Abstract
Purpose
Stem cells are characterized by the capability of self-renewal and multi-differentiation. Normal stem cells, which are important for tissue repair and tissue regeneration, can be divided into embryonic stem cells (ESCs) and somatic stem cells (SSCs) depending on their origin. As a subpopulation of cells within cancer, cancer stem cells (CSCs) are at the root of therapeutic resistance. Tumor-initiating cells (TICs) are necessary for tumor initiation. Caveolin1 (Cav1), a membrane protein located at the caveolae, participates in cell lipid transport, cell migration, cell proliferation, and cell signal transduction. The purpose of this review was to explore the relationship between Cav1 and stem cells.
Results
In ESCs, Cav1 is beneficial for self-renewal, proliferation, and migration. In SSCs, Cav1 exhibits positive or/and negative effects on stem cell self-renewal, differentiation, proliferation, migration, and angiogenic capacity. Cav1 deficiency impairs normal stem cell-based tissue repair. In CSCs, Cav1 inhibits or/and promotes CSC self-renewal, differentiation, invasion, migration, tumorigenicity ability, and CSC formation. And suppressing Cav1 promotes chemo-sensitivity in CSCs and TICs.
Conclusion
Cav1 shows dual roles in stem cell biology. Targeting the Cav1-stem cell axis would be a new way for tissue repair and cancer drug resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells are characterized by the capacities to self-renew and produce multiple differentiated progenies (Rigaud et al. 2020). There are two main types of normal stem cells based on their origin, embryonic stem cells (ESCs), which can be isolated from the inner cell mass of early mammalian blastocysts (Harvey et al. 2019), and somatic stem cells (SSCs), which can be isolated from developed tissues and organs (Ly et al. 2020). Recognized as pluripotent stem cells, ESCs cannot create extra-embryonic tissues. Nevertheless, ESCs can produce somatic cell types, like hepatocytes, endothelial cells, hematopoietic cells, etc. (Semba et al. 2020). SSCs include hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), neural stem cells (NSCs), adipose-derived stem cells (ADSCs), epidermal stem cells (EpiSCs), mammary stem cells (MaSCs), and intestinal stem cells (ISCs) (Wang et al. 2020a; Tweedell 2017). Besides, progenitor cells are also considered to be SSCs (Rebuzzini et al. 2015). SSCs are known as multipotent stem cells and possess more limited differentiation potential than ESCs. SSCs can create several terminally differentiated cells that maintain tissue homeostasis (Semba et al. 2020). Recently, scientists can reprogram somatic cells to create pluripotent stem cells in a state similar to ESCs. These cells are called induced pluripotent stem cells (iPSCs) and can be used for regenerative medicine, disease modeling, and drug testing (Takahashi and Yamanaka 2006; Soman and Vijayavenkataraman 2020).
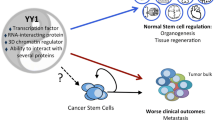
Nowadays, despite advances in cancer diagnosis and treatment, cancer is still considered to be the second leading cause of death in the world (Ahmed et al. 2020). Cancer stem cells (CSCs), a rare subset of cancer cells within the tumor bulk, were first launched in the researches about primary human acute myeloid leukemia (Lapidot et al. 1994). Accumulative studies demonstrate that CSCs can come from normal stem cells, progenitor cells, differentiated tissue cells, or tumor cells (Chiodi and Mondello 2020). It is determined that, based on the surface markers CD44, CD24, CD133, etc., CSCs can be collected from several malignant cancers (e.g., breast, lung, liver, prostate, pancreatic, colorectal, gastric, etc.) through the techniques of flow cytometry and Fluorescence-Activated Cell Sorting (FACS) (Thankamony et al. 2020). CSCs can form three-dimensional spheres that are also called tumorspheres. The tumorsphere formation assay can be used for CSC isolation and identification in vitro (Zhu et al. 2017). In addition to the stemness properties (i.e., self-renewal and differentiation), CSCs also have stemness-related signal pathways similar to normal stem cells. In normal stem cells, the signal pathways maintaining stemness are strictly controlled without the mutation. While in CSCs, due to genetic and epigenetic mutations, the signals are usually dysfunctional (Nimmakayala et al. 2019). CSCs are involved in tumor initiation, metastasis, relapse, and therapeutic resistance (Clara et al. 2020). At present, the significance of CSC-based anti-cancer treatment cannot be underestimated.
Caveolae, which are defined as flask-shaped membrane invaginations located at the plasma membrane, are rich in cholesterol and sphingolipids (Skotland et al. 2020). Caveolin 1 (Cav1), a 21 kDa membrane protein composed of 178 amino acids, is one of the basic components in caveolae and is pivotal for the formation of caveolae. The encoding gene CAV1 is located on human chromosome 7q31.1 (Yan et al. 2020). Cav1 highly exists in adipocytes, fibroblasts, endothelial cells, muscle cells, and epithelial cells (Williams and Lisanti 2004). Cav1 has the N-terminal domain, the caveolin scaffolding domain (CSD), the intramembrane domain (IMD), and the C-terminal spanning domain (Root et al. 2015). Many studies emphasize that the CSD region can modulate the interaction between Cav1 and other signaling proteins, such as adenylyl cyclase, phosphatidylinositol-3-kinase (PI3K), endothelial nitric oxide synthase (eNOS), mitogen-activated protein kinase (MAPK), protein kinase A and C (Patel et al. 2008). It is proved that Cav1 can regulate stem cell biology, tissue repair, and tissue regeneration (Baker and Tuan 2013). Besides, Cav1 also displays both suppressive and oncogenic effects on cancer aggressive behaviors according to tumor type and environment (Singh and Lamaze 2020). And it is suggested that Cav1 correlates with tumor cell proliferation, migration, invasion, metastasis, stemness, and drug resistance (Qian et al. 2019).
In this review, we collected the evidence to discuss the interaction between Cav1 and stem cells, altogether describing the roles of Cav1 in stem cell self-renewal, differentiation, proliferation, and migration. We also found that Cav1 has a role in modulating CSC formation and chemoresistance.
Caveolae and Cav1
Described as small (50–80 nm) invaginations, caveolae play vital roles in endocytosis, cell signal transduction, membrane trafficking, and dynamics (Filippini and D’Alessio 2020). The Cavs and cavins are the main components of the caveolae. Cavs are composed of three isoforms: Cav1, Cav2, and Cav3. Cav1 and Cav2 are expressed in non-muscle cells. Cav3 mainly exists in muscle cells (Chidlow and Sessa 2010). And the cavin family includes cavin-1 (polymerase I and transcript release factor, PTRF), cavin-2 (serum deprivation response protein, SDPR), cavin-3 (serum deprivation response factor-related gene product that binds to C-kinase, SRBC), cavin-4 (muscle-restricted coiled-coil protein, MURC). Cavin-1 is expressed in all tissues and is essential for caveolae formation (Kovtun et al. 2015). It is proved that cavin-1 can interact with Cav1 to prevent Cav1 degradation, thus, to shape caveolae structure (Hansen and Nichols 2010; Yamaguchi et al. 2016).
It is indicated that Cav1 has two isoforms: Cav1α and Cav1β. Cav1α contains 178 amino acids and Cav1β contains residues 32–178. Both Cav1β and Cav1α can be phosphorylated on serine 80 (Lajoie and Nabi 2010). One study suggested that Cav1α has additional two phosphorylation sites, tyrosine 14 and serine 37. And Cav1β can also undergo phosphorylation on serine 5 (Vainonen et al. 2004). The tyrosine 14 in Cav1 is the target of non-receptor tyrosine kinases Src, Fyn, and c-Abl in response to certain stimuli. The Cav1 phosphorylation on tyrosine 14 can promote cell migration, invasion, and metastasis (Wong et al. 2020). And the Cav1 phosphorylation on serine 80 is implicated in cholesterol transport, protein retention in the endoplasmic reticulum, and protein secretion (Campos et al. 2019). It is shown that mechanical stress can come from the cell’s cytoskeleton or external loads applied to the cell surface. And mechanical stress is implicated in gene expression, cell division, cell migration, cell differentiation, cell adhesion, etc. (Belaadi et al. 2016; Nogueira et al. 2015). Accumulative evidence reveals that under mechanical stress, caveolae open and flatten, thereby, to prevent cell membrane rupture (Cheng and Nichols 2016). The Cav1–cavin-1 interaction is impaired by mechanical stress. When the mechanical stress is disappeared, Cav1 and cavin-1 are reassembled in the plasma membrane and promote caveolae formation (Sinha et al. 2011; Nassoy and Lamaze 2012). Teo et al. reported that Cav1 depletion recruits the formin FMNL2 to stabilize filamentous actin (F-actin), which can elevate epithelial contractile tension to reduce oncogenic cell extrusion. Finally, Cav1 absence represses the elimination of tumor cells from the epithelium monolayer (2020). Cav1 phosphorylation can be stimulated via periodic mechanical stress, leading to the activation of insulin-like growth factor-1 receptor (IGF-1R) and extracellular signal-regulated kinase 1/2 (ERK1/2). Cav1 phosphorylation eventually potentiates chondrocyte proliferation through the IGF-1R/ERK1/2 pathway in response to periodic mechanical stress (Ren et al. 2018).
Cav1 and embryonic stem cells
Found in the early blastocysts, ESCs are capable of multidirectional differentiation, self-renewal, and proliferation properties (Wang et al. 2019a). ESCs can create all of the ectodermal, mesodermal, and endodermal cell layers (Soleimani et al. 2020). ESC self-renewal and pluripotency are involved in both proliferation and maintaining an undifferentiated state. And the ESC self-renewal potential is unlimited (Romeo et al. 2012).
Self-renewal
Down-regulated Cav1 can attenuate ESC proliferation and diminish messenger RNAs (mRNAs) of pluripotent effectors like octamer-binding transcription factor 4 (Oct4), Sox2, FoxD3, and Rex1. These suggest a positive relationship between Cav1 and ESC self-renewal (Lee et al. 2010). LacdiNAc (GalNAcβ1-4GlcNAc) is a cell surface glycan involved in ESC self-renewal. Cav1 can interact with the LacdiNAc-expressed leukemia inhibitory factor (LIF) receptor (LIFR) and glycoprotein 130 (gp130), leading to strong transduction of LIF/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Eventually, the improved LIF/STAT3 signal transduction can promote mouse ESC self-renewal (Sasaki et al. 2011).
Proliferation
Estradiol-17β (E2), one of the female sex steroid hormones, has been found to stimulate mouse ESC migration (Yun et al. 2012). One study shows that Cav1 is a downstream mediator of E2 in mouse ESC cells. E2 can increase Cav1 expression via activating PI3K/protein kinase B (AKT) and ERK1/2 pathways. Inhibiting Cav1 can repress proto-oncogene (c-fos, c-Myc, and c-jun) transcription and decrease cell cycle regulator (cyclin D1, cyclin E, cyclin-dependent kinase 4 (CDK4), and CDK2) expressions. As a result, Cav1 can contribute to E2-modulated ESC proliferation (Park et al. 2009). Galectin-1 is an animal lectin that can enhance stem cell proliferation (Yanagisawa and Yu 2007). Src kinase can phosphorylate Cav1 on tyrosine 14 (Cao et al. 2002). It is shown that Galectin-1 can activate Src to induce Cav1 activation. Activated Cav1 can promote mouse ESC proliferation through activating AKT and mammalian target of rapamycin (mTOR) (Lee et al. 2009). Cav1 expression can also be up-regulated by high glucose through p38 MAPK phosphorylation. Cav1 can stimulate F-actin reorganization through β1 integrin/focal adhesion kinase (FAK)/PIP complex (PINCH1/2, integrin-linked kinase (ILK), and α-parvin) signaling pathway, ultimately, leading to potentiating ESC proliferation under high glucose (Lee et al. 2011). Fibronectin, one of the extracellular matrix (ECM) components, can promote cell proliferation and migration. One report observed that Cav1 phosphorylation can be induced by fibronectin through Src/FAK signal pathway in mouse ESCs. Subsequently, Cav1 can activate Rho-associated protein kinase (ROCK) and RhoA, which can contribute to ESC proliferation through PI3K/AKT and ERK1/2 signals (Park et al. 2011).
Migration
Park et al. proved that fibronectin-induced Cav1 can augment MMP-2 and F-actin expressions via RhoA/ROCK/ERK1/2 signal, resulting in increased migration in mouse ESCs (Park et al. 2012). Epidermal growth factor (EGF), a well-known mitogen, can bind to the EGF receptor (EGFR) to activate the MAPK pathway, which plays a critical role in intercellular communication in mouse ESC colonies (Fong et al. 2014). Cav1 in mouse ESCs is phosphorylated by the EGF/Src signal. And Cav1 can improve mouse ESC proliferation and migration via the PI3K/AKT/ERK1/2/matrix metalloproteinase-2 (MMP-2) pