Abstract
Animal body size and sex are requisite data for understanding population structure and demography. Little information exists regarding Nile crocodile, Crocodylus niloticus, morphometrics, sex ratios of wild populations, sexual size dimorphism and standing crop biomass. We captured 322 C. niloticus at Lake St Lucia and Ndumo Game Reserve, South Africa, the two largest crocodile populations at the southern extent of the species’ range in Africa, and measured a suite of physical characteristics to create predictive models of body length from other morphological attributes and body mass. Our sample included 118 hatchlings, 91 subadults and 113 adults. Strong positive allometric relationships were found between body length metrics (total length and snout-vent length) and other morphometrics. All morphometric regressions were linear, with the exception of the relationship of body length to body mass, which was logarithmic. Among relationships of cranial morphology and body length, we found considerable individual variation among all size classes. The mean head width-to-head length ratio was 1.9 ± 1.6, and mean head length-to-total length ratio was 0.14 ± 0.005. The sex ratios for non-hatchling individuals at both populations were essentially 1:1, but adult sex ratios were male biased. We calculated a total standing crop biomass of 96,867.18 kg (161.45 kg/km) and 52,640.40 kg (1504.01 kg/km) for C. niloticus at Lake St Lucia and Ndumo Game Reserve, respectively, and an estimated 3650 non-hatchling individuals for the province of KwaZulu-Natal. The data presented here will help inform crocodile management and population surveys in South Africa, where C. niloticus is an important apex predator that partitions aquatic resources and occasionally comes into conflict with human beings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of the seven proposed crocodilian species in Africa (Shirley et al. 2014), the Nile crocodile, Crocodylus niloticus (Laurenti 1768), is the most iconic and widespread, ranging throughout sub-Saharan Africa to its continental southern range limit in subtropical KwaZulu-Natal, South Africa. As an apex predator, C. niloticus has extensive ecological influences on both aquatic and terrestrial food webs (Warner 2015). The species is also economically valuable in the commercial leather trade (Cott 1961; Blake and Jacobsen 1992). Despite the species’ fearsome reputation, C. niloticus is still an understated human health concern in rural Africa where numerous communities share water resources with crocodiles, and fatalities likely number in the hundreds annually.
Crocodylus niloticus is currently under threat in South Africa and IUCN red-listed as Vulnerable (Marais 2014). In addition to traditional threats of habitat alteration and destruction (Leslie and Spotila 2001; Combrink et al. 2011) and poaching (Calverley and Downs 2014a), environmental pollution has emerged as an insidious new threat to C. niloticus, highlighted by the pansteatitis outbreak and resultant deaths of hundreds of crocodiles from the greater Olifants River watershed (Ferreira and Pienaar 2011; Lane et al. 2013).
In South Africa outside of the Kruger National Park, remaining viable crocodile populations are limited to northern KwaZulu-Natal Province, where the highest densities of C. niloticus occur in protected areas at Lake St Lucia within the UNESCO World Heritage iSimangaliso Wetland Park (Combrink 2014; Warner 2015), Ndumo Game Reserve (Calverley 2013) and at the Pongola River inlet at Pongolapoort (Jozini) Dam (Champion 2011; Summers 2015). Numerous smaller populations persist at the periphery of these areas, including uMkhuze Game Reserve, Tembe Elephant Reserve, Hluhluwe-iMfolozi Park, various waterbodies in greater iSimangaliso Wetland Park (Kosi Bay, Lake Sibaya, Lake Bhangazi North and South), and in the Enseleni, Zinkwazi and Tugela Rivers.
Knowledge of individual body size and sex is necessary data for analyses of animal population ecology and structure. Basic morphometric parameters (e.g., head shape, total length, mass) can help researchers answer complex questions about sexual size dimorphism (Shine 1986; Zamudio 1998), energetic constraints (Wikelski et al. 1997), hybridization (Brede et al. 2000), adaptation to different ecological niches (McMaster and Downs 2006; Ousterhout 2015), and the identification and conservation of cryptic species (Sanders et al. 2015; Davis et al. 2016). Conservation management of crocodilians in particular relies heavily on knowledge of individual body size and population size-class structure because size, rather than age, is the primary driver of demographic and reproductive processes (Webb and Smith 1987).
Despite a legacy of conservation-based research on the species (Cott 1961; Pooley 1982a, b; Leslie 1997; Champion 2011; Calverley 2013; Combrink 2014; Summers 2015; Warner 2015), with the exception of Hutton (1987a, b, c) scant published information exists regarding morphometrics of free-ranging C. niloticus, sex ratios of wild populations, and sexual size dimorphism. In this study, we present an analysis of these variables from data collected in field studies. Additionally, we estimate the current metapopulation size and standing crop biomass of C. niloticus at its African range limit in KwaZulu-Natal based on recent ecological and population studies. Our findings are presented in light of their significance to crocodile research and management in South Africa.
Materials and methods
We captured C. niloticus during 2009-13 at Lake St Lucia and Ndumo Game Reserve, KwaZulu-Natal, South Africa, in conjunction with crocodile population surveys (Champion 2011), and studies of habitat use (Calverley 2013), spatial ecology (Combrink 2014), ecotoxicology (Warner et al. 2016) and stable isotope ecology (Warner 2015). The Lake St Lucia estuarine system is 67 km in total length and contains a main lake basin (6 km wide at capacity) connected to the Indian Ocean via a 27-km-long “Narrows” channel. Lake St Lucia is a highly dynamic environment over space and time with cyclical, but often unpredictable annual fluctuations between drought and high rainfall (Stretch et al. 2013). Due to a decade-long drought, at the time of our sampling the estuary mouth was closed and significant sections of the lake were hypersaline or exposed due to evaporation, thereby rendering the surrounding freshwater pools and feeder streams important habitat for crocodiles (Combrink et al. 2013). The 10,000-ha Ndumo Game Reserve is located on the southern Mozambique border, with the Usuthu and Phongola Rivers forming the northern and eastern boundaries of the reserve, respectively. During the rainy season (Nov–Mar), up to 40 % of the reserve may be inundated, including 12 permanent and semi-permanent floodplain lakes that host large congregations of crocodiles (Calverley and Downs 2014b). Lake St Lucia and Ndumo Game Reserve represent the two largest C. niloticus populations in KwaZulu-Natal.
We captured crocodiles at night and during the day, usually by direct noosing or snagging with a barbless weighted treble hook attached to Kevlar rope or fishing line (Combrink et al. 2012). Hatchlings were captured by hand in nursery areas in late February, March and early April within 30 days of nest emergence. After individuals were safely restrained and tissue samples were taken, we recorded the following measurements:
-
1.
Total length (TL): distance from the tip of the snout to the tip of the tail, measured dorsally.
-
2.
Snout-vent length (SVL): distance from the tip of the snout to the posterior margin of the cloacal vent, measured dorsally.
-
3.
Head width (HW): maximum distance between the surangular bones at the level of jaw articulation.
-
4.
Head length (HL): maximum distance between the tip of the snout to the posterior edge of the supraoccipital bone.
-
5.
Neck girth (NG): maximum circumference of the neck, measured between the base of the skull and front legs.
-
6.
Front-leg body girth (FBG): circumference of the pectoral region, measured immediately posterior to the front legs.
-
7.
Middle body girth (MBG): maximum circumference of the middle torso.
-
8.
Hind-leg body girth (HBG): circumference of the pelvic region, measured immediately anterior to the hind legs.
-
9.
Tail girth (TG): maximum circumference of the tail posterior to the hind legs.
-
10.
Horizontal–ventral scale girth (HVG): tail circumference where the two rows of horizontal tail scutes transition into a single vertical scute row.
-
11.
Body mass (BM).
Head length, TL and SVL are used as body length indicators in many crocodilian studies (Fukuda et al. 2013), whereas NG, FBG, MBG, HBG, TG and HVG are volumetric indicators of body condition (Zweig et al. 2004). The body measurements collected during this study are consistent with other crocodilian morphometric studies (Webb and Messel 1978; Montague 1984; Hutton 1987a; Hall and Portier 1994; Platt et al. 2009, 2011), and for comparative purposes with other species, we generally followed the statistical approach of Hutton (1987a) and Platt et al. (2009, 2011). Cranial morphometrics (HW, HL) were measured with tree calipers for adults (±1 mm) and digital calipers for hatchlings (±0.1 mm). All other body measurements were collected using a fiberglass measuring tape or plastic sewing tape (±1 mm). Non-hatchling crocodiles were weighed in the field with a digital crane scale (±0.1 kg) attached to an adjustable aluminum tripod. Individuals were first stabilized with towing bands and then hoisted with a block and tackle until they were suspended from the ground, allowing accurate mass to be recorded (Appendix of Fig. 6). The mass of the bands and d-ring attached to the scale were subtracted from the total mass. Hatchling crocodiles were weighed with an Ohaus digital scale (±0.1 g). Non-hatchling C. niloticus were sexed by manual probing of the cloacal cavity (Brazaitis 1968).
