Abstract
In some species, sperm is stored within the female reproductive tract for months to years, and yet remains viable to fertilize eggs and produce offspring. Female red-sided garter snakes store sperm for over 7 months of winter dormancy. In previous work, we demonstrated that these stored sperm account for an average of 25 % paternity of a litter when the female mates with a male at spring emergence. Here, we tested whether last-male sperm precedence was prevalent when a female mates with two males during the spring. On average, paternity was shared equally among the first (P1 proportion of paternity of the first male to mate) and second males (P2) to mate in the spring, and stored sperm (Pss), but the variance in paternity was high. Thus, last male sperm precedence may diminish when a female has more than two mates. Male size did not affect paternity, but, as the interval between matings increased, P1 increased at the expense of Pss. Interestingly, as the second spring male’s copulation duration increased, P1 also increased at the expense of P2. This result suggests that female influence over sperm and/or copulatory plug transfer during matings may also affect which male fathers her offspring in response to coercive matings as we assisted females to mate for their second mating. Finally, all females were spring “virgins”; consequently, sperm stored from autumn matings (and/or previous spring matings) remain competitive even when faced with two rivals in sperm competition and is likely the driver of the evolution of sperm longevity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sperm storage within the female reproductive tract is a prerequisite for sperm competition and is ubiquitous throughout the animal kingdom (Birkhead and Møller 1998; Simmons 2001). Sperm storage for long periods (months to years) is less common but also widespread (Birkhead and Møller 1993; Holt and Lloyd 2010; Orr and Zuk 2012) and is predicted to affect male and female reproductive strategies as well as mating system evolution (Birkhead and Møller 1993; Alonzo and Pizzari 2013; Parker and Birkhead 2013). Thus, it is important to characterize the use of sperm stored for prolonged periods in a range of systems in order to fully appreciate how postcopulatory sexual selection affects the evolution of mating systems in the wild (Shuster and Wade 2003; Engqvist 2013; Parker and Birkhead 2013; Shuster et al. 2013), which often involves sperm storage across mating seasons (Birkhead and Møller 1993). Numerous studies have estimated mate order effects using virgin females (Birkhead and Møller 1998; Simmons 2001). However, in order to understand sperm competition dynamics in wild populations, we need to know the average fitness payoff accrued via long-term female sperm storage. This is essential for estimating the strength of selection on male traits such as sperm longevity and/or complex traits such as specialized sperm storage organs in females. However, with the exception of the social insects (Hölldobler and Wilson 1990; Pamilo 1991; Boomsma et al. 2005) and a handful of other taxa such as bats, salamanders, lizards, snakes, turtles, and guppies (Hosken 1998; Zamudio and Sinervo 2000; Adams et al. 2005; Olsson et al. 2009; Sakaoka et al. 2011; Friesen et al. 2013a; López-Sepulcre et al. 2013; Uller et al. 2013), the effect of long-term sperm storage on patterns of paternity has not been adequately assessed (Birkhead and Møller 1993, 1998; Simmons 2001; Uller et al. 2010; Orr and Zuk 2013).
Reptiles are ideal models for studying traits related to long-term sperm viability/storage within the female reproductive tract, because long-term female sperm-storage is ubiquitous within this ecologically diverse taxon (months to years in many species, Birkhead and Møller 1993; Uller and Olsson 2008). Reptiles exhibit a wide array of mating behaviors and degree of sexual size dimorphism (Duvall et al. 1993; Shine 1994; Olsson and Madsen 1998; Shanbhag 2003; Shine 2003) which is ideal for comparative studies to link ecological variables to the evolution of long-term sperm storage. However, this taxon is underrepresented in studies of sexual selection in general and postcopulatory selection in particular (Uller and Olsson 2008; Uller et al. 2010).
Among the reptiles, snakes exhibit some of the longest periods of sperm storage in any vertebrate (years in some cases; Birkhead and Møller 1993; Uller and Olsson 2008; Uller et al. 2010). Snakes are difficult to study due to their cryptic nature (Seigel 1987; Duvall et al. 1993; Gibbs and Weatherhead 2001), and only one study of a wild population (Friesen et al. 2013a) has employed an experimental approach in which the order of mating males is known which can have strong effects on paternity share. Other studies on wild populations have found mixed evidence for male size effects on paternity (e.g., Prosser et al. 2002; Weatherhead et al. 2002; Blouin-Demers et al. 2005; Kissner et al. 2005), but, without knowing the mate-order, neither the prevalence of stored sperm usage nor the role of mate order effects on sperm precedence could be established. As some populations of snakes predictably aggregate annually at the same locations and exhibit robust courtship behavior even while they are observed, this feature can be exploited during the spring breeding period to assess patterns of paternity (Gregory 1974; Friesen et al. 2013a).
Red-sided garter snakes (Thamnophis sirtalis parietalis) of the Interlake region of Manitoba, Canada, emerge en masse from communal limestone hibernacula (dens) in late April each year (Gregory 1974) to form large breeding aggregations. Within these large aggregations, male mating success is essentially random with respect to male size (Joy and Crews 1988; Shine et al. 2000c), and female precopulatory choice is limited (Shine et al. 2000a). Multiple paternity is common within the genus Thamnophis (Blanchard and Blanchard 1941; Gibson and Falls 1975; Schwartz et al. 1989; McCracken et al. 1999; King et al. 2001; Garner and Larsen 2005; Wusterbarth et al. 2010). Thus, females likely mate again later or use stored sperm. Females may have the opportunity to ‘trade-up’ by instigating sperm competition (cryptic female choice in sensu; Thornhill 1983; Simmons 1987). Females may also mitigate the lack of precopulatory choice in large, spring mating aggregations by using sperm stored over winter (Friesen et al. 2013a). Fortunately, as males display robust courtship in controlled mating trials (e.g., Whittier et al. 1985; Shine et al. 2000c; LeMaster and Mason 2002; Friesen et al. 2013b), the last male to mate can be easily identified.
The current study builds upon our previous work documenting the use of sperm stored over 7–8 months of winter dormancy (>85 % of litters had offspring fathered by stored sperm; Friesen et al. 2013a). Our main aims were (1) to assess the effect of multiple spring matings on long-term stored sperm usage, (2) to assess last male sperm precedence of within-season matings, and (3) to test for postcopulatory male size advantages. The sperm from early matings seem to first fill posterior-most and presumably less favorable sperm storage tubules (Fox 1956; Halpert et al. 1982; Devine 1984). Males also invest heavily in the production of a copulatory plug to prevent second matings and sperm loss (Shine et al. 2000b; Friesen et al. 2013b; CRF et al. unpublished data). Therefore, we, like Devine (1984), predicted that the last male to mate would have precedence in this species. In addition, we also assessed the effects that copulation duration and the interval between matings have on paternity.
