Abstract
We describe here for the first time the body size and age of the Common Toad (Bufo bufo) from high (Trabzon, 1090 m and Kastamonu, 925 m above sea level) and low (Yalova, 65 m above sea level) altitudes in Turkey using skeletochronology. The specimens from Trabzon were significantly smaller and younger than the ones from the Kastamonu and Yalova populations. Age at sexual maturity was three years for females while it varied between two to three years in males in all three populations. We found the females to be significantly larger than males in all three populations. The sexual dimorphism indices (SDI) were biased toward the female populations. Even after accounting for the influence of age, the snout-vent length (SVL) differed significantly among the populations. Age and SVL were closely correlated in the male populations for all the three localities (Yalova, Kastamonu, and Trabzon) and for female individuals in the Trabzon population. In this first report, we reveal the demographic structure of B. bufo from the Anatolia region in Turkey and provide comparative data regarding this species for further discussion and determined that age and body size of Common Toad not differentiated among different altitudes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The size of animals generally follows the tenets of Bergmann’s rule states that the variation in body size is dependent on the environmental temperature (animals to be larger in colder environments) [1]. However, the short growing season at higher altitudes and latitudes may limit the time available for growth, resulting in converse Bergmann size clines [2, 3]. In addition, environmental conditions, especially climate change, are inducing body size changes in animals [4] and there is no generally accepted temperature-size relationship in amphibians [5].
The age of amphibians is crucial for determining various demographic parameters such as longevity, age at sexual maturity of populations, and other ecological factors [6]. Skeletochronology is a reliable and relatively harmless method for estimating age based on annual rings of periods of active growth in bones (LAGs). These rings form due to the variations in growth rates during warm and cold seasons and show the seasonal changes in growth rate [7].
The Bufonidae family is comprised of 52 genera, distributed globally [8]. The Common Toad (Bufo bufo L., 1758) is the representative member of this family. Several reports on the life-history traits like ecology [9], toxicology [10], genetic structure [11], even age structure [12–14] of this taxon have been performed in Europe, however, demographics of the populations of Bufo bufo in Anatolia remains a mystery.
In the present study, we present the first reported data on the age structure of Bufo bufo using skeletochronology for three populations from different altitudes in Anatolia. We also tested differences in the body size and age structure variation with the altitude and temperature at the different sites based on the estimates of age at sexual maturity, SVL and SDI.
MATERIAL AND METHODS
A total of 62 adult common toads (13 ♀♀, 49 ♂♂) from three different localities ranging from 65 to 1090 m above sea level in Turkey (Yalova; 65 m a.s.l., Kastamonu; 925 m a.s.l., Trabzon; 1090 m a.s.l.) (Fig. 1) were collected by hand from regional breeding ponds varying in March to May (depending on the mating season). The animals were released back into their respective habitats following sex determination, measurement of snout-vent length (SVL), and toe-clipping (the 4th toe of the hind limb was taken for each toad). SVL was measured to the nearest 0.1 mm for each specimen by using digital calipers, and sex was determined according to the presence of the nuptial pad and body size. Clipped toes were preserved in 95% ethanol at room temperature. The geographic and climatic conditions of the three localities are presented in Table 1.
The established procedure of skeletochronology as described by Castanet and Smirina [15] was followed. The tissue samples were decalcified in 5% nitric acid for approximately 1.5 h and cross-sections of 17–18 µm were taken with a freezing microtome (Thermo). After staining with Ehrlich’s hematoxylin, cross-sections were imaged using an Olympus BX51 microscope. For counting of the growth rings, two independent researchers (T. Ergül Kalaycı and N. Özdemir) determined the ring count in selected sections in which the size of the medullary cavity was at its minimum and that of the periosteal bone was at its maximum.
Sexual dimorphism index (SDI) was calculated according to the formula introduced by Lovich and Gibbons [16<]: (mean length of the larger sex/mean length of the smaller sex) ±1, (SDI>0 when females are larger than males, SDI<0 when males are larger than females).
The distribution of age and body size in each population was determined using the Kolmogorov-Smirnov test. After determining normality of the distribution, intra-population variations of body size and age were analyzed using Student’s-t Test. Variation of body size and age between populations was determined by the ANOVA test. We also used analysis of covariance (ANCOVA) to test whether body size was still significantly different in the absence of the age covariate.
Correlation between body size and age was calculated by Pearson’s correlation coefficient. All statistical analyses were conducted using SPSS software version 21.0.
RESULTS
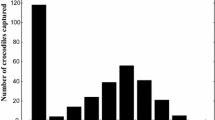
We determined the age of 62 adult individuals on the basis of the numbers of Lines of the Arrested Growth (LAG) (Fig. 2). Some of our LAGs look paler than the others. It has been generally hypothesized that the growth marks visible in the bones are the result of a genetically controlled growth rhythm which is synchronized and reinforced by particular environmental conditions, such as seasonality [17]. The variation among LAGs might be the consequence of differentiation of the temperature (or other environmental condition) in the hibernation period. Descriptive statistic of study populations and comparisons with literature data were given Table 2. The maximum age was 7 years for the Teşvikiye (Yalova) and Uzungöl (Trabzon) populations and 6 years for the Taşlık (Kastamonu) population. On average, the age of males was 5.20 ± (S.D.) 0.77, 4.47 ± 1.06, and 4.26 ± 0.87 years for Teşvikiye (Yalova), Taşlık (Kastamonu), and Uzungöl (Trabzon), respectively. For females, it was found to be 5.67 ± 1.15, 5.20 ± 0.45, and 5.00 ± 2.34 years for Teşvikiye (Yalova), Taşlık (Kastamonu), and Uzungöl (Trabzon), respectively. The age was differentiated at the inter-population level (F2,61= 3.42, P < 0.05). Further, the age of males demonstrated significant variations at the three sites (F2,48= 4.75, P < 0.05) and male individuals of the Teşvikiye (Yalova) population were significantly older than the males of the Uzungöl (Trabzon) population (as confirmed by a Bonferroni post-hoc test, P < 0.05). In the females, no such significant difference was found (Bonferroni post-hoc test, P > 0.05). The low sample size of females could give rise to this discrepancy. We also did not find any differences in ages between the sexes at the intra-population level (Student’s-t test; Yalova: t = 0.89, df = 16; Kastamonu: t = 1.48, df = 18; Trabzon: t = 0.69, df = 4.30; P > 0.05). The age frequency distributions of the individual specimens are given in Fig. 3.
A significant decrease in the growth at the age of maturity [18] was observed, which varied between two and three years for all three populations.
The mean body size (SVL) of males was 83.03 ± 4.42, 79.21 ± 7.13, and 69.20 ± 4.45 mm for the Teşvikiye (Yalova), Taşlık (Kastamonu), and Uzungöl (Trabzon), respectively. The mean SVLs for females was determined to be 115.98 ± 7.07, 110.32 ± 3.59, and 80.53 ± 20.41 mm in the Teşvikiye (Yalova), Taşlık (Kastamonu), and Uzungöl (Trabzon), respectively. SVL significantly varied between the sexes in all populations as analyzed by Student’s t-Test (Teşvikiye, Yalova: t= 10.78, df = 16, P < 0.05; Taşlık, Kastamonu: t = 9.25, df = 18, P < 0.05; Uzungöl, Trabzon: t = 2.31, df = 22, P < 0.05). The females of the species were significantly larger than males in all populations. Among the three sites, SVL differed significantly (One-way ANOVA F2,61 = 11.44, P < 0.05), as did the average SVLs between the males and females in the three populations (male: F2,48 = 30.17; female: F2,12 = 8.88, P < 0.05). The individuals collected at higher altitudes (Uzungöl, Trabzon population; 1090 m) were significantly smaller than those found at lower altitudes (Bonferroni post-hoc test, P < 0.05). Upon removing the factor of the age parameter, the mean SVLs still varied significantly among the populations (ANCOVA: F2,62 = 11.01, P < 0.05).
The SDI values generally showed an increasing trend from 0.16 at the highest altitude site (east: Uzungöl, Trabzon; 1090 m a.s.l.) to 0.40 at the lowest altitude site (west: Teşvikiye, Yalova; 65 m a.s.l.), most likely a result of the decrease in both male and female size with the increase in altitude. The reported SDI values indicate that the sexual size dimorphism (SSD) is more well defined in the west than the east.
The age and SVL were significantly correlated in the males for all three locations (Teşvikiye; Yalova, Taşlık; Kastamonu, and Uzungöl; Trabzon) and females for Uzungöl, Trabzon (Pearson’s correlation coefficient, Yalova: r = 0.69, Kastamonu: r = 0.92, Trabzon: r = 0.63, P < 0.05 for males; and r = 0.99, P < 0.01 for females).
DISCUSSION
The present study is the first report that identifies the geographical variations involved in two key life-history parameters of B. bufo, collected from three different altitudes in Turkey.
