Abstract
The reproductive cycle of Crotalus durissus is markedly seasonal and synchronous between individuals. The start of vitellogenesis occurs at the end of the summer and coincides with copulation. However, given that the copulation is dissociated from ovulation, sperm storage is obligatory in females. In viperids, sperm storage in the female reproductive tract is reported to occur in two regions: (1) the posterior infundibulum, which presents sperm storage glands; and (2) the nonglandular uterus where sperm is stored in crypts by means of the uterine muscular twisting (UMT). The mechanisms that allow the survival of sperm in the female reproductive tract of snakes are still unknown. In this study, we investigated five regions of the reproductive tract of C. durissus, searching for the presence of spermatozoa and sperm storage structures in different oviductal portions. Additionally, we used histological techniques to verify the occurrence of hypertrophy of the infundibular and uterine glands during the processes of vitellogenesis, as well as histochemical techniques to investigate the nature of the secretion produced in the nonglandular uterus and posterior infundibulum. Storage sperm were observed in the nonglandular uterus and although the posterior infundibulum had storage receptacles, sperm were not observed in that region. Both sperm storage regions presented granules testing positive for acidic and neutral polysaccharides, in vitellogenic and previtellogenic females. This presence of guaranteeing conditions for sperm storage. Histochemical analysis revealed the possible storage capacity of sperm in the nonglandular uterus. In addition, the UMT was observed in all the females with storage sperm, which assures the maintenance of sperm in the nonglandular uterus until ovulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The female reproductive tract (Agkistrodon, Bothrops, Crotalus) of pit vipers presents two ovaries and two oviducts arranged asymmetrically (Almeida-Santos and Orsi 2002; Siegel et al. 2011; Barros et al. 2014). The anatomy of the oviduct has been divided into anterior infundibulum, posterior infundibulum, nonglandular uterus, glandular uterus, and pouch (Siegel et al. 2011). Regarding the relationship among morphology and reproductive cycles, the posterior infundibulum and the nonglandular uterus can be considered one of the most intriguing and less studied regions of the oviduct (Siegel et al. 2011). It has been suggested that these two oviduct regions represent the locations that allow the impressive long period sperm storage in snakes (Girling 2002; Holt and Fazeli 2016; Levine et al. 2021). Therefore, several studies have described the morphological and histochemical characteristics of the oviduct throughout the reproductive cycle (Hoffman and Wimsatt 1972; Rojas et al. 2015; Silva et al. 2019). So far, it is well known that the oviduct of vitellogenic females increases the production of secretory granules (e.g., polysaccharides, proteins) in the presence of sperm (Siegel and Sever 2008; Rojas et al. 2015; Silva et al. 2019). However, specificities and roles of these secretions in storing and keeping viable sperm cells are still poorly known.
In general, pit vipers present sperm storage in the nonglandular uterus occurring in crypts, which can be facilitated through uterine contractions, known as uterine muscular twisting (UMT) (Ludwig and Rahn 1943; Almeida-Santos and Salomão 1997; Barros et al. 2012; Silva et al. 2019). On the other hand, sperm storage in the posterior infundibulum occurs in specialized tubular receptacles (Siegel and Sever 2008; Silva et al. 2019). Some authors have presented evidence that the oviductal secretion provides a chemical refuge and a place of nutrition for the stored sperm (Siegel and Sever 2008; Marinho et al. 2009). The vitellogenesis phase of the South American rattlesnake, Crotalus durissus (Linnaeus 1758), is markedly seasonal and synchronic between individuals (Almeida-Santos and Orsi 2002; Barros et al. 2012). The onset of vitellogenesis occurs at the end of summer and autumn and coincides with the mating period (Almeida-Santos and Orsi 2002; Barros et al. 2012). However, because of the dissociation between copulation and ovulation periods, sperm storage is mandatory for at least four months (Almeida-Santos and Orsi 2002; Barros et al. 2012).
Several histochemical and ultrastructural aspects of the oviduct of pit viper snakes are still little explored (Siegel and Sever 2008; Silva et al. 2019; Souza and Almeida-Santos 2022), and the posterior infundibulum of C. durissus has never been properly studied. The morphological description of the oviduct of C. durissus and its sperm receptacles is a key improvement for the understanding of the reproductive strategies of Crotalus. The sperm storage in this genus is the longest known among vertebrates, reaching up to six years (Booth and Schuett 2011; Levine et al. 2021). Moreover, the stored spermatozoa can allow consecutive parturitions in this genus (Levine et al. 2021). Specifically in C. durissus, spermatozoa have been found in the nonglandular uterus (Almeida-Santos and Salomão 1997; Barros et al. 2012), and present a uterine contortion until now exclusive to viper snakes (Nilson and Andrén 1982; Yamanoye et al. 2004; Muniz-da-Silva et al. 2018; Silva et al. 2019).
