bicanthalis (Squamata: Phrynosomatidae). Theriogenology 76:1090–1099
Guillette JL Jr (1981) On the occurrence of oviparous and viviparous forms of the Mexican lizard Sceloporus aeneus. Herpetologica 37:11–15
Guillette JL Jr, Casas-Andreu G (1981) Seasonal variation in fat body weights of the Mexican high elevation lizard Sceloporus grammicus microlepidotus. J Herpetol 15:366–371
Guillette JL Jr, Guillette RE (1985) Ovarian, oviductual, and placental morphology of the reproductively bimodal lizard Sceloporus aeneus. J Morphol 184:85–98
Hernández-Gallegos O (1995) Estudio comparativo del patrón reproductor de los machos de dos especies de lagartijas emparentadas con distinto modo reproductor: Sceloporus aeneus y S.bicanthalis. Tesis de Licenciatura en Biología, Facultad de Ciencias, Universidad Nacional Autónoma de México, México
Hernández-Gallegos O, Méndez-de la Cruz FR, Villagrán-Santa Cruz M, Andrews RM (2002) Continuos spermatogenesis in the lizard Sceloporus bicanthalis (Sauria: Phrynosomatidae) from high elevation habitat of central México. Herpetologica 58:415–421
Kiernan JA (1981) Histochemical and histochemical methods: theory and practice. Pergamon, Oxford
Leache AD (2010) Species trees for spiny lizards (genus Sceloporus): identifying points of concordance and conflict between nuclear and mitochondrial data. Mol Phylogenet Evol 54:162–171
Manríquez-Morán NL (1995) Estrategias reproductoras en las hembras de dos especies hermanas de lacetilios: Scelporus aeneus y S. bicanthalis. Tesis de icenciatura en Biología. Facultad de Ciencias. Universidad Nacional Autónoma de México, México
Mendoza-Cruz E (2006) Morfología del oviducto y formación de la membrana de la cáscara en el lacertilio vivíparo Sceloporus bicanthalis (Sauria: Phrynosomatidae). Tesis de Maestría en Ciencias. Facultad de Ciencias, Universidad Nacional Autónoma de México, México
Mendoza-Cruz and Villagrán-Santa Cruz M (2007) Participación del oviducto en la formación y estructura de la membrana de la cáscara en la lagartija vivípara Sceloporus bicanthalis. S3-BYQ16 Memorias del IV encuentro Participación de la Mujer en la Ciencia, León Guanajuato, México
Murphy KS, Hudson S, Shea G (2006) Reproductive seasonality of three cold-temperate viviparous skinks from Southeastern Australia. J Herpetol 40:454–464
Nogueira KOPC (2008) Morfologia e ultra-estructura de oviducto de Hemidactylus mabouia (Moreau de Jonnès, 1818) (Reptilia, Squamata, Sauria, Gekkonidae) durante o ciclo reproductivo. Dissertação. Universidade Federal de Viçosa, MG
Nogueira KOPC, Souza-Rodrígues S, Alvano-Araújo V, Andrade Neves C (2011) Oviductal structure and ultrastructure of the oviparous gecko, Hemidactylus mabouia (Moreau De Jonnés, 1818). Anat Rec 294:883–892
Olsson M, Madsen T (1998) Sexual selection and sperm competition in reptiles. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, London, pp 503–564
Ortega-León A, Villagrán-Santa Cruz M, Zúñiga-Vega JJ, Cueva del Castillo R, Méndez-de la Cruz FR (2009) Sperm viability in the reproductive tract of females in a population of Sceloporus mucronatus exhibiting asynchronous reproduction. West North Am Nat 69:96–104
Palmer BD, DeMarco VC, Guillette JL Jr (1993) Oviductal morphology and the eggshell formation in the lizard, Sceloporus woodi. J Morphol 217:205–217
Rheubert JL, Touzinsky K, Hernández-Gallegos O, Granados-González G, Gribbins KM (2012) Ontogenic development of spermatids during spermiogenesis in the high altitude bunchgrass lizard (Sceloporus bicanthalis). Spermatogenesis 2:1–10