The mean age of toads at the high altitude and northern population (Uzungöl, Trabzon; 1090 m a.s.l.) was significantly less than those collected at the lower altitude and southern region (Teşvikiye, Yalova; 65 m a.s.l.). Cvetković et al. [13] noted the highest mean age for the southern populations of B. bufo in the different parts of Europe, indicating that the growth potentials of animals may be limited by harsher conditions at higher altitudes [19]. Although altitude exerts a strong influence on the age structure in B. bufo [13, 20], it is difficult to define just one specific cause for such variations in the life-history traits. Urbanization is another major influence on such life-history traits. While two of the study sites (Teşvikiye; Yalova and Taşlık; Kastamonu) are mostly rural, the rapid urbanization of Uzungöl (Trabzon) is a problem, which might have caused loss of resident species. Very limited information is available on the adaptations that the surviving species may inculcate to thrive in urban environments [21]. We found seven years as the maximum age recorded for the females and 6 years for the males. The similar maximum age structure has been reported in a few European Common Toad populations (Netherland and Germany populations) [13], although the majority of the European populations seem to have a life-span of more than seven years [12–14]. Each population has its own characteristic response to the changes in environmental conditions such as the duration of activity period, food availability and quality and climatic conditions. The variations among other reported populations and ours may be a result of such discrepancy.
We reported age at sexual maturity to be three years for females and between two and three years for males, although it did not vary among the three populations. Hemelaar [12] has previously reported that the males of B. bufo are able to reach sexual maturity at least one year earlier than the females. The average reported the minimum age of maturation in the Common Toad B. bufo from six different European populations varied from 2.1 to 5.9 years and was not found to be significantly different among the populations [13]. The differences in life-history traits between the populations, such as the age at maturity, might be dependent on intrinsic (genetic) characteristics of the species and/or can be the result of one or more environmental factors [22].
Contrary to our results, previous study have shown that B. bufo specimens from higher altitudes are actually larger than those found in the lowland populations [12]. We found significant differences between the lowland (Yalova) and highland populations (Trabzon). The mean body length of the toads in the Trabzon population was smaller than those of the Yalova population. Cvetković et al. [13] reported no variation among the populations from 15 m to 1850 m a.s.l. in terms of body length in different B. bufo populations in Europe. There were no significant differences among the B. bufo individuals, in terms of body length, found in geographically closed populations in England. There is more than one reason for the variation in the adult body size in anurans, such as age, food availability, and temperature, all depend on geographical location [23]. Oromi et al. [24] proposed that at higher elevations, short annual activity periods directly correlate to the the reduced size of animals and it is a result of the reduced feeding period between the metamorphosis and first hibernation in natterjack toads (B. calamita). In addition, in anurans, body condition is negatively correlated with the amount of snowfall [25]. Uzungöl location is characterized by snowy and cold winter period [26] Also, there is an opinion which confirmed in Fowler’s toads (Anaxyrus fowleri), animals are getting bigger while the temperature increases [4].
In anurans, the majority (~90%) shows female-biased sexual size dimorphism [27, 28]. In our study too, significant size differences were noted in all sexes and in all populations, and females were larger than males in all of them. This is a well-known fact that with respect to sexual dimorphism in body size of toads, females are significantly larger than males [28]. A possible reason for this is that females, who are viviparous, require more energy than males for the development of the ovaries and eggs [30].
Age and body size are not always correlated in amphibians. Sometimes they show a significant correlation in both sexes [31, 32], or it was showed in one sex, usually in all male populations [33]. This observation is also in line with the fact that the females tend to choose larger males because they are older and fitter, as proven by their survival to reach a considerable body size [34]. In our study, however, only in Uzungöl (Trabzon) population, the females were significantly correlated in terms of age and body size. Höglund and Säterberg [35] also found a positive correlation between the age and body length only in females of a B. bufo population.
CONCLUSION
In this study, we provide the first report that B. bufo species from different altitudes show different demographic structures. Comparative studies of populations living in climatologically different regions are necessary to determine the effects of differentiation of regional temperature on population demography. Further investigations are required to define the underlying effects of terrestrial microclimate and activity period temperature. Obviously, animals’ life circle is impressed by more than one ecological parameters.
REFERENCES
Bergmann, C., Ueber die Verhältnisseder Wärmeökonomie der thiere zu ihrer grösse, Göttinger Stud., 1847, vol. 3, no. 1, pp. 595–708.