In this study, we provide new evidence about the anatomy and reproductive cycle of C. durissus. We investigate five regions of the female reproductive tract (pouch, nonglandular uterus, glandular uterus, posterior infundibulum, and anterior infundibulum), inspecting the presence of spermatozoa and sperm storage structures in these different oviductal portions. Furthermore, we also use histological techniques to verify the occurrence of hypertrophy of the infundibular and uterine glands during the processes of vitellogenesis. Finally, we use histochemical techniques to investigate the histochemical nature of the secretion produced in the nonglandular uterus and in the posterior infundibulum.
Materials and methods
We examined 55 females of C. durissus collected throughout the southeastern region of Brazil and preserved in scientific collections (Appendix I). We measured snout–vent length (SVL) of each specimen before performing dissections.
We recorded the diameter of the largest ovarian follicle using a digital caliper (± 0.1 mm) and the presence of UMT. We conducted histological analyses of the right side of the oviduct (pouch, nonglandular uterus, glandular uterus, posterior infundibulum, anterior infundibulum). Tissue samples were dehydrated through a series of increasing ethanol concentrations, cleared in xylene, embedded in paraffin, and stained with hematoxylin and eosin (Junqueira and Carneiro 2008). In addition, we collected the tissues only from adult females according to the morphological characteristics of the ovaries (presence of vitellogenic follicles or corpus luteum) and/or characteristics of the oviducts (presence of embryos, UMT, or folded oviducts indicating parturition; Silva et al. 2023).
To assess the nature of the secretion produced in the nonglandular uterus and posterior infundibulum, we performed several histochemical techniques. The periodic acid-Schiff (PAS) reaction was performed to identify neutral carbohydrates, and Alcian blue (AB) 8GX (pH 2.5) was used to identify carboxyl and sulfate–ester groups of acid mucosubstances (Kiernan 2008). To detect proteins, we used Coomassie brilliant blue (CBB) R250 (Kiernan 2008) diluted at 0.04 mg/mL (Braz et al. 2018).
We calculated the mean diameter of the uterine and infundibular glands by taking 5–10 measurements from females in vitellogenesis (follicles > 10 mm) and previtellogenesis (follicles ≤ 10 mm; see Almeida-Santos and Orsi 2002; Vieira et al. 2010) using the software ImageJ, version 1.51d (Abràmoff et al. 2004). Values for all measured variables were then averaged to obtain a mean value per individual. Before the analysis, all variables were tested for normality of distribution using the Kolmogorov–Smirnov test. Linear regression was performed to analyze the relationship between the independent variable (follicle diameter) and the dependent variable (secretory infundibular gland diameter and uterine gland diameter).
Results
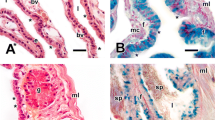
To facilitate the description, we divided the infundibulum of C. durissus into anterior and posterior portions. The anterior infundibulum histologically presented thin muscular tissue, irregular folds in the epithelium, and the absence of glands in the connective (Fig. 1a). The epithelial tissue is presented as a simple cuboidal aggregated, consisting mainly of ciliated cells interspersed with secretory cells. On the other hand, the posterior infundibulum differed from the anterior infundibulum by presenting many ciliated and secretory glands in the connective tissue (Fig. 1b). The epithelium of the posterior infundibulum presented ciliated and secretory cells with oval and rounded nuclei. The epithelium of posterior infundibulum of C. durissus showed histochemical reaction to AB and PAS in vitellogenic and previtellogenic females (Fig. 1c).
Photomicrograph of the infundibulum and uterus of Crotalus durissus. The anterior infundibulum (a). The posterior infundibulum highlights the presence of glands in the connective tissue (b). The posterior infundibulum showing epithelial cells stained for AB (c). The glandular uterus with spermatozoa in the lumen (d). AB+ positive reaction to alcian blue, C connective tissue, Ep luminal epithelium, Cg ciliated tubular glands, L lumen, Lp lamina propria, M muscular tissue, S spermatozoa, Sg secretory tubular glands, Sh sperm head, Ug uterine glands
In addition, the posterior infundibulum presented thicker lamina propria with deeper tubular-like folds forming glandular grooves. The secretory tubular glands in posterior infundibulum (Fig. 1b) indicated seasonal variation in relation to their diameters, showing hypertrophy and increased secretions in vitellogenic females (Table 1) only in the months of January, March, May, June, July, September, and November. These secretory glands presented a significant correlation with the diameter of the ovarian follicle (r = 0.5, P = 0.03).
We did not find spermatozoa stored in the lumen or within the infundibular glands (receptacles of sperm). However, we observed spermatozoa in the glandular uterus in the months of July and September, in two females with ovarian follicles measuring 27 mm and 35 mm, respectively, indicating that spermatozoa ascend to the infundibular region before ovulation (Fig. 1d).
The glandular uterus of C. durissus presented a lamina propria and a well-developed muscular layer (Fig. 2a, b). The inner layer of the glandular uterus was constituted by ciliated and secretory cells of the simple cuboidal type, which is the most abundant in this portion of the oviduct. The connective tissue of the glandular uterus showed many uterine glands (or eggshell glands). These uterine glands were bigger in vitellogenic females (mean 33 ± 4 mm of diameter, range = 27–36, n = 4) than in previtellogenic females (mean 17 ± 5 mm of diameter, range = 11–26, n = 5). Additionally, the uterine glands presented a significant correlation with the diameter of the ovarian follicle (r = 0.8, P = 0.01).
The nonglandular uterus of C. durissus presented folds in the mucosa and deep crypts, which are lined with simple columnar to pseudostratified epithelium constituted by ciliated cells with spherical or elongated nuclei, and several mucus-secreting goblet cells (Fig. 3a, b). The epithelium of nonglandular uterus of C. durissus indicated histochemical reaction to AB and PAS in vitellogenic and previtellogenic females (Fig. 3c, d). Muscle tissue was well developed, composed of smooth muscle with fusiform cells and central nucleus. The innermost layer was circular, and the outermost layer was longitudinal.
Photomicrograph of the nonglandular uterus of Crotalus durissus. Midsagittal section showing uterine muscular twisting of the nonglandular uterus. Note that the lumen is not presented in linear arrangement, instead it is presented as a curved structure. The curves are showed sectioned as three independent lumina (a). Higher magnification of the nonglandular uterus with storage sperm (b). The nonglandular uterus showing epithelial cells stained for AB (c). The nonglandular uterus showing epithelial cells stained for PAS (d). AB+ positive reaction to alcian blue, L lumen, Lp lamina propria, M muscular tissue, S spermatozoa, PAS+ positive reaction to periodic acid-Schiff
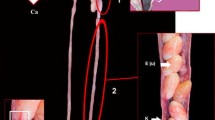
The nonglandular uterus presented a UMT in vitellogenic and previtellogenic females (Figs. 3a, 4a). Spermatozoa in this region were observed in three previtellogenic females (January and August) and nine vitellogenic females (January, May, July, August, and September) (Fig. 3b). Frequently, spermatozoa were associated with the epithelium with their heads oriented toward the cilia (Fig. 3b). All females with stored sperm presented UMT (Figs. 3a, 4a). Remarkably, the female of July presented spermatozoa in three regions of the oviduct, in the pouch (Fig. 4b), in the nonglandular uterus (Fig. 3b), and in the lumen of the glandular uterus (Fig. 1d). The pouch (Fig. 4a, c) was characterized by an epithelium composed of columnar secretory cells with basal nuclei (Fig. 4b), a thick lamina propria, and external musculature.