We used regression analysis to determine predictive relationships between morphometric data (HW, HL, NG, FBG, MBG, HBG, TG, HVG, BM) and body size (TL and SVL), with the former treated as independent variables. For comparison with other crocodilian species, we also regressed the ratio of HL/HW against SVL, and the ratio of HL/TL against TL to examine potential ontogenetic changes in cranial morphology and the consistency of the HL/TL ratio across size classes (Tucker et al. 1996; Platt et al. 2009, 2011).
Similar to other crocodilians, male C. niloticus attain greater body sizes than females (Cott 1961; Pooley 1982a; Hutton 1987b). To confirm this quantitatively, we used a t test to test the hypothesis that adult male SVL is significantly greater than adult female SVL (for free-ranging crocodiles in South Africa both sexes reach maturity at approximately 1400 mm SVL; Combrink 2014). We used a compressed sexual size dimorphism index (SDI) to quantify the degree of sexual size dimorphism between adult males and females (Lovich and Gibbons 1992; Platt et al. 2009, 2011). The SDI is a dimensionless number calculated by dividing the mean size of the larger sex by the mean size of the smaller sex and then adding one to this value if males are the larger sex, or subtracting one if females are larger (Lovich and Gibbons 1992). Although SDI can theoretically be based on any measurement or mass, we used SVL given its ubiquity in herpetological research and in keeping with previous crocodilian studies (Platt et al. 2009, 2011).
We estimated standing crop biomass (total mass of all individuals) for C. niloticus at Ndumo Game Reserve and Lake St Lucia by calculating the mean body mass for subadults and adults using the TL-BM regression equation, multiplied by the estimated number of individuals for each cohort for each population, and then summed to determine standing crop biomass (Thorbjarnarson 1988; Platt et al. 2009, 2011). The estimated number of individuals for each population was derived from spotlight and aerial survey data with correction factors (Calverley 2013; Combrink 2014). Biomass estimates for crocodilians are usually reported as kg/km of shoreline (Hutton 1987b; Thorbjarnarson 1988; Platt et al. 2009, 2011; Fukuda et al. 2011); we report biomass in both kg/km and kg/ha. Lake St Lucia is approximately 35,000 ha at mean lake level (Taylor 2006) with 600 km of shoreline observed during crocodile surveys (Combrink 2014). Shoreline length varies over time at the 10,117-ha Ndumo Game Reserve, as it is bordered by two meandering rivers and contains numerous ephemeral floodplain habitats. Taking into account the boundaries of the reserve and seasonal movement ecology of crocodiles at Ndumo Game Reserve (Calverley 2013), we established a shoreline length of 35 km for biomass estimates, although this number is likely an underestimate. Population size for the province of KwaZulu-Natal was aggregated from survey data in Champion (2011), Calverley (2013), Combrink et al. (2011), Combrink (2014) and unpublished reports of standardized aerial and spotlight counts.
Results
We captured 322 C. niloticus from Lake St Lucia and Ndumo Game Reserve for morphometric analyses (Fig. 1). Our sample included 118 hatchlings with a mean TL, SVL and BM (±SD) of 316 ± 25 mm (range 272–414 mm), 146 ± 12 mm (range 131–193 mm) and 73 ± 19 g (range 17–182 g), respectively. For 91 subadults captured, mean TL and SVL were 1958 ± 486 mm (range 753–2702 mm) and 1001 ± 258 (range 358–1392), respectively, and for 113 adults mean TL and SVL were 3172 ± 389 mm (range 2590–4136 mm) and 1710 ± 225 mm (range 1408–2460 mm), respectively. Mean BM for non-hatchlings was 90.3 ± 70.6 kg (range 7.0–341.0 kg), and hatchlings were excluded from body mass regressions and biomass calculations due to extensive variance (Platt et al. 2009, 2011). Strong positive allometric relationships were found between body length metrics (TL and SVL) and other morphometrics (r 2 ≥ 0.91); not all variables were recorded for each individual (Table 1; Figs. 2, 3). Morphometric regressions were linear, with the exception of the relationship of TL and SVL to BM, which was logarithmic (Table 1; Figs. 2, 3). For the relationships of HW/HL to SVL and HL/TL to TL we found considerable individual variation among all size classes, although there were slight negative linear trends for both relationships (Fig. 4). The mean HW/HL ratio was 1.9 ± 1.6 (range 1.39–2.50), and mean HL/TL was 0.14 ± 0.005 (range 0.12–0.16).