Methods
Animal collection and mating trials
Male T. s. parietalis were collected by hand from a population near Inwood, MB, and taken to the Chatfield Research Station 16 km away early in the season before females began to emerge in large numbers. It is unlikely, but possible, that the males had mated prior to capture that spring. All females were collected immediately upon emergence from their winter dormancy before spring mating. Thus, they did not have the opportunity to mate with any males except those they may have mated with prior to brumation (i.e., the previous autumn or possibly the previous spring). These previous matings would be the likely source of stored sperm in our paternity analysis (Friesen et al. 2013a). The sexes were housed separately in outdoor nylon arenas (1 × 1 × 1 m) and were provided water ad libitum. Over the course of the next 17 days (25 April to 12 May, Fig. 1), a single group of 24 randomly selected males were allowed to court and mate with the females in 1 × 1 × 1 m semi-natural arenas on each day that was warm enough to allow vigorous courtship (≥13 °C). Females were added sequentially and replaced if they had not mated within 30 min of being introduced to the enclosure. Thus, at any one time, two females were in the enclosure with the males. When a pair began mating, we placed the pair in a smaller arena where they were under constant observation in order to time copulation duration (±10 s). Courtship and copulation duration are unaffected by the translocation (Friesen et al. 2013b, 2014a). The 24 males were allowed unlimited mating opportunities; they mated with 52 females, which is an average of 2.17 matings per male (three males were especially successful: Two males mated five times, and one male mated four times). These matings were the first spring mating for each of the 52 females, but from the first to the fifth spring matings for each of the 24 males; male mating history may thus affect paternity.
The timing of matings relative to average ovulation. The solid points represent first matings, and the arrow indicates all second matings. The interval between matings as also represents the time between the first male’s mating and ovulation. All sperm were stored in the female for >40 days before ovulation in late June (modified from Whittier and Crews 1986)
After the initial mating, the females were kept separate from any male until May 14th, when each female was tested for attractivity and receptivity. All females were attractive (i.e., males courted them), but none of the females were receptive, as none mated after 30 min with 24 males in an arena (Whittier et al. 1985; Whittier and Crews 1989). This is important, because unreceptive and receptive females may exhibit different sperm utilization patterns. We note that females have days (or weeks) to mate in secrecy during migration to feeding grounds once they leave the den after their first mating (CRF and RTM pers. obs.), but previous work by Shine et al (2000b) found them to be relatively unreceptive to second matings (~7 % remate) in outdoor nylon arenas, although they readily mated in these same arenas for first matings.
Assisted matings of each female to a second male were then carried out on 15 May (e.g., Friesen et al. 2014b) (Fig. 1). These assisted rematings allowed us to partially control the timing of the last mating relative to ovulation, in late June in this population, although individuals will vary (Garstka et al. 1982; Whittier and Crews 1986), which is significant because this timing is known to affect sperm precedence patterns in other species (e.g., Parker 1984; Zeh and Zeh 1994; Olsson and Madsen 1998). In addition, the assisted mating method also allowed us to conduct all of the second matings on a day when weather permitted mating trials. Twenty-four actively courting males were placed in an arena with one female. Assisted female remating was accomplished by gently gaping the female’s cloaca as a courting male aligned with her. We used a blunt probe to lift the ventral scale that covers the opening to her cloaca such that a male could easily intromit one of his two hemipenes (Friesen et al. 2014b). Typically, a courting male would then evert one hemipene into the female’s cloaca a few seconds after the ventral scale was lifted. Copulation duration was measured as previously described for the first matings. Each male’s size (mass ±0.1 g and snout to vent length (SVL) ±1 mm) was recorded. Tail tips were collected from each male for genotyping (Garner et al. 2004; Friesen et al. 2013a). Female size (SVL and mass) was recorded after the second mating. Females were then returned to laboratory facilities at Oregon State University, where gravid females were either kept alone or with a non-gravid female until they gave birth in late summer; thus, maternity was certain. At this time, tail tip tissue (≤3 mm) was collected from each mother and her offspring.
Molecular methods
We followed the same protocols for paternity analysis as Friesen et al. (2013a). Briefly, DNA was extracted from male, female, and offspring tail tip tissue (Garner et al. 2004; Friesen et al. 2013a). We used three microsatellite loci to exclude the focal male from paternity: Ts1 (McCracken et al. 1999) and Nsμ2 and Nsμ3 (Prosser et al. 1999). All three loci were multiplexed in a single 12 μl polymerase chain reaction (PCR) reaction (see Friesen et al. 2013a for details). Reaction products were analyzed in an ABI 3100 genetic analyzer, and the alleles were visualized using ABI Genotyper software. Genotypes were assigned manually. For each offspring missing a maternal allele we re-extracted DNA from the tissue and conducted a new PCR reaction of that offspring, the mother, and a random subset of siblings to check for errors. In total, 26 families and all 409 offspring were successfully genotyped with a maternal allele.
We used an exclusion-based protocol for paternity assignment. We estimated average exclusion probability using CERVUS 3.0.3. (Kalinowski et al. 2007). The three loci chosen for this study were all highly polymorphic. The average exclusion probabilities, based on the genotypes from 56 random adults from a previous study in the same subspecies (T. s. paretalis), were: Ts1 (1–0.12) = 0.88 exclusion probability (second parent); there were 26 alleles found in 56 genotyped individuals; observed heterozygosity, H 0 = 0.96. Nsμ2 (1–0.17) = 0.83 exclusion probability (second parent); there were 20 alleles found in 56 individuals, H 0 = 0.54. Nsμ3 (1–0.25) = 0.75 exclusion probability (second parent); there were 12 alleles found in 56 individuals, H 0 = 0.89 (Friesen et al. 2013a). The combined average exclusion probability using all three alleles is (1–0.015) = 0.99, and any pair of loci yields greater than 0.95 confidence of correctly excluding our known males. Analysis of the adults within this study established that the single locus exclusion probability of locus Ts1 was >0.98, provided the maternal and focal male’s genotypes did not match. Thus, we relied on this locus alone when the other loci were uninformative.
A conservative estimate of the minimum number of fathers per litter was calculated by dividing the number of paternal alleles by 2 after excluding maternal alleles and those assigned to the known males. Any remaining (“extra”) alleles could not have come from our focal males and were thus assigned as stored sperm. If there were over two alleles we could not account for (i.e., from mother or focal males), we inferred that sperm from two males was used from stores within the female (Friesen et al. 2013a).