Photographs of the tract reproductive of Crotalus durissus. Nonglandular uterus presenting the uterine muscular twisting (a). Pouch lumen with spermatozoa (b). Macroscopic view of the pouch with a part of the uterus (c). Ep luminal epithelium, GU glandular uterus, L lumen, NU nonglandular uterus, S spermatozoa, UMT uterine muscular twisting
Discussion
The sperm storage has been commonly observed in the nonglandular uterus in vitellogenic and previtellogenic females of C. durissus (Almeida-Santos and Salomão 1997; Barros et al. 2012). Our study extends the knowledge about sperm storage in pit vipers through the identification of spermatozoa in three regions (pouch, nonglandular uterus, and glandular uterus) of the reproductive tract of C. durissus. These histological results—found only in July—may suggest that some females could have mated recently, because they still present spermatozoa in the pouch (Fig. 5). On the other hand, the presence of spermatozoa in the glandular uterus in some individuals may indicate that sperm can ascend to the infundibular region before ovulation (Fig. 5). Therefore, for C. durissus, sperm storage can occur in different portions of the oviduct, a strategy that can increase the reproductive success in this species. Sperm storage in both regions, nonglandular uterus and posterior infundibulum, has been described for several species (Braz and Almeida-Santos 2022), and these morphological adaptations in the oviduct have been described as essential to the strategy of storage of sperm derived from different males (Hoggren and Tegelstrom 1995; Friesen et al. 2020).
Overview of female reproductive aspects of Crotalus durissus. Solid circles: diameter of the largest ovarian follicle of females without spermatozoa in the oviduct. Open circles: diameter of the largest embryo. Open squares: diameter of the largest ovarian follicles of females presenting sperm storage in the nonglandular uterus. Solid squares: diameter of the largest ovarian follicles of females presenting spermatozoa in the nonglandular uterus, and in the lumen of the glandular uterus (some females also presented spermatozoa in the pouch in July). Shaded rectangles indicate the period of reproductive events as described by Barros et al. (2012). Blue rectangle indicates period related to the birth of neonates; yellow rectangle indicates the mating period; Green rectangle indicates the overlap period between birth and mating; and red rectangle indicates the period of ovulation
The UMT is another remarkable morphological adaptation of the female reproductive tract of pit vipers, which is usually macroscopically noticeable, especially in adult females in the vitellogenesis stage (Yamanouye et al. 2004; Muniz-da-Silva et al. 2018). Our findings about the presence of UMT in vitellogenic and previtellogenic females in C. durissus indicate that its presence is not directly related with ovulation. However, the UMT is under hormonal influence—a balance between estradiol and progesterone—suggesting its dependence of particular stages of the reproductive cycle that can be associated with the maintenance and survival of spermatozoa in the reproductive tract (Yamanouye et al. 2004).
The contact between the spermatozoa acrosome and the oviduct epithelium has been described as a necessary phenomenon to activate the synthesis of substances related to the maintenance and survival of spermatozoa in the oviduct (Holt and Fazeli 2016). Usually, when we identified sperm storage in the nonglandular uterus of C. durissus, the acrosomes were observed orientated toward the oviduct epithelium—and the presence of UMT was also observed. Studies have shown that acidic glycoproteins can reduce the phagocytosis of spermatozoa by polymorphonuclear leukocytes (Holt and Fazeli 2016). The neutral and acidic glycoproteins can stain to PAS and AB, and in C. durissus, we identified that the epithelial cells of the nonglandular uterus synthesize AB and PAS-positive secretory granules (Almeida-Santos and Salomão 1997). Although we are not able to confirm the specific mechanisms that provide the environment for sperm survival in the oviduct, through morphological and histochemical analyzes of the nonglandular uterus of C. durissus, we can conclude that this region presents the necessary glycoproteic conditions to store spermatozoa for a long period.
The posterior infundibulum of C. durissus has several infundibular glands (receptacles of sperm), which represent small invaginations into the lamina propria, forming simple tubular glands (Siegel et al. 2009, 2011). Other species of vipers of the genera Agkistrodon, Crotalus, Bothrops, Cerastes, and Vipera also present sperm storage in tubular glands (Saint-Girons 1957, 1962; Siegel and Sever 2008; Silva et al. 2020). These glands and the epithelium of the posterior infundibulum of several groups of snakes have positive secretory granules composed by polysaccharides and proteins (Siegel and Sever 2008; Rojas et al. 2015; Silva et al. 2019). In Agkistrodon piscivorus (Lacépède 1789), the infundibular glands have a lipoid material produced in the secretory cells that are either diffuse and unorganized or tightly packed into denser lipid droplets (Siegel et al. 2009). The presence of these granules is often associated with sperm storage, as a possible sperm survival mechanism (Siegel and Sever 2008; Rojas et al. 2015, 2017; Silva et al. 2019). For example, in Bothrops and Agkistrodon, infundibular glands are positive for PAS in the presence of sperm (Siegel and Sever 2008; Silva et al. 2019). Although we did not find spermatozoa in the infundibular glands of C. durissus, we found several sperm receptacles in the infundibulum. Based on the presence of these glands in the posterior infundibulum, together with the histochemical results, we suppose that C. durissus can store spermatozoa in this region, in a similar way presented by its congeners (Siegel and Sever 2008; Silva et al. 2019, 2020).