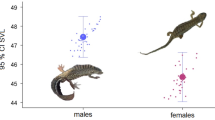
We determined the sex of 191 non-hatchling C. niloticus (96 female, 95 male). At Ndumo Game Reserve, we captured 35 subadult and 9 adult females, and 23 subadult and 20 adult males. The female-to-male observed sex ratio for all individuals at Ndumo Game Reserve was even (1:0.97), but the observed adult sex ratio was male biased (0.45:1). At Lake St Lucia, we captured 15 subadult and 37 adult females, and 5 subadult and 47 adult males, with an even sex ratio (1:1) for all individuals. The observed adult sex ratio was male biased (0.79:1). For both Ndumo Game Reserve and Lake St Lucia populations (which represent the majority of C. niloticus in KwaZulu-Natal), the observed sex ratio for all individuals was essentially even (1:0.99). Males were larger than females; the largest female SVL was 1782 mm, while 49 % of adult males captured were greater than 1800 mm. The mean SVL of adult males was significantly greater than that of adult females (t = −6.52, df = 111, p < 0.001; Fig. 5), and an SDI of 2.19 was calculated for the species.
Equation 18 from Table 1 was used to calculate standing crop biomass of C. niloticus at Ndumo Game Reserve (52,640.40 kg; Table 2) and Lake St Lucia (96,867.18 kg; Table 3). These values were then divided by the respective ha of habitat and km of shoreline for both populations and reported in relation to other studies (Table 4). We estimate the total C. niloticus non-hatchling metapopulation size in KwaZulu-Natal to be approximately 3650 individuals, with Lake St Lucia (1005 ± 137 individuals; Combrink 2014), Ndumo Game Reserve (846 ± 59 individuals; Calverley 2013) and Pongolapoort Dam (549 individuals; Summers 2015) accounting for most crocodiles in the province.
Discussion
We did not include an assessment of morphometric variance between Lake St Lucia and Ndumo Game Reserve in our study because although these two populations are allopatric, there is a documented history in Zululand of C. niloticus being transported from source populations and released into foreign ones, particularly between uMkhuze Game Reserve, Ndumo Game Reserve and Lake St Lucia (Pooley 1982a, b). Furthermore, “problem” crocodiles captured by local conservation authorities in peripheral waterbodies outside of protected areas are frequently released at Lake St Lucia, as well as presumed farmed and pet escapees captured as far away as the uMsunduzi River near Durban (Warner et al. pers. obs.). Discussion of the conservation merit of these practices is beyond the scope of this manuscript, but any minor morphological or especially genetic difference(s) found between current Zululand C. niloticus populations are almost certainly not a reflection of ecological or evolutionary processes.
Similar to other morphometric studies of C. porosus (Webb and Messel 1978), C. siamensis (Chentanez et al. 1983), C. novaeguineae (Montague 1984), C. niloticus (Hutton 1987a), C. moreletii (Platt et al. 2009) and C. acutus (Platt et al. 2011), we found strong relationships between measures of body length (TL and SVL) and other physical attributes, with greater variance in body form found among larger individuals (Figs. 2, 3). The considerable individual variation found among all size classes for the relationships of HW/HL, HW/HL to SVL and HL/TL to TL revealed a significant degree of morphological plasticity for C. niloticus in KwaZulu-Natal. While such variability is not unusual for crocodilian hatchlings (Milnes et al. 2001; Murray et al. 2013; Fig. 4), our study adds to a growing body of literature that suggests there is an inherent high degree of variation between and among C. niloticus populations that probably exceeds that of all other crocodilian species (Salem 2011; Pearcy and Wijtten 2011; Hekkala et al. 2011; Nestler 2012). For example, Nestler (2012) found that based on statistical analyses of skull characteristics alone, C. niloticus in the Congo River Basin alone showed as much morphological variation as C. porosus did across the entirety of its geographic range (the most extensive among crocodilians). It is therefore possible that C. niloticus is actually a cryptic species complex consisting of multiple taxa (Hekkala et al. 2010, 2011), and the recent elevation of C. suchus in West Africa to specific status supports this (Hekkala et al. 2011). Additional morphometric and molecular data for C. niloticus across the species’ range are needed to delineate potential taxa synonymized under C. niloticus and to properly address the region-specific conservation challenges these crocodiles face (Shirley et al. 2014). For southern Africa, the predictive models we present here (Table 1) will be helpful to future studies in assessing C. niloticus body length from skulls found in the field, detecting ontogenetic shifts in morphology, and estimating crocodile biomass at population and regional scales.