Statistical methods
Statistical analyses were conducted using SigmaPlot 11.0. Proportion of offspring fathered was arcsine-square root transformed and male snout-to-vent length (SVL) was ln-transformed to equalize variance for regression analyses. When equalized variance could not be achieved by transforming data, we used nonparametric methods. We tested whether the first males to mate in the spring differed in their share of paternity across successive matings using repeated-measures ANOVA, and when we found no significant effect of male ID on paternity, we dropped male ID as a factor from the analyses. We used backward stepwise regression model selection (BSRMS) in SigmaPlot to assess which combination of explanatory variables best explained variation in the proportion of paternity attributable to spring-male mating order [P1, P2, or stored sperm (Pss)]. Our initial model included male mate number, male and female size, the first and second copulation duration, and the interval between matings. Although the proportion of paternity attributable to different males within a litter is not independent, we performed BSRMS on each proportion (i.e., P1, P2, or/and Pss) separately because no other statistical approach was appropriate to analyze three separate non-independent responses simultaneously. In addition, it is unlikely that such an analysis would change or add to the interpretation of our results. To visualize the relationships and aid in our interpretation of the sources of variation in paternity, we plotted separate regressions of standardized partial residuals for each significant independent variable in Sigma Plot, and we present R 2 for each graph as a measure of effect size after accounting for the other significant predictor variable(s) (Grissom and Kim 2012).
Results
No effect of male mate number on paternity
As noted above, most of the first males to mate had mated multiply. For seven of these males, two of the females they mated with gave birth. Therefore, we were able to compare a male’s share of paternity for his first mating with that of a subsequent mating. We used repeated measures to assess the effect of the first male’s mating history on his ability to defend paternity against a random second competitor (which was the case for all of the females’ second matings). The number of times a male mated (male mate number of the first male to mate) did not affect his paternity (i.e., P1) (paired t test; t df = 7 = 0.727, p = 0.491). Mixed-model approaches did not yield different results.
Distribution of paternity and mate-order
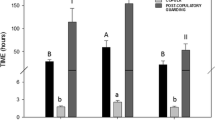
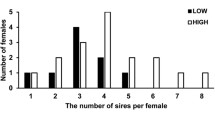
Each male mated an average of 2.17 times (0 − 5 matings per male). Of the 52 doubly mated females, 26 (50 %) gave birth, which is a typical parturition rate for capital breeders that rely on stored energy to determine their reproductive state (Gregory 2006). The first male’s number of matings did not affect the likelihood of a female giving birth (Fisher’s exact test, p = 0.424). Litter sizes ranged from four to thirty one (\( \overline{X} \) ± SEM; 15.73 ± 1.26), with a total of 409 offspring produced. Litter sizes were relatively large as 85 % of the litters contained more than ten offspring (22/26 litters). We detected an average of 2.62 fathers per litter (1–4 fathers, SEM ± 0.170); see Fig. 2.
On average, the first male to mate in the spring (henceforth P1) fathered 33 % (\( \overline{X} \) ± SEM; 0.33 ± 0.063) of the offspring across litters. The second male to mate in the spring (henceforth, P2) fathered 34 % (0.34 ± 0.064) of the offspring. We could not assign paternity to 33 % (0.33 ± 0.058) of the offspring; thus, they were attributed to stored sperm (henceforth, Pss) from autumnal or potentially previous spring matings; see Fig. 3. Ninety-two percent (24/26) of litters showed evidence of stored sperm use, and, in all but two cases, stored sperm was attributed to a single male. There was evidence of two sires from stored sperm in two litters.
There was no effect of male size or female size on proportion of paternity for any males. There was no effect of the minimum number of fathers on proportion of paternity: ANOVA P1 (F1, 24 = 0.333, p = 0.333), P2, (F1, 24 = 0.029, p = 0.865) or Pss (F1, 24 = 0.931, p = 0.345). Larger litters were not more likely to have more fathers than smaller litters (SLR, F1, 24 = 0.664, p = 0.424), although multiple paternity is more difficult to detect in small litters, most litters had > 10 offspring.
Effect of the interval between matings and the second male’s copulation duration on paternity share
There was no difference in copulation duration between a female’s first and second mating (Signed rank test W = 235, P = 0.244). We conducted backward stepwise regression model selection (BSRMS) on arcsine(sqrt) transformed proportions of offspring fathered, which revealed that only the second male’s copulation duration (CD2) and mating interval (MI) were significant predictors of P1 (multiple regression; P1 ~ CD2 + MI; Adj. R 2 = 0.528, F2, 24 = 13.289, p < 0.001; Fig. 4a). BSRMS identified the second male’s copulation duration as the only significant predictor of P2 (multiple regression; P2 ~ CD2; Adj. R 2 = 0.258, F1,21 = 8.646, p = 0.008; Fig. 4b). BSRMS identified male mate-number (MM#) as a significant predictor of Pss (Pss ~ MM#; Adj. R 2 = 0.135, F1,21 = 8.646, p = 0.048). However, this relationship was driven by a single male’s fifth mating, in which all the offspring were attributed to stored sperm. If this observation is removed from the analysis, then male mate-number is not significant (Adj. R 2 = 0.000, F1,21 = 0.392, p = 0.537).
Individual regressions of standardized, partial residuals of the proportion of offspring fathered. Each panel represents the source of paternity: Pss , P1, and P2 (top to bottom, respectively) after accounting for mating interval (a) and copulation duration of the second male (b). a The effect of the second male to mate’s copulation duration on paternity accounting for the effect of mating interval. b The effect of the interval between first and second matings after accounting for copulation duration of the second male. Since second matings occurred on the same day (May, 15th), longer intervals indicate first matings that occurred earlier in the season
Discussion
Consistent use of sperm stored over winter
Our current study confirms that sperm stored over winter dormancy are an important source of paternity (\( \overline{X} \) = 33 %) when females mate with more than one male in the spring. This stored sperm paternity rate is similar to that found in our previous study with a single spring mating (\( \overline{X} \) = 25 %, Friesen et al. 2013a). Over 90 % of litters showed evidence of long-term stored sperm usage, which is also similar to our previous work on this species (85 %, Friesen et al. 2013a). Although the average proportion of paternity attributable to stored sperm usage (Pss) is relatively low compared with potential paternity from spring matings (Friesen et al. 2013a), in some individual cases, Pss was quite large (e.g., in one litter 100 % of paternity was attributed as Pss). These relatively low mean paternity pay-offs of autumn matings (\( \overline{X} \) of Pss ≈ 25 − 33 %) may have low opportunity costs, with few constraints imposed by energy and sperm allocation budgets.