Crotalus durissus shows the presence of glands in the posterior infundibulum and glandular uterus that undergo hypertrophy during vitellogenesis. It is noteworthy that the infundibular secretory glands and the uterine glands increase substantially in diameter under the influence of the vitellogenesis processes. Therefore, it is probable that these glands are hypertrophy under the influence of increased levels of plasmatic estradiol. The glandular uterus and the sperm storage tubules are the only two regions of the oviduct that exhibit dramatic seasonal changes in diameter in pit vipers (e.g., Agkistrodon piscivorus; Siegel and Sever 2008).
The relationship between the vitellogenesis processes and the hypertrophy of the infundibular and uterine glands is supported by several studies (Rojas et al. 2017; Braz et al. 2018). In C. durissus, the estradiol levels are significantly higher in vitellogenic females (Almeida-Santos et al. 2004) similar to the results presented for other species of Viperidae (Bonnet et al. 1994, 2001; Schuett et al. 2004; Taylor et al. 2004). The vitellogenesis process ends with a decrease in estradiol and a subsequent increase in progesterone (Taylor et al. 2004). These hormonal changes are associated with anatomic and secretory alterations in the oviduct, supporting the conclusion that the estradiol influences the vitellogenesis process, oviductal hypertrophy, increase in the number of mucous glands, oviductal secretory activity, and vascularization (Girling 2002).
In conclusion, our study indicates that the morphology of the female reproductive tract of C. durissus follows similar patterns already described for viperids (Siegel and Sever 2008; Barros et al. 2014; Silva et al. 2019). Sperm storage in C. durissus occurs in the nonglandular uterus and probably in the posterior infundibulum. Although spermatozoa were not observed in the latter, the region has tubular glands specialized in sperm storage. Furthermore, the observation of spermatozoa in the lumen of the glandular uterus before the period of ovulation indicates that sperm may also remain in the infundibular region. Because of the fragile nature of the spermatozoa, we suggest that more advanced microscopic techniques (e.g., electron microscopy, scanning microscopy) should be considered to advance the investigation of the infundibular region of animals fixed in scientific collections. Regarding some hypotheses about divergent sperm storage time among different regions, the pouch region likely has the shortest sperm storage time, while the nonglandular uterus region likely has the longest sperm storage duration. The infundibulum region likely presents a more variable sperm storage duration (maybe shorter than the nonglandular region), which can extend until the sperm migration before ovulation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics 11:36–42
Almeida-Santos SM, Orsi AM (2002) Ciclo reprodutivo de Crotalus durissus e Bothrops jararaca (Serpentes, Viperidae): morfologia e função do oviduto. Rev Bras Reprodução Anim 26:109–112
Almeida-Santos SM, Salomão MG (1997) Long-term sperm storage in the female neotropical Rattlesnake Crotalus durissus terrificus (Viperidae: Crotalinae). Jpn J Herpetol 17:46–52
Almeida-Santos SMD, Abdalla FMF, Silveira PF, Yamanouye N, Breno MC, Salomão MG (2004) Reproductive cycle of the Neotropical Crotalus durissus terrificus: I. Seasonal levels and interplay between steroid hormones and vasotocinase. Gen Comp Endocrinol 139(2):143–150. https://doi.org/10.1016/j.ygcen.2004.09.001
Barros VA, Sueiro LR, Almeida-Santos SM (2012) Reproductive biology of the neotropical rattlesnake Crotalus durissus from northeastern Brazil: a test of phylogenetic conservatism of reproductive patterns. Herpetol J 22:97–104
Barros VA, Rojas CA, Almeida-Santos SM (2014) Reproductive biology of Bothrops erythromelas from the Brazilian Caatinga. Adv Zool 2014:1–11. https://doi.org/10.1155/2014/680861