The neutral sex ratio found among non-hatchlings at both Ndumo Game Reserve and Lake St Lucia was unexpected. We hypothesized some degree of skewness among males and females simply because we could not capture and account for the sex of every individual in the population. Additionally, sex among C. niloticus embryos is determined by temperature (similar to other crocodilians), and the nesting substrate and aspects of the species’ nesting ecology differ substantially between the two populations (Calverley 2013; Combrink 2014). The observed even ratio we found for both populations is indicative that our sampling was probably truly random and reflective of the actual sex ratio, as observed deviations from a 1:1 pairing in reptile populations with temperature-sex determination is often due to sampling bias (Mrosovsky and Pieau 1991). However, intraspecific population biases do occur in crocodilians (Thorbjarnarson 1997) and for C. niloticus at the higher (colder) elevation of Lake Ngezi in Zimbabwe, Hutton (1987b) found a strongly female-biased sex ratio among all size classes and embryos.
Despite congruity in the overall sex ratio at Ndumo Game Reserve, the strong male bias we observed among adults is concerning with regard to long-term recruitment in the face of the ongoing C. niloticus population decline there (Calverley and Downs 2014a). For Lake St Lucia, Leslie and Spotila (2001) documented the shading effect of invasive triffid weed or paraffin bush (Chromolaena odorata) at C. niloticus nesting sites and warned that “unless immediate action is taken, a female-biased sex ratio in all nesting areas will result in eventual extirpation of the Nile crocodile from the Lake St. Lucia ecosystem.” 15 years on, the sex ratios found in our study do not support this claim, and in fact we found a male-biased adult population. However, this should not diminish the ongoing serious threat of C. odorata invasion to suitable nesting habitat at Lake St Lucia, which requires active management in the form of plant removal and other proactive strategies.
The significant sexual size dimorphism we found for C. niloticus (Fig. 5), with males obtaining larger body sizes, conforms to the findings of previous research on the species (Hutton 1987a, b) and the normal trend among crocodilians (Webb and Messel 1978; Platt et al. 2009, 2011). Numerous hypotheses have been posited for the crocodilian size differential among sexes, but the most likely explanation is that female growth slows at sexual maturity so that resources can be diverted into reproduction, whereas large body size in males confers a fitness advantage in mating competition and territory defense (Platt et al. 2011). Across species males are approximately 20 % larger than females (Platt et al. 2009), and the SDI of 2.19 we calculated for C. niloticus is comparable to C. moreletii (2.12; Platt et al. 2009) and C. acutus (2.10; Platt et al. 2011). From an ancillary study of stable isotope analyses, we found no dietary differences between sexes that could be responsible for differences in growth (Warner 2015).
For Zululand, the largest female captured in this study was 3.22 m TL, which probably is close to the maximum female size for the region. Although male C. niloticus reach lengths of 5 m in the Kruger National Park in South Africa (D. Pienaar, pers. comm.), a 4-m male is an exceedingly large crocodile for the province of KwaZulu-Natal. For our study at Ndumo Game Reserve and Lake St Lucia, there were only five individuals captured >4.0 m, none of which were >4.2 m. However, at the outset of our research in 2009 two males >4.2 m (not included in morphometric analyses) were captured; a 4.30-m male from Pongolapoort Dam and a 4.72-m male from Ndumo Game Reserve. The latter, “Beauty,” was a relatively famous crocodile in the region known for his enormous body size, severe facial scarring and reputation as a man-eater. Beauty was in noticeably poor body condition at the time of capture and died several months later at Lake Inyamithi in Ndumo Game Reserve, presumably of old age. From years of C. niloticus surveillance and monitoring data in Zululand, we find it unlikely that there are currently more than five crocodiles >4.3 m, and extremely unlikely that there are any individuals >5.0 m.
Our estimate of biomass for Lake St Lucia (161.45 kg/km) is comparable with other C. niloticus populations (Table 4), although it is important to note that given the current severe drought in the region most individuals are now restricted to southern portions of the lake exposed to freshwater inputs (e.g., the Nkazana stream mouth at Catalina Bay) and the 27-km-long Narrows channel. In light of the current adult bias at Lake St Lucia (Table 3), the survival of hatchlings to the subadult stage is a critical factor to the stability of the C. niltocus population over the next 20 years. For Ndumo Game Reserve, the biomass of 1504.01 kg/km we found is over four times the amount recorded for any other crocodilian population in the world (Table 4), but this is potentially due to a restocking program for the species in the late 1960s and early 1970s. Unless mitigative management actions are taken in the area, we predict the biomass at Ndumo Game Reserve to decline in the future primarily due to poaching and the destruction of nesting habitat (Calverley and Downs 2014a). The viable future of the estimated 3650 C. niloticus in KwaZulu-Natal will largely depend on proactive crocodile management practice and protection underpinned by continuing demographic and ecological studies at Lake St Lucia, Ndumo Game Reserve and Pongolapoort Dam.