During the spring, the males’ testes are quiescent because of the temporally dissociated reproductive pattern of this species (Crews 1984; Crews et al. 1984), which may leave males vulnerable to sperm depletion after repeated matings (Friesen et al. 2014a) and, in turn, leave females sperm limited. In addition, as the snakes are aphagous during the spring mating season (O’Donnell et al. 2004), their energy stores may limit the time and energy a male can commit to courtship and mating without starving (Shine and Mason 2005). However, during the autumn, males can replenish sperm while the energetically expensive process of spermatogenesis is ongoing (Olsson et al. 1997) and eat while food is still available before winter, and thus autumn matings should not have the same opportunity costs as spring matings.
In addition to allowing paternity success from low-cost autumn matings, sperm storage (or longevity) can facilitate posthumous male reproductive success, a phenomenon known to occur, for example, in Trinidadian guppies (Poecilia reticulata, López-Sepulcre et al. 2013), side-blotched lizards (Uta stansburiana, Zamudio and Sinervo 2000), and dragon lizards (Ctenophorus pictus, Olsson et al. 2009; Ctenophorus fordi, Uller et al. 2013). For red-sided garter snakes, males that die during brumation in the harsh winter cold will have no further mating opportunities, and yet sperm storage can still allow them to gain posthumous paternity.
In a system with a dissociated reproductive pattern, the chance of mortality over winter, as well as the prospect of inseminating females while spermatogenesis is ongoing and food is abundant in autumn, may select for long-term viability of ejaculates (Uller et al. 2013), rather than specialization of the female oviduct for sperm storage. Specialized female sperm storage organs are predicted to evolve in response to low mate encounter rates (Birkhead and Møller 1993; Duvall et al. 1993), but males of this species reliably aggregate at den sites during both the autumn and at spring emergence. Furthermore, sperm storage receptacles within snakes are simple invaginations of the oviductal wall with few specialized secretory cells to sustain sperm (Sever and Hamlett 2002; Siegel et al. 2011a). Therefore, in snakes, the male ejaculate is the more likely target of selection rather than the female reproductive tract (Uller et al. 2010, 2013). The short breeding seasons and harsh overwintering conditions of high-latitude, temperate regions may be ecological factors which first favor the evolution of a dissociated reproductive pattern, which in turn favors sperm longevity. Further comparative work on a variety of taxa across latitudinal gradients would help to address this hypothesis.
Multiple paternity
Within the genus Thamnophis, multiple paternity has been established in five of the six species studied thus far (Wusterbarth et al. 2010). There have been five studies of T. sirtalis that used molecular methods to assign paternity, in which 73 % (85/116) of the litters genotyped display multiple paternity (range, 37 − 100 %: Schwartz et al. 1989; McCracken et al. 1999; King et al. 2001; Garner et al. 2002; most reviewed in Uller and Olsson 2008; Friesen et al. 2013a). Given that females in this study mated with two males, it is not surprising that nearly 90 % of the litters in this study were multiply sired. It may be more surprising that 10 % were singly sired, which may indicate female control of sperm used for fertilization (i.e., cryptic female choice; Eberhard 1996, 1998). Thus, instances of single paternity do not necessarily indicate that a female only mated with a single male as she may mate with several males but only use the sperm from one of them.
We found that two to three fathers per litter was common, and although the females in this study were unreceptive and coerced to mate, the number of fathers per litter is similar to other studies within the genus Thamnophis (McCracken et al. 1999; King et al. 2001; Garner and Larsen 2005). While it is impossible to assign the total number of female matings using paternity data alone, ours and other studies strongly suggest that female garter snakes generally mate with at least two to three males between litters, which may be 1 to 2 years, depending on her fat reserves (Gregory 2006). Females readily mate in arena trials for first spring matings (e.g., Shine et al. 2000b; Friesen et al. 2013a, 2014a). Shine et al (2000b) found that 7 % of females remated over the 20 days that they were kept in areas such as those used in this study. Shine et al. (2000b) also found the same percentage of females (7 %) had multiple mating plugs in the wild around the dens. Nevertheless, the frequency of second spring matings around the den or during migration to feeding grounds remains unknown and is difficult to assess because females are more evasive and secretive after their first mating in the dens (CRF and RTM pers. obs.). It is possible that females rely on sperm from autumn matings to hedge their bets. However, it seems likely that T. s. parietalis also mate more than once in the spring, as mated females (i.e., those bearing a mating plug) are courted within small aggregations (one to six males) in the aspen grooves surrounding the dens (CRF and RTM pers. obs.), and the range of multiple fathers in our study closely matches those from previous studies conducted with wild caught female garter snakes. Females may wait until the operational sex ratio is less biased towards males in the woodlands along migration routes, when predation risk is lower and where they may be better able to exert control in smaller, secluded mating aggregations. However, in this study, we failed to uncover a strong last male precedence; instead we, found high variance in paternity. This study removed any possible mechanism for precopulatory female choice during second matings, and the high variance in paternity may reflect postcopulatory mechanisms under female control (Eberhard 1996, 1998).
Mate order
Patterns of sperm use and the mechanisms that generate these patterns are particularly well understood in a limited range of taxa, such as birds and insects (Wigby and Chapman 2004; Andersson and Simmons 2006; Birkhead et al. 2008; Lüpold et al. 2012; Manier et al. 2013). A simple, yet fundamental, principle of sperm competition has come from this work: A male’s fitness increases with the number of sperm he inseminates relative to his rivals (Parker 1990). However, in most taxa, sperm quickly become inviable after insemination because the female reproductive tract is inhospitable (Poiani 2006; Suarez and Pacey 2006; Suarez 2008; Pitnick et al. 2009). Thus, sperm from the last male to mate often have a competitive advantage because of the attrition, loss, or displacement of his competitors’ sperm within the female (Birkhead 1998; Birkhead and Biggins 1998; Simmons and Siva-Jothy 1998). This typically results in last male sperm precedence, in which the last male to mate fathers most or all of the offspring produced by the female (Boorman and Parker 1976); however, first male sperm precedence is also prevalent in some taxa (reviewed in Birkhead and Møller 1998). Consistent patterns of sperm precedence affect the allocation of paternity and thus are important for male and female reproductive tactics and mating system evolution (Shuster and Wade 2003; Parker and Birkhead 2013; Shuster et al. 2013).