Mousseau, T.A., Ectotherms follow the converse Bergmann’s rule, Evolution, 1997, vol. 51, no. 2 pp. 630–632.
Blanckenhorn, W.U. and Demont, M., Bergmann and converse Bergmann latitudinal clines in arthropods: Two ends of a continuum?, Integr. Comp. Biol., 2004, vol. 44, no. 6, pp. 413–424.
Green, D. M. and Middleton, J., Body size varies with abundance, not climate, in an amphibian population, Ecography, 2013, vol. 36, pp. 947–955.
Tryjanowski, P., Sparks, T., Rybacki, M., and Berger, L., Is body size of the water frog Rana esculenta complex responding to climate change? Naturwissenschaften, 2006, vol. 93, no. 3, pp. 110–113.
Duellman, W.E. and Trueb, L., Biology of Amphibians, NewYork: McGraw-Hill, 1994.
Bionda, C.D.L., Kost, S., Salas, N.E., Lajmanovich, R.C., Sinsch, U., and Martino, A.L., Age structure, growth and longevity in the common toad, Rhinella arenarum, from Argentina, Acta Herpetol., 2015, vol. 10, no. 1, pp. 55–62.
The IUCN Red List of Threatened Species. Version 2017-2. www.iucnredlist.org. Cited November 27, 2017.
Reading, C.J., The effect of winter temperatures on the timing of breeding activity in the common toad Bufo bufo, Oecologia, 1998, vol. 117 no. 4, pp. 469–475.
Brunelli, E., Bernabò, I., Berg, C., Lundstedt-Enkel, K., Bonacci, A., and Tripepi, S., Environmentally relevant concentrations of endosulfan impair development, metamorphosis and behaviour in Bufo bufo tadpoles, Aquat. Toxicol., 2009, vol. 91 no. 2, pp. 135–142.
Seppä, P. and Laurila, A., Genetic structure of island populations of the anurans Rana temporaria and Bufo bufo, Heredity, 1999, vol. 82, no. 3, pp. 309–317.
Hemelaar, A.M.S., Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes, J. Herpetol., 1988, vol. 22, pp. 369–388.
Cvetković, D., Tomašević, N., Ficetola, G.F., Crnobrnja-Isailović, J., and Miaud, C., Bergmann’s rule in amphibians: Combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufo, J. Zool. Syst. Evol. Res., 2009, vol. 47, no. 2, pp.171–180.
Tomasevic, N., Cvetkovic, D., Miaud, C., Aleksic, I., and Crnobrnja-Isailovic, J., Interannual variation in life history traits between neighbouring populations of the widespread amphibian Bufo bufo, Revue D'écologie, 2008, vol. 63, pp. 371–381.
Castanet, J., and Smirina, E., Introduction to the skeletochronological method in amphibians and reptiles, Ann. Sci. Nat. Zool. Biol. Anim., 1990, vol. 11, no. 4, pp. 191–196.
Lovich, J.E., and Gibbons, J.W., A review of techniques for quantifying sexual size dimorphism, Growth Dev. Aging, 1992, vol. 56, pp. 269–281.
Castanet, J., Francillon-Vieillot, H., Meunier, F.J., and de Ricqlés, A., in Bone and Individual Aging, Hall, B.K., Ed., Boca Raton: CRC Press, 1993, pp. 245–283.
Ryser, J., Determination of growth and maturation in the common frog, Rana temporaria, by skeletochronology, J. Zool., 1988, vol. 216, no. 4, pp. 673–685.
Dmitriew, C.M., The evolution of growth trajectories: What limits growth rate? Biol. Rev., 2011, vol. 86, no. 1, pp. 97–116.
Grossenbacher, K., First results of a 20-year study on common toad Bufo bufo in the Swiss Alps, Biota, 2002, vol.3, pp. 43–48.
Jennette, M.A., and Snodgrass, J.W., Effects of urbanization on life history traits in two anurans, 95th ESA Annual Meeting, Pittsburgh, PA, 2010, PS 78-107.
Guarino, F.M., Lunardi, S., Carlomagno, M., and Mazzotti, S., A skeletochronological study of growth, longevity, and age at sexual maturity in a population of Rana latastei (Amphibia, Anura), J. Biosci., 2003, vol. 28, no. 6, pp. 775–782.
Gramapurohit, N.P., Shanbhag, B.A., and Saidapur, S.K., Growth, sexual maturation and body size dimorphism in the Indian bullfrog, Hoplobatrachus Tigerinus (Daud), Herpetologica, 2004,vol. 60, no. 4, pp. 414–419.