Bonnet X, Naulleau G, Mauget R (1994) The influence of body condition on 17-beta estradiol levels in relation to vitellogenesis in female Vipera aspis (Reptilia, Viperidae). Gen Comp Endocrinol 93:424–437. https://doi.org/10.1006/gcen.1994.1047
Bonnet X, Naulleau G, Bradshaw D, Shine R (2001) Changes in plasma progesterone in relation to vitellogenesis and gestation in the viviparous snake Vipera aspis. Gen Comp Endocrinol 121:84–94. https://doi.org/10.1006/gcen.2000.7574
Booth W, Schuett GW (2011) Molecular genetic evidence for alternative reproductive strategies in North American pit vipers (Serpentes, Viperidae): long-term sperm storage and facultative parthenogenesis. Biol J Linn Society 104:934–942. https://doi.org/10.1111/j.1095-8312.2011.01782.x
Braz HB, Almeida-Santos SM (2022) The Evolution of Sperm-Storage Location in Squamata with Particular Reference to Snakes. Origin Early Evol Hist Snakes 90:389
Braz HB, Almeida-Santos SM, Murphy CR, Thompson MB (2018) Uterine and eggshell modifications associated with the evolution of viviparity in South American water snakes (Helicops spp.). J Exp Zool B Mol Dev Evol 330:165–180. https://doi.org/10.1002/jez.b.22800
Friesen CR, Kahrl AF, Olsson M (2020) Sperm competition in squamate reptiles. Phil Trans R Soc B 375:20200079. https://doi.org/10.1098/rstb.2020.0079
Girling JE (2002) The reptilian oviduct: a review of structure and function and directions for future research. J Exp Zool 293:141–170. https://doi.org/10.1002/jez.10105
Hoffman LH, Wimsatt WA (1972) Histochemical and electron microscopic observations on the sperm receptacles in the garter snake oviduct. Am J Anat 134:71–96. https://doi.org/10.1002/aja.1001340107
Hoggren M, Tegelstrom H (1995) DNA fingerprinting shows within-season multiple paternity in the adder (Vipera berus). Copeia 1995:271–277. https://doi.org/10.2307/1446890
Holt WV, Fazeli A (2016) Sperm storage in the female reproductive tract. Annu Rev Anim Biosci 4:291–310
Junqueira CL, Carneiro J (2008) Histologia Básica. Guanabara Koogan, Rio de Janeiro
Kiernan JA (2008) Histological and histochemical methods: theory and practice. Scion Publishing, Oxfordshire
Lacépède BGE (1789) Histoire Naturelle des Quadrupèdes Ovipares et de Serpens. Imprimerie du Roi, Paris
Levine BA, Schuett GW, Booth W (2021) Exceptional long-term sperm storage by a female vertebrate. PLoS ONE 16(6):e0252049. https://doi.org/10.1371/journal.pone.0252049
Linnaeus C (1758) Systema naturae, per regna tria naturae: classes secundum, ordines, genera, espécies cum characteribus, differentiis, synonimis, locis. Tomus I. Laurentii Salvii, Holmiae
Ludwig M, Rahn H (1943) Sperm storage and copulatory adjustment in the prairie rattlesnake. Copeia 1943:15–18
Marinho CE, Almeida-Santos SM, Yamasaki SC, Silveira PF (2009) Peptidase activities in the semen from the vas deferens and uterus of the neotropical rattlesnake Crotalus durissus terrificus. J Comp Physiol 79:635–642
Muniz-da-Silva DF, Passos J, Siegel DS, Almeida- Santos SM (2018) Caudal oviduct coiling in a viperid snake, Crotalus durissus. Acta Zool 2018:1–9
Nilson G, Andrén C (1982) Function of renal sex secretion and male hierarchy in the adder, Vipera berus, during reproduction. Horm Behav 16(4):404–413
Rojas CA, Barros VA, Almeida-Santos SM (2015) Sperm storage and morphofunctional bases of the female reproductive tract of the snake Philodryas patagoniensis from southeastern Brazil. Zoomorphology 134:577–586
Rojas CA, Barros VA, Almeida-Santos SM (2017) A histological and ultrastructural investigation of the female reproductive system of the water snake (Erythrolamprus miliaris): Oviductal cycle and sperm storage. Acta Zool 2017:1–12. https://doi.org/10.1111/azo.12234
Saint-Girons HS (1957) Le cycle sexuel chez Vipera aspis dans l´ouest de la France. Bull Biol Fr Belg 91:284–350
Saint-Girons HS (1962) Le cycle reproducteur de la vipère à cornes, Cerastes cerastes (L.), dans la nature et en captivité. Bull Biol Fr Belg 87:41–51