In the only other study to address mating order in any snake, captive Vipera berus, Höggren and Tegelström (2002) found first-male advantage in within-season matings. However, we hypothesized that last male precedence would be strong in red-sided garter snakes because males invest in a gelatinous copulatory plug that occludes the female’s cloaca after mating (Devine 1975, 1977). Mate guarding devices, such as copulatory plugs, are expected to evolve when there is risk of sperm competition, and the first male to mate is likely to lose paternity in the absence of such a device (Parker 1984, 1998; but see Simmons and Siva-Jothy 1998; Simmons 2001). The copulatory plug of T. s. parietalis decreases female remating rates for up to 2 days before it fully dissolves in arena trials (Shine et al. 2000b). As the plug dissolves, sperm migrate to sperm storage receptacles (SSRs) in the anterior infundibulum of the oviduct 24–48 h after mating (Halpert et al. 1982), with sperm tending to fill the more posterior SSRs first (Fox 1956). Based on these observations, Devine (1984) suggested that last male precedence should be the rule if the anterior SSRs were filled by sperm from successive inseminations, putting them closer to the ova at the time of ovulation. Friesen et al. (2013a) found that sperm from single spring matings (i.e., last male) had precedence. However, in the current study, we found no consistent effect of mate order within spring matings; rather, average paternity was shared equally among the first (P1) and last male (P2) to mate, and stored sperm (Pss). Although average paternity share was equal, variance in fertilization success was extremely high. Male mating history did not explain this variation, but our small sample size may have limited our ability to detect a trend if the effect was minor.
Male body size
It is interesting that we found no effect of male body size, as it has been shown to affect paternity success in a number of taxa (e.g., Simmons and Parker 1992; Bissoondath and Wiklund 1997; Arnqvist and Danielsson 1999; Bangham et al. 2002; delBarco-Trillo and Ferkin 2004). Indeed, male size is a predictor of paternity in some species of snakes [black rat snake (Blouin-Demers et al. 2005); water snake (Kissner et al. 2005); Eurasian viper (Ursenbacher et al. 2009); but see water snake (Weatherhead et al. 2002)]. However, the two species that exhibited a strong male-size advantage, the black rat snake (Elaphe obsoleta) and Eurasian viper (V. berus), display male–male combat (Blouin-Demers et al. 2005; Ursenbacher et al. 2009). Therefore, male size advantage may reflect a pre-copulatory mating advantage rather than postcopulatory processes influenced by mating order, sperm competitiveness, or cryptic female choice.
In the current study, like that of Friesen et al. (2013a), male size did not affect the probability of paternity in this population of red-sided garter snakes. This may be because, in this population, neither male nor female size has an effect on the number of sperm a male inseminates (Friesen et al. 2014a). However, in a smaller, less dense population of red-sided garter snakes, in Manitoba, Canada, larger males had a significant paternity advantage over stored sperm (Friesen et al. 2013a). As yet, there are no data on sperm numbers from this population. Population size and density may explain why some populations exhibit different patterns of size-dependent reproductive success while others do not (Prosser et al. 2002; Weatherhead et al. 2002; Kissner et al. 2005; Friesen et al. 2013a): Sexual coevolution in small populations is predicted to occur rapidly along a line of equilibrium due to drift (Lande 1981; Gavrilets 2000; Uyeda et al. 2009), while large populations are predicted (Gavrilets 2000) and demonstrated (Martin and Hosken 2003; Gay et al. 2010) to be more responsive to selection promoted by sexual conflict.
Mechanisms
Although more controlled experiments are necessary to fully elucidate mechanisms of sperm usage in this species, our results provide a foundation for future experiments. We found parity in paternity among potential fathers and a significant increase of the variance in male fertilization success when females mated with two spring males versus one male (Friesen et al. 2013a). Studies of other taxa have found that mate-order effects are attenuated and the inflation of variance in paternity as a female mate with more than two males. For example, in harlequin beetle-riding pseudoscorpions, among male variance in fertilization success increased, and mate order effects vanished when females were mated with three males and when the interval between matings was increased (Zeh and Zeh 1994; but see Lewis and Jutkiewicz 1998). Similarly, our analyses revealed that the interval between mating and the second male to mate’s copulation duration explained some of the variation in paternity.
Mating interval
Timing of ovulation relative to insemination has previously been shown to influence paternity patterns (reviewed in Birkhead and Pizzari 2002). Since all the second matings took place at the same time, longer mating intervals indicate that the first mating took place earlier in the season. The first male’s paternity significantly increased the earlier he mated. The second male’s paternity was unaffected by the interval between matings, probably because all second matings occurred on the same day relative to ovulation. Although not a significant result, as the interval between matings increases, Pss decreases as P1 increases. We propose that first matings that occur earlier in the season allow more time for those sperm to displace or overlay sperm that were stored prior to winter dormancy.
Copulation duration
It may seem paradoxical that longer copulations by the second male reduced his paternity: Longer copulation durations are positively correlated with the amount of sperm transferred in at least two species of lizard (Tokarz 1999; Olsson 2001), as well as many other taxa (Birkhead and Møller 1998; Simmons 2001). However, copulation duration does not affect sperm numbers in this population of garter snakes (Friesen et al. 2014a). Copulation duration does correlate with the mass of the sperm-free section of the plug, which may be a source of sexual conflict (Friesen et al. 2014b), and long copulation durations may not be a target for strong selection in this species. In fact, there may be selection to shorten copulation duration to decrease opportunity costs for males which is facilitated by depositing a large copulatory plug (Shine et al. 2000b; Friesen et al. 2013b). There are a few intriguing hypotheses to explain why, as the second male’s copulation duration increased, P2 decreased while P1 increased with no effect on Pss. First, the muscular contractions or twisting of the oviduct (Nilson and Andrén 1982; Siegel and Sever 2006) caused by the second male’s insemination may aid transport of the first male’s sperm. Second, the second male’s ejaculate physically moves the first male’s sperm further into the oviduct. Third, the female may have a mechanism to reduce the paternity of a coercive male, for example, by constriction of the vaginal pouch (references in Siegel and Sever 2006). We propose that this last explanation is most likely because the second insemination was assisted, not voluntary, and, thus, could be considered a coerced mating. These hypotheses are not mutually exclusive, and the second male to mate may unwittingly aid his rival, by increasing oviductal peristalsis or twisting during copulation, which moves the first male’s sperm farther up the oviduct (Nilson and Andrén 1982; Yamanouye et al. 2004; Siegel and Sever 2006).
Red-sided garter snake females lack precopulatory choice in mating aggregations in the den (Shine et al. 2000a), and it might benefit female garter snakes to reduce plug and sperm transfer during copulation, which may explain the reduced paternity associated with longer copulation duration seen here. In jungle fowl, females are able to eject the sperm of an unwanted suitor of low social rank (Pizzari and Birkhead 2000), and female dunnocks eject sperm in response to cloaca-pecking by their dominant mate (Davies 1983). The vaginal pouch of T. s. parietalis is thickened with muscle (Siegel et al. 2011a, b), so a mechanism similar to that found in dunnocks and fowl may exist in snakes (Friesen et al. 2014b). Increased copulation duration could indicate female resistance to sperm transfer and plug deposition. The plug prevents sperm leakage (Friesen et al. 2013b), and female resistance may increase the chance of leakage and/or a poor fit of the copulatory plug within the female vagina (Friesen et al. 2013b; Friesen et al. 2014b). The vaginal pouch is highly muscularized and limits plug transfer (Friesen et al. 2014b) and prevents sperm from entering the oviducts (Friesen unpublished data).