References
Blake DK, Jacobsen NHG (1992) The conservation status of the Nile crocodile (Crocodylus niloticus) in South Africa. In: Smith GA, Marais J (eds) Conservation and utilisation of the Nile crocodile in South Africa—handbook on crocodile farming. The Crocodilian Study Group of Southern Africa, Pretoria
Botha PJ (2006) The ecology and population dynamics of the Nile crocodile Crocodylus niloticus in the Flag Boshielo Dam, Mpumalanga province, South Africa. Unpublised M.Sc. thesis, University of Pretoria
Brazaitis PJ (1968) The determination of sex in living crocodilians. Br J Herpetol 4:54–58
Brede EG, Thorpe RS, Arntzen JW, Langton TES (2000) A morphometric study of a hybrid newt population (Triturus cristatus/T. carnifex): Beam Brook Nurseries, Surrey, U.K. Biol J Linn Soc 70:685–695
Calverley P (2013) The conservation ecology of the Nile crocodile (Crocodylus niloticus) at Ndumo Game Reserve in northeastern KwaZulu-Natal and the Rio Maputo floodplain in southeastern Mozambique. Unpublished D. Phil. thesis, University of KwaZulu-Natal, Pietermaritzburg
Calverley PM, Downs CT (2014a) Population status of Nile crocodiles in Ndumo Game Reserve, KwaZulu-Natal, South Africa (1971–2012). Herpetologica 70:417–425
Calverley PM, Downs CT (2014b) Habitat use by Nile crocodiles in Ndumo Game Reserve, South Africa: a naturally patchy environment. Herpetologica 70:426–438
Champion G (2011) The ecology of Nile Crocodile (Crocodylus niloticus) in Pongolapoort Dam, northern KwaZulu-Natal, South Africa. Unpublished MSc thesis, University of KwaZulu-Natal, Pietermaritzburg
Chentanez T, Huggins SE, Chentanez V (1983) Allometric relationships of the Siamese crocodile (Crocodylus siamensis). J Sci Soc Thail 9:5–26
Combrink X (2014) Spatial and reproductive ecology and population status of the Nile Crocodile (Crocodylus niloticus) in the Lake St Lucia estuarine system, South Africa. Unpublished D. Phil. thesis, University of KwaZulu-Natal, Pietermaritzburg
Combrink X, Korrubel JL, Kyle R, Taylor R, Ross P (2011) Evidence of a declining Nile crocodile (Crocodylus niloticus) population at Lake Sibaya, South Africa. S Afr J Wildl Res 41:145–157
Combrink X, Warner JK, Hofmeyr M, Govender D, Ferreira SM (2012) Standard operating procedure for the monitoring, capture and sampling of Nile Crocodiles (Crocodylus niloticus). South African National Parks, Skukuza
Combrink X, Warner J, Taylor R, Downs CT (2013) Nile crocodiles. In: Perissinotto R, Stretch D, Taylor RH (eds) Ecology and conservation of estuarine ecosystems: lake St Lucia as a global model. Cambridge University Press, Barcelona, pp 333–353
Cott HB (1961) Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodylus niloticus) in Uganda and northern Rhodesia. Trans Zool Soc Lond 29:211–356
Davis MA, Douglas MR, Collyer ML, Douglas ME (2016) Deconstructing a species-complex: geometric morphometric and molecular analyses define species in the Western Rattlesnake (Crotalus viridis). PLoS One. doi:10.1371/journal.pone.0146166
Ferreira SM, Pienaar D (2011) Degradation of the crocodile population in the Olifants River Gorge of Kruger National Park, South Africa. Aquat Conserv 21:155–164
Fukuda Y, Webb G, Manolis C, Delaney R, Letnic M, Lindner G (2011) Recovery of saltwater crocodiles following unregulated hunting in tidal rivers of the Northern Territory, Australia. J Wildl Manag 75:1253–1266
Fukuda Y, Saalfeld K, Lindner G, Nichols T (2013) Estimation of total length from head length of saltwater crocodiles (Crocodylus porosus) in the Northern Territory, Australia. J Herpetol 47:34–40
Graham AD (1968) The Lake Rudolf crocodile (Crocodylus niloticus) population. Report to Kenya Game Department
Hall PM, Portier KM (1994) Cranial morphology of New Guinea crocodiles (Crocodylus novaeguineae): ontogenetic variation in relative growth of the skull and an assessment of its utility as a predictor of the sex and size of individuals. Herpetol Monogr 8:203–225
Hekkala ER, Amato G, DeSalle R, Blum MJ (2010) Molecular assessment of population differentiation and individual assignment potential of Nile crocodile (Crocodylus niloticus) populations. Conserv Genet 11:1435–1443