Rodríguez-Romero F, Smith GR, Méndez-Sánchez F, Hernández-Gallegos O, Sánchez-Nava P, Méndez-de la Cruz FR (2011) Demography of a semelparous, high-elevation population of Sceloporus bicanthalis (Lacertilia: Phrynosomatidae) from the Nevado de Toluca Volcano, México. Southwest Nat 56:71–77
Saint-Girons H (1962) Presence de receptacles seminaux chez les cameleons. Beaufortia 9:165–172
Saint-Girons H (1973) Sperm survival and transport in the female genital tract of reptiles. In: Hafez ESE, Thibault CG (eds) The biology of spermatozoa. Karger, Basel, pp 105–113
Schuett GW (1992) Is long-term sperm storage an important component of the reproductive biology of temperate pitvipers? In: Campbell JA, Brodic ED Jr (eds) Biology of the Pitvipers. Selva, Austin, pp 169–184
Sever DM, Hamlett WC (2002) Female sperm storage in reptiles. J Exp Zool 292:187–199
Sever DM, Hopkins WA (2004) Oviductal sperm storage in the ground skink Scincella laterale Holbrood (Reptilia: Scincidae). J Exp Zool 301A:599–611
Sever DM, Ryan TJ (1999) Ultrastructure of the reproductive system of the black swamp snake (Seminatrix pygaea). Part I Evidence for oviductal sperm storage. J Morphol 241:1–18
Sever DM, Ryan TJ, Morris T, Patton D, Swafford S (2000) Ultrastructure of the reproductive system of the black snake (Seminatrix pygaea). Part II annual oviductal cycle. J Morphol 245:146–160
Siegel DS, Miralles A, Chabarria RE, Aldridge RD (2011) Female reproductive anatomy: cloaca, oviducts, and sperm storage. In: Aldridge RD, Sever DM (eds) Reproductive biology and phylogeny of snakes. CRC Press, Florida
Siegel DS, Miralles A, Rheubert JL, Sever DM (2015) Female reproductive anatomy: cloaca, oviduct and sperm storage. In: Rheubert JL (ed) Reproductive biology and phylogeny of lizards and tuatara. Taylor & Francis Group, Oxfordshire, pp 144–195
Villagrán-Santa Cruz M, Méndez-de la Cruz FR, Cuellar O (1992) Obligatory sperm storage in the lizard Sceloporus grammicus. Acta Zool Mex 49:23–31
Villagrán-Santa Cruz M, Hernández-Gallegos O, Méndez-de la Cruz FR (2009) Reproductive cycle of the lizard Sceloporus mucronatus with comments on intraspecific geographic variation. West North Am Nat 69:437–446
Vitt LJ (1986) Reproductive tactic of sympatric gekkonid lizards with a comment on the evolutionary and ecological consequences of invariant clutch size. Copeia 1986:773–786
Acknowledgments
We thank Teresa F López for the histological processing; Misol-ja Méndez and Dzilam Méndez for their help with the graphic design and digital preparation of the figures. The Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México (UNAM), provided financial support through a fellowship granted to Mendoza-Cruz E.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights
This article does not contain any studies with human.
Statement on the welfare of animals
All laboratory procedures were undertaken following ethical norms for animal experiments, and the study met the relevant legal regulations as well as institutional procedures for research of the Universidad Nacional Autónoma de México, and Ley de protección a los animales del Distrito Federal, México.
Rights and permissions
About this article
Cite this article
Villagrán-SantaCruz, M., Mendoza-Cruz, E., Granados-González, G. et al. Sperm storage in the viviparous lizard Sceloporus bicanthalis (Squamata: Phrynosomatidae), a species with continuous spermatogenesis. Zoomorphology 136, 85–93 (2017). https://doi.org/10.1007/s00435-016-0327-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0327-6