Conclusion
We have established that sperm stored overwinter is a consistent source of male fertilization success in red-sided garter snakes, even if females mate multiply in the spring. Engqvist (2013) points to the shortcomings of using standardized males in studies of sperm competition and the determinants of male fertilization success. Similarly, we suggest that the use of virgin females ignores the effect of long-term sperm storage, which is a ubiquitous and important factor in wild populations that should not be overlooked (see for example, Olsson et al. 2009; Friesen et al. 2013a; López-Sepulcre et al. 2013; Uller et al. 2013). Inflated variance in paternity with increased female mating and stored sperm use is not necessarily a random effect, which suggests it may be important for mating system evolution as well as selection on male sperm traits and mating strategies. In addition, our assisted mating technique has revealed that females may exert control over copulatory plug and/or sperm transfer, which is also a potential source of sexual conflict (Friesen et al. 2014b). In the future, comparisons of paternity patterns, sperm counts, plug mass, and copulation duration between assisted matings and natural single spring matings may reveal whether females exert control of sperm transfer or copulatory plug material to bias paternity after coerced matings.
References
Adams EM, Jones AG, Arnold SJ (2005) Multiple paternity in a natural population of a salamander with long-term sperm storage. Mol Ecol 14:1803–1810
Alonzo SH, Pizzari T (2013) Selection on female remating interval is influenced by male sperm competition strategies and ejaculate characteristics. Phil Trans R Soc B 368(1613):20120044. doi:10.1098/rstb.2012.0044
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Arnqvist G, Danielsson I (1999) Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav Ecol 10:358–365
Bangham J, Chapman T, Partridge L (2002) Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim Behav 64:915–921
Birkhead TR (1998) Sperm competition in birds: mechanisms and function. In: Birkhead T, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, CA, pp 579–622
Birkhead TR, Biggins JD (1998) Sperm competition mechanisms in birds: models and data. Behav Ecol 9:253–260
Birkhead T, Hosken D, Pitnick S (eds) (2008) Sperm biology: an evolutionary perspective. Elsevier/Academic Press, London
Birkhead TR, Møller AP (1993) Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol J Linn Soc 50:295–311
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic Press, San Diego
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3:262–273
Bissoondath CJ, Wiklund C (1997) Effect of male body size on sperm precedence in the polyandrous butterfly Pieris napi L. (Lepidoptera: Pieridae). Behav Ecol 8:518–523
Blanchard FN, Blanchard FC (1941) The inheritance of melanism in the garter snake Thamnophis sirtalis sirtalis (Linnaeus), and some evidence of effective autumn mating. Mich Acad Sci Arts Lett 26:177–193
Blouin-Demers G, Gibbs H, Weatherhead P (2005) Genetic evidence for sexual selection in black ratsnakes, Elaphe obsoleta. Anim Behav 69(1):225–234
Boomsma JJ, Baer B, Heinze J (2005) The evolution of male traits in social insects. Annu Rev Entomol 50:395–420
Boorman E, Parker GA (1976) Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol Entomol 1:145–155
Crews D (1984) Gamete production, sex hormone secretion, and mating behavior uncoupled. Horm Behav 18:22–28
Crews D, Camazine B, Diamond M, Mason R, Tokarz RR, Garstka WR (1984) Hormonal independence of courtship behavior in the male garter snake. Horm Behav 18:29–41
Davies NB (1983) Polyandry, cloaca-pecking and sperm competition in dunnocks. Nature 302:334–336
delBarco-Trillo J, Ferkin MH (2004) Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature 431:446–449
Devine MC (1975) Copulatory plugs in snakes: enforced chastity. Science 187:844–845
Devine MC (1977) Copulatory plugs, restricted mating opportunities and reproductive competition among male garter snakes. Nature 267:345–346
Devine MC (1984) Potential for sperm competition in reptiles: behavioral and physiological consequences. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Orlando, pp 509–521
Duvall D, Schuett GW, Arnold SJ (1993) Ecology and evolution of snake mating systems. In: Seigel RA, Collins JT (eds) Snakes: ecology and behavior. McGraw-Hill, New York, pp 165–200
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Eberhard WG (1998) Female roles in sperm competition. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, CA, pp 91–116
Engqvist L (2013) A general description of additive and nonadditive elements of sperm competitiveness and their relation to male fertilization success. Evolution 67:1396–1405
Fox W (1956) Seminal receptacles of snakes. Anat Rec 124:519–539
Friesen CR, Mason RT, Arnold SJ, Estes S (2013a) Patterns of sperm use in two populations of red-sided garter snake (Thamnophis sirtalis parietalis) with long-term female sperm storage. Can J Zool 92:33–40
Friesen CR, Shine R, Krohmer RW, Mason RT (2013b) Not just a chastity belt: the functional significance of mating plugs in garter snakes, revisited. Biol J Linn Soc 109:893–907
Friesen CR, Squire MK, Mason RT (2014a) Intrapopulational variation of ejaculate traits and sperm depletion in red-sided garter snakes. J Zool 292:192–201
Friesen CR, Uhrig EJ, Squire MK, Mason RT, Brennan PLR (2014b) Sexual conflict over mating in red-sided garter snakes (Thamnophis sirtalis) as indicated by experimental manipulation of genitalia. Proc R Soc B 281:20132694
Garner TWJ, Gregory PT, McCracken GF, Burghardt GM, Koop BF, McLain SE, Nelson RJ (2002) Geographic variation of multiple paternity in the common garter snake (Thamnophis sirtalis). Copeia 2002:15–23
Garner TWJ, Larsen KW (2005) Multiple paternity in the western terrestrial garter snake, Thamnophis elegans. Can J Zool 83:656–663
Garner TWJ, Pearman PB, Gregory PT, Tomio G, Wischniowski SG, Hosken DJ (2004) Microsatellite markers developed from Thamnophis elegans and Thamnophis sirtalis and their utility in three species of garter snakes. Mol Ecol Notes 4:369–371
Garstka WR, Camazine B, Crews D (1982) Interactions of behavior and physiology during the annual reproductive cycle of the red-sided garter snake (Thamnophis sirtalis parietalis). Herpetologica 38:104–123