Hekkala E, Shirley MH, Amato G, Austin JG, Charter SL, Thorbjarnarson J, Vliet KA, Houck ML, Desalle R, Blum MJ (2011) An ancient icon reveals new mysteries: mummy DNA resurrects a cryptic species within the Nile crocodile. Mol Ecol 20:4199–4215
Hutton JM (1987a) Morphometrics and field estimation of the size of the Nile crocodile. Afr J Ecol 25:225–230
Hutton JM (1987b) Growth and feeding ecology of the Nile crocodile Crocodylus niloticus at Ngezi, Zimbabwe. J Anim Ecol 56:25–38
Hutton JM (1987c) Incubation temperatures, sex ratios and sex determination in a population of Nile crocodiles (Crocodylus niloticus). J Zool 211:143–155
Kleynhans CJ, Engelbrecht JS (1993) A summarised assessment of the conservation status of the Olifants River (Limpopo system) from the Loskop Dam to the Kruger National Park. Unpublished report, Transvaal Chief Directorate of Nature and Environmental Conservation, Pretoria
Lane EP, Huchzermeyer FW, Govender D, Bengis RG, Buss PE, Hofmeyr M, Myburgh JG, Steyl JC, Pienaar DJ, Kotze A (2013) Pansteatitis of unknown etiology associated with large-scale Nile Crocodile (Crocodylus niloticus) mortality in Kruger National Park, South Africa: pathologic findings. J Zoo Wildl Med 44:899–910
Leslie A (1997) The ecology and physiology of the Nile crocodile, Crocodylus niloticus, in Lake St Lucia KwaZulu Natal, South Africa. Ph.D. thesis, Drexel University, Philadelphia
Leslie AJ, Spotila JR (2001) Alien plant threatens Nile crocodile (Crocodylus niloticus) breeding in Lake St Lucia, South Africa. Biol Conserv 98:347–355
Lovich JE, Gibbons JW (1992) A review of techniques for quantifying sexual size dimorphism. Growth Dev Aging 56:269–281
Marais J (2014) Crocodylus niloticus (Laurenti, 1768). In: Bates MF, Branch WR, Bauer AM, Burger M, Marais J, Alexander GJ, de Villiers MS (eds) Atlas and red list of the reptiles of South Africa, Lesotho and Swaziland. South African National Biodiversity Institute, Pretoria
McMaster MK, Downs CT (2006) Population structure and density of leopard tortoises (Geochelone pardalis) on farmland in the Nama-Karoo. J Herpetol 40:495–502
Milnes MR, Woodward AR, Guillette LJ (2001) Morphological variation in hatchling American alligators (Alligator mississippiensis) from three Florida lakes. J Herpetol 35:264–271
Montague JJ (1984) Morphometric analysis of Crocodylus novaeguineae from the Fly River drainage, Papua New Guinea. Aust Wildl Res 11:395–414
Mrosovsky N, Pieau C (1991) Transitional range of temperature, pivotal temperatures and thermosensitive stages for sex determination in reptiles. Amphib Reptil 12:169–179
Murray CM, Easter M, Merchant M, Cooper A, Crother BI (2013) Can reproductive allometry assess population marginality in crocodilians? a comparative analysis of Gulf Coast American alligator (Alligator mississippiensis) populations. Copeia 2013:268–276
Nestler JH (2012) A geometric morphometric analysis of Crocodylus niloticus: evidence for a cryptic species complex. M.Sc. thesis, University of Iowa, Iowa
Ousterhout BH, Anderson TL, Drake DL, Peterman WE, Semlitsch RD (2015) Habitat traits and species interactions differentially affect abundance and body size in pond-breeding amphibians. J Anim Ecol 84:1–11
Parker ISC, Watson RM (1970) Crocodile distribution and status in the major waters of western and central Uganda. E Afr Wildl J 8:85–103
Pearcy A, Wijtten Z (2011) A morphometric analysis of crocodilian skull shapes. Herpetol J 21:213–218
Platt SG, Rainwater TR, Thorbjanarson JB, Finger AG, Anderson TA, McMurry ST (2009) Size estimation, morphometrics, sex ratio, sexual size dimorphism, and biomass of Morelet’s crocodile in northern Belize. Carribean J Sci 45:1–14
Platt SG, Rainwater TR, Thorbjanarson JB, Finger AG, Anderson TA, McMurry ST (2011) Size estimation, morphometrics, sex ratio, sexual size dimorphism, and biomass of Crocodylus acutus in the coastal zone of Belize. Salamandra 47:179–192
Pooley AC (1982a) The ecology of the Nile crocodile Crocodylus niloticus in Zululand. M.Sc. thesis, University of Natal, Pietermaritzburg