Oromi, N., Sanuy, D., and Sinsch, U., Altitudinal variation of demographic life-history traits does not mimic latitudinal variation in natterjack toads (Bufo calamita), Zoology, 2012, vol. 115, no. 1, pp. 30–37.
Pope, K.L. and Matthews, K.R., Influence of anuran prey on the condition and distribution of Rana muscosa in the Sierra Nevada, Herpetologica, 2002, vol. 58, no. 3, pp. 354–363.
Verep, B., Şahin, C., Çiloğlu, E. and İmamoğlu, H.O., Uzungöl'ün İklimi ve Çevresel Sorunları Üzerine Bir Çalışma, Atatürk Üniversitesi Ziraat Fakültesi Dergisi, 2002, vol. 33, no. 4, pp. 353–358.
Demirel, G.D., Development and conservation of cultural properties in rural areas of eastern Black Sea region: A case study in Karacakaya village. M.S. Thesis in Restoration, Middle East Technical University, Ankara, Turkey, 2010.
Kupfer, A., In Sex, Size and Gender Roles: Evolutionary Studies of Sexual Size Dimorphism, Fairbairn, D., Blanckenhorn, W., and Szekely, T., Eds., Oxford: Oxford Univ. Press, 2007, pp. 50–59.
Cvetković, D.D., Tomašević, N., Aleksić, I.D., and Crnobrnja-Isailović, J.M., Assessment of age and intersexual size differences in Bufo bufo, Arch. Biol. Sci., 2005, vol. 57, no. 2, pp.157–162.
Kutrup, B., Cakir, E., Colak, Z., Bulbul, U., and Karaoglu, H., Age and growth of the green toad, Bufo viridis (Laurenti, 1768) from an island and a mainland population in Giresun, Turkey, J. Anim. Vet. Adv., 2011, vol. 10, no. 11, pp. 1469–1472.
Miaud, C., Guyétant, R., and Elmberg, J., Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): A literature review and new data from the French Alps, J. Zool., 1999, vol. 249, no. 1, pp. 61–73.
Gül, S., Olgun, K., and Kutrup, B., Body size and age structure of Pelophylax ridibundus populations from two different altitudes in Turkey, 2011, Amphibia-Reptilia, vol. 32, no. 2, pp. 287–292.
Cherry, M.I. and Francillon-Vieillot, H., Body size, age and reproduction in the leopard toad, Bufo pardalis, J. Zool.,1992, vol. 228, no. 1, pp. 41–50.
Trivers, R.L., Parental Investment And Sexual Selection, Aldine, Chicago: B. Campbell, 1972, pp. 136–179.
Höglund, J., and Säterberg, L., Sexual selection in common toads: Correlates with age and body size, J. Evol. Biol., 1989, vol. 2, no. 5, pp. 367–372.
Frétey, T. and Le Garff, B., Skeletochronological study in Bufo bufo in Brittany, CR. Acad. Sci. III, 1996, vol. 319, pp. 295–299.
Kuhn, J, Lebensgeschichte und Demographie von Erdkrotenweibchen Bufo bufo bufo (L.), Z. Feldherpet., 1994, vol 1., pp. 3–87.
Schabetsberger, R., Langer, H., Jersabek, C.D., and Goldschmid, A., On age structure and longevity in two populations of Bufo bufo (Linnaeus 1758) at high altitude breeding sites in Austria, Herpetozoa, 2000, vol. 13, pp. 187–191.
Gittins, S.P., Parker, A.G., and Slater, F.M., Population characteristics of the common toad (Bufo bufo) visiting a breeding site in Mid-Wales, J. Anim. Ecol., 1980, vol. 49, pp. 161–173.
Gittins, S.P., Steeds, J.E., and Williams, R., Population age structure of the common toad (Bufo bufo) at a lake in Mid-Wales determined from annual growth rings in the phalanges, Br. J. Herpetol.,1982, vol. 6, pp. 249–252.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. We collected the animals with the approval of the local ethics committee (Recep Tayyip Erdoğan University, decision number: 2017/27).
Rights and permissions
About this article
Cite this article
Tuğba Ergül Kalayci, Gül, S., Dursun, C. et al. Age Structure and Body Size Variation in Common Toad (Bufo bufo, Linnaeus 1758) from Three Different Altitudes in Turkey. Russ J Ecol 50, 397–403 (2019). https://doi.org/10.1134/S106741361904009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106741361904009X