Gavrilets S (2000) Rapid evolution of reproductive barriers driven by sexual conflict. Nature 403:886–889
Gay L, Hosken DJ, Eady P, Vasudev R, Tregenza T (2010) The evolution of harm—effect of sexual conflicts and population size. Evolution 65:725–737
Gibbs HL, Weatherhead PJ (2001) Insights into population ecology and sexual selection in snakes through the application of DNA-based genetic markers. J Hered 92:173
Gibson AR, Falls JB (1975) Evidence for multiple insemination in the common garter snake, Thamnophis sirtalis. Can J Zool 53:1362–1368
Gregory P (2006) Influence of income and capital on reproduction in a viviparous snake: direct and indirect effects. J Zool 270:414–419
Gregory PT (1974) Patterns of spring emergence of the red-sided garter snake (Thamnophis sirtalis parietalis) in the Interlake region of Manitoba. Can J Zool 52:1063–1069
Grissom RJ, Kim JJ (2012) Effect sizes for research: univariate and multivariate applications. Routledge, New York
Halpert AP, Garstka WR, Crews D (1982) Sperm transport and storage and its relation to the annual sexual cycle of the female red-sided garter snake, Thamnophis sirtalis parietalis. J Morphol 174:149–159
Höggren M, Tegelström H (2002) Genetic evidence for first male mating advantage in the adder (Vipera berus). In: Schuett G, Höggren M, Douglas M, Greene H (eds) Biology of the vipers. Eagle Mountain Press, Salt Lake City, UT, pp 235–242
Hölldobler B, Wilson EO (1990) The ants. Belknap Press of Harvard University Press, Cambridge
Holt W, Lloyd R (2010) Sperm storage in the vertebrate female reproductive tract: how does it work so well? Theriogenology 73:713–722
Hosken DJ (1998) Sperm fertility and skewed paternity during sperm competition in the Australian long‐eared bat Nyctophilus geoffroyi (Chiroptera: Vespertilionidae). J Zool 245:93–100
Joy JE, Crews D (1988) Male mating success in red-sided garter snakes: size is not important. Anim Behav 36:1839–1841
Kalinowski S, Taper M, Marshall T (2007) Revising how the computer program Cervus accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
King RB, Milstead WB, Gibbs HL, Prosser MR, Burghardt GM, McCracken GF (2001) Application of microsatellite DNA markers to discriminate between maternal and genetic effects on scalation and behavior in multiply-sired garter snake litters. Can J Zool 79:121–128
Kissner KJ, Weatherhead PJ, Gibbs HL (2005) Experimental assessment of ecological and phenotypic factors affecting male mating success and polyandry in northern watersnakes, Nerodia sipedon. Behav Ecol Sociobiol 59:207–214
Lande R (1981) Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci U S A 78:3721–3725
LeMaster MP, Mason RT (2002) Variation in a female sexual attractiveness pheromone controls male mate choice in garter snakes. J Chem Ecol 28:1269–1285
Lewis SM, Jutkiewicz E (1998) Sperm precedence and sperm storage in multiply mated red flour beetles. Behav Ecol Sociobiol 43:365–369
López-Sepulcre A, Gordon SP, Paterson IG, Bentzen P, Reznick DN (2013) Beyond lifetime reproductive success: the posthumous reproductive dynamics of male Trinidadian guppies. Proc R Soc B 280:20132694
Lüpold S, Manier MK, Berben KS, Smith KJ, Daley BD, Buckley SH, Belote JM, Pitnick S (2012) How multivariate ejaculate traits determine competitive fertilization success in Drosophila melanogaster. Curr Biol 22:1667–1672
Manier MK, Lüpold S, Pitnick S, Starmer WT (2013) An analytical framework for estimating fertilization bias and the fertilization set from multiple sperm-storage organs. Am Nat 182:552–561
Martin OY, Hosken DJ (2003) The evolution of reproductive isolation through sexual conflict. Nature 423:979–982
McCracken GF, Burghardt GM, Houts SE (1999) Microsatellite markers and multiple paternity in the garter snake Thamnophis sirtalis. Mol Ecol 8:1475–1479
Nilson G, Andrén C (1982) Function of renal sex secretion and male hierarchy in the adder, Vipera berus, during reproduction. Horm Behav 16:404–413
O’Donnell RP, Shine R, Mason RT (2004) Seasonal anorexia in the male red-sided garter snake, Thamnophis sirtalis parietalis. Behav Ecol Sociobiol 56:413–419
Olsson M (2001) ‘Voyeurism’ prolongs copulation in the dragon lizard Ctenophorus fordi. Behav Ecol Sociobiol 50:378–381
Olsson M, Madsen T, Shine R (1997) Is sperm really so cheap? Costs of reproduction in male adders Vipera berus. Proc R Soc B 264:455–459
Olsson M, Madsen T (1998) Sexual selection and sperm competition in reptiles. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, pp 503–578
Olsson M, Schwartz T, Uller T, Healey M (2009) Effects of sperm storage and male colour on probability of paternity in a polychromatic lizard. Anim Behav 77:419–424
Orr TJ, Zuk M (2012) Sperm storage. Curr Biol 22:R8–R10
Orr TJ, Zuk M (2013) Does delayed fertilization facilitate sperm competition in bats? Behav Ecol Sociobiol 67:1903–1913
Pamilo P (1991) Life span of queens in the ant Formica exsecta. Insect Soc 38:111–119
Parker GA (1984) Sperm competition and the evolution of animal mating strategies. In: Smith RL (ed) Sperm competition and the evolution of animal mating systems. Academic Press, New York, pp 1–60
Parker GA (1990) Sperm competition games: raffles and roles. Proc R Soc Lond B 242:120–126
Parker GA (1998) Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, pp 3–49
Parker GA, Birkhead TR (2013) Polyandry: the history of a revolution. Philos T Roy Soc B 368:1–8
Pitnick S, Wolfner MF, Suarez SS (2009) Ejaculate–female and sperm–female interactions. In: Birkhead TR, Hosken DJ, Pitnick S (eds) Sperm biology: an evolutionary perspective. Elsevier/Academic Press, London, pp 247–304
Pizzari T, Birkhead TR (2000) Female feral fowl eject sperm of subdominant males. Nature 405:787–789
Poiani A (2006) Complexity of seminal fluid: a review. Behav Ecol Sociobiol 60:289–310
Prosser MR, Gibbs HL, Weatherhead PJ (1999) Microgeographic population genetic structure in the northern water snake, Nerodia sipedon sipedon detected using microsatellite DNA loci. Mol Ecol 8:329–333
Prosser MR, Weatherhead PJ, Gibbs HL, Brown GP (2002) Genetic analysis of the mating system and opportunity for sexual selection in northern water snakes (Nerodia sipedon). Behav Ecol 13:800–807
Sakaoka K, Yoshii M, Okamoto H, Sakai F, Nagasawa K (2011) Sperm utilization patterns and reproductive success in captive loggerhead turtles (Caretta caretta). Chelonian Conserv Biol 10:62–72