Pooley AC (1982b) Discoveries of a crocodile man. Collins, London
Salem A (2011) Morphometric measurement and field estimation of the size of Crocodylus niloticus in Lake Nasser (Egypt). Transylv Rev Syst Ecol Res 12:53–74
Sanders KL, Schroeder T, Guinea ML, Rasmussen AR (2015) Molecules and morphology reveal overlooked populations of two presumed extinct Australian sea snakes (Aipysurus: Hydrophiinae). PLoS One 10:e0115679
Shine R (1986) Sexual differences in morphology and niche utilization in an aquatic snake, Acrochordus arafurae. Oecologia 69:260–267
Shirley MH, Vliet KA, Carr AN, Austin JD (2014) Rigorous approaches to species delimitation have significant implications for African crocodilian systematics and conservation. Proc R Soc B 281:20132483
Stretch D, Chrystal CP, Chrystal RA, Maine CM, Pringle JJ (2013) Estuary and lake hydrodynamics. In: Perissinotto R, Stretch DD, Taylor RH (eds) Ecology and conservation of estuarine ecosystems: lake St Lucia as a global model. Cambridge University Press, Barcelona, pp 112–149
Summers MK (2015) Aspects of Nile crocodile (Crocodylus niloticus) population ecology and thermal biology in Pongolapoort Dam, KwaZulu-Natal. M.Sc. thesis, University of KwaZulu-Natal, Pietermaritzburg
Taylor RH (2006) Ecological responses to changes in the physical environment of the St Lucia estuary. Ph.D. thesis, Norwegian University of Life Sciences, As, Norway
Thorbjarnarson JB (1988) The status and ecology of the American crocodile in Haiti. Bull Fla State Mus 33:1–86
Thorbjarnarson JB (1997) Are crocodilian sex ratios female biased? The data are equivocal. Copeia 2:451–455
Tucker AD, Limpus CJ, McDonald KR, McCallum HI (1996) Ontogenetic dietary partitioning by Crocodylus johnstoni during the dry season. Copeia 4:978–988
Warner JK (2015) Morphometrics, ecotoxicology and stable isotope ecology of Nile crocodiles (Crocodylus niloticus) in KwaZulu-Natal, South Africa. Ph.D. thesis, University of KwaZulu-Natal, Pietermaritzburg
Warner JK, Combrink X, Myburgh JG, Downs CT (2016) Blood lead concentrations in 358 free-ranging Nile crocodiles (Crocodylus niloticus) from South Africa. Ecotoxicology 25(5):950–958
Webb GJW, Messel H (1978) Morphometric analysis of Crocodylus porosus from the north coast of Arnhem Land, northern Australia. Aust J Zool 26:1–27
Webb GJW, Smith AMA (1987) Life history parameters, population dynamics, and the management of crocodilians. In: Manolis SC, Whitehead PJ, Webb GJW (eds) Wildlife management: crocodiles and alligators. Surrey Beatty & Sons, Ltd, Sydney
Wikelski M, Carrillo V, Trillmich F (1997) Energy limits to body size in a grazing reptile, the Galapagos marine iguana. Ecology 78:2204–2217
Zamudio K (1998) The evolution of female-biased sexual size dimorphism: a population-level comparative study in horned lizards (Phrynosoma). Evolution 52:1821–1833
Zweig CL, Mazzotti FJ, Rice KG, Brandt LA, Abercrombie CL (2004) Evaluation of field measurements of the American alligator for use in morphometric studies. Herpetol Rev 35:43–44
Acknowledgments
We thank R. Taylor, J. Myburgh and S. Kyle for advisory support. The success of this project was reliant on a great number of volunteers that assisted with fieldwork, but we would like to thank F. Myburgh and M. Robertson in particular for their logistical support and provision of boats. The Ford Wildlife Foundation and the South African Water Research Commission provided vehicle and financial support. We thank for Ezemvelo KwaZulu-Natal Wildlife and the iSimangaliso Wetland Park Authority for hosting this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Appendix
Appendix
See Fig. 6.
Rights and permissions
About this article
Cite this article
Warner, J.K., Combrink, X., Calverley, P. et al. Morphometrics, sex ratio, sexual size dimorphism, biomass, and population size of the Nile crocodile (Crocodylus niloticus) at its southern range limit in KwaZulu-Natal, South Africa. Zoomorphology 135, 511–521 (2016). https://doi.org/10.1007/s00435-016-0325-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0325-8