Schuett GW, Grober MS, Van Kirk EA, Murdoch WJ (2004) Long-term sperm storage and plasma steroid profile of pregnancy in a Western diamond-backed Rattsnake (Crotalus atrox). Herpetol Rev 35:328–333
Siegel DS, Sever DM (2008) Seasonal variation in the oviduct of female Agkistrodon piscivorus (Reptilia: Squamata): an ultrastructural investigation. J Morphol 269(8):980–997. https://doi.org/10.1002/jmor.10638
Siegel DS, Sever DM, Rheuvert JL, Gribbins KM (2009) Reproductive biology of Agkistrodon piscivorus (Squamata, Serpentes, Viperidae, Crotalinae). Herpetol Monogr 23:74–107. https://doi.org/10.1655/08-031.1
Siegel DS, Miralles A, Chabarria RE, Aldridge RD (2011) Female reproductive anatomy: cloaca, oviduct, and sperm storage. In: Aldridge RD, Sever DM (eds) Reproductive biology and phylogeny of snakes. Science Publishers, Enfield, pp 347–409
Silva KMP, Barros VA, Rojas CA, Almeida-Santos SM (2019) Infundibular sperm storage and uterine muscular twisting in the Amazonian lancehead, Bothrops atrox. Anat Rec 2019:1–10. https://doi.org/10.1002/ar.24309
Silva KMP, Braz HB, Kasperoviczus KN et al (2020) Reproduction in the pitviper Bothrops jararacussu: large females increase their reproductive output while small males increase their potential to mate. Zoology 142:125816. https://doi.org/10.1016/j.zool.2020.125816
Silva KMP, Almeida-Santos SM, Bravo-Veja CA, Mahmood S (2023) Sexual maturity of Bothrops asper (Serpentes: Viperidae) from Costa Rica. Phyllomedusa. https://doi.org/10.11606/issn.2316-9079.v21i2pxx-xx
Souza E, Almeida-Santos SM (2022) Reproduction in the bushmaster (Lachesis muta): uterine muscular coiling and female sperm storage. Acta Zool 103(2):244–255. https://doi.org/10.1111/azo.12369
Taylor EN, DeNardo DF, Jennings DH (2004) Seasonal steroid hormone levels and their relation to reproduction in the Western Diamond-backed Rattlesnake, Crotalus atrox (Serpentes: Viperidae). Gen Comp Endocrinol 136:328–337. https://doi.org/10.1016/j.ygcen.2004.01.008
Vieira S, Romero-De-Perez G, Ramirez-Pinilla MP (2010) Ultrastructure of the ovarian follicles in the placentotrophic Andean Lizard of the genus Mabuya (Squamata: Scincidae). J Morphol 271:738–749. https://doi.org/10.1002/jmor.10830
Yamanouye N, Silveira PF, Abdalla FMF, Almeida-Santos SM, Breno MC, Salomão MG (2004) Reproductive cycle of the neotropical Crotalus durissus terrificus: II. Establishment and maintenance of the uterine muscular twisting, a strategy for long-term sperm storage. Gen Comp Endocrinol 139(2):143–150. https://doi.org/10.1016/j.ygcen.2004.09.001
Acknowledgements
We thank Giuseppe Puorto, Felipe G. Grazziotin and Valdir Germano from the Herpetological collection “Alphonse Richard Hoge” from Instituto Butantan; for allowing assistance and access to specimens under their care. We are grateful to Carlos Jared and Marta Antoniazzi from the Instituto Butantan, for providing access and preparation of the optical microscopy techniques. We also thank Adriana da Costa Neves, Dener Madeiro de Souza and Eduardo Osorio Frare from the Instituto Butantan, for providing access and help with the microscope equipment. This study was completed in partial fulfillment of a scientific initiation by Rafaella Jurkfitz and funded by CNPq. Selma M. Almeida-Santos have a research fellowship granted by the Brazilian Agency for Scientific Research (CNPq) (process number 310357/2018-7).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RJ and KS. The first draft of the manuscript was written by RJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jurkfitz, R.C., Silva, K.M.P. & Almeida-Santos, S.M. Sperm storage in Crotalus durissus (Serpentes: Crotalinae): histological insights about the female reproductive tract of pit vipers. Zoomorphology 142, 487–496 (2023). https://doi.org/10.1007/s00435-023-00613-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00613-8