Schwartz JM, McCracken GF, Burghardt GM (1989) Multiple paternity in wild populations of the garter snake, Thamnophis sirtalis. Behav Ecol Sociobiol 25:269–273
Seigel RA (1987) Snakes: ecology and evolutionary biology. Macmillan, New York
Sever DM, Hamlett WC (2002) Female sperm storage in reptiles. J Exp Zool 292:187–199
Shanbhag BA (2003) Reproductive strategies in the lizard Calotes versicolor. Curr Sci 84:646–652
Shine R (1994) Sexual size dimorphism in snakes revisited. Copeia 1994:326–346
Shine R (2003) Reproductive strategies in snakes. Proc R Soc Lond B 270:995–1004
Shine R, Mason RT (2005) Do a male garter snake’s energy stores limit his reproductive effort? Can J Zool 83:1265–1270
Shine R, O'Connor D, Mason RT (2000a) Sexual conflict in the snake den. Behav Ecol Sociobiol 48:392–401
Shine R, Olsson MM, Mason RT (2000b) Chastity belts in gartersnakes: the functional significance of mating plugs. Biol J Linn Soc 70:377–390
Shine R, Olsson MM, Moore I, LeMaster MP, Greene M, Mason RT (2000c) Body size enhances mating success in male gartersnakes. Anim Behav 59:F4–F11
Shuster SM, Briggs WR, Dennis PA (2013) How multiple mating by females affects sexual selection. Philos T Roy Soc B 368:1–28
Shuster SM, Wade MJ (2003) Mating systems and strategies. Monographs in behavior and ecology. Princeton University Press, Princeton
Siegel DS, Miralles A, Chabarria RE, Aldridge RD (2011a) Female reproductive anatomy: cloaca, oviduct, and sperm storage. In: Aldridge RD, Sever DM (eds) Reproductive biology and phylogeny of snakes. CRC Press, Enfield, pp 347–409
Siegel DS, Miralles A, Trauth SE, Aldridge RD (2011b) The phylogenetic distribution and morphological variation of the ‘pouch’ in female snakes. Acta Zool 93:400–408
Siegel DS, Sever DM (2006) Utero-muscular twisting and sperm storage in viperids. Herpetol Conserv Biol 1:87–92
Simmons LW (1987) Sperm competition as a mechanism of female choice in the field cricket, Gryllus bimaculatus. Behav Ecol Sociobiol 21:197–202
Simmons LW, Parker GA (1992) Individual variation in sperm competition success of yellow dung flies, Scatophaga stercoraria. Evolution 46:366–375
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton
Simmons LW, Siva-Jothy MT (1998) Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, pp 323–383
Suarez S (2008) Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol 52:455–462
Suarez S, Pacey A (2006) Sperm transport in the female reproductive tract. Hum Reprod Update 12:23–37
Thornhill R (1983) Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am Nat 122:765–788
Tokarz RR (1999) Relationship between copulation duration and sperm transfer in the lizard Anolis sagrei. Herpetologica 55:234–241
Uller T, Olsson M (2008) Multiple paternity in reptiles: patterns and processes. Mol Ecol 17:2566–2580
Uller T, Schwartz T, Koglin T, Olsson M (2013) Sperm storage and sperm competition across ovarian cycles in the dragon lizard, Ctenophorus fordi. J Exp Zool 319:404–408
Uller T, Stuart-Fox D, Olsson M (2010) Evolution of primary sexual characters in reptiles. In: Leonard JL, Córdoba-Aguilar A (eds) Evolution of primary sexual characters in animals. Oxford University Press, Oxford, pp 425–452
Ursenbacher S, Erny C, Fumagalli L (2009) Male reproductive success and multiple paternity in wild, low-density populations of the Adder (Vipera berus). J Hered 100:365–370
Uyeda JC, Arnold SJ, Hohenlohe PA, Mead LS (2009) Drift promotes speciation by sexual selection. Evolution 63:583–594
Weatherhead PJ, Prosser MR, Gibbs HL, Brown GP (2002) Male reproductive success and sexual selection in northern water snakes determined by microsatellite DNA analysis. Behav Ecol 13:808–815
Whittier JM, Crews D (1986) Ovarian development in red-sided garter snakes, Thamnophis sirtalis parietalis: relationship to mating. Gen Comp Endocrinol 61:5–12
Whittier JM, Crews D (1989) Mating increases plasma levels of prostaglandin F2 alpha in female garter snakes. Prostaglandins 37:359–366
Whittier JM, Mason RT, Crews D (1985) Mating in the red-sided garter snake, Thamnophis sirtalis parietalis: differential effects on male and female sexual behavior. Behav Ecol Sociobiol 16:257–261
Wigby S, Chapman T (2004) Sperm competition. Curr Biol 14:100–103
Wusterbarth T, King RB, Duvall MR, Grayburn WS, Burghardt GM (2010) Phylogenetically widespread multiple paternity in New World natricine snakes. Herpetol Conserv Biol 2010:86–93
Yamanouye N, Silveira P, Abdalla FMF, Almeida-Santos SM, Breno MC, Salomao MG (2004) Reproductive cycle of the Neotropical Crotalus durissus terriicus: II. Establishment and maintenance of the uterine muscular twisting, a strategy for long-term sperm storage. Gen Comp Endocrinol 139:151–157
Zamudio KR, Sinervo B (2000) Polygyny, mate-guarding, and posthumous fertilization as alternative male mating strategies. Proc Natl Acad Sci U S A 97:14427–14432
Zeh JA, Zeh DW (1994) Last-male sperm precedence breaks down when females mate with three males. Proc R Soc Lond B 257:287–292
Acknowledgments
We thank Dave Roberts (Manitoba Dept. of Natural Resources) for logistical support, and the residents of Chatfield (especially Al and Gerry Johnson, our thoughts are with you) for encouragement. M. Rockwell Parker, Ruth Nesbitt, and Marie Tubello assisted with data collection, and Emily Uhrig, Sarah L. Eddy, Camilla Whittington, and five anonymous reviewers who provided invaluable comments on previous versions of this manuscript. Financial support was provided by the National Science Foundation Doctoral Dissertation Improvement Grant (1011727) to CRF and 060125NSF grant IOB-0620125 to RTM.
Ethical standards
Research was conducted under the authority of Oregon State University Institutional Animal Care and Use Committee Protocol No. ACUP-3738 and under permit No. WB12405 issued by Manitoba Conservation.
Conflicts of interest
We, the authors, declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Madsen
Rights and permissions
About this article
Cite this article
Friesen, C.R., Kerns, A.R. & Mason, R.T. Factors influencing paternity in multiply mated female red-sided garter snakes and the persistent use of sperm stored over winter. Behav Ecol Sociobiol 68, 1419–1430 (2014). https://doi.org/10.1007/s00265-014-1749-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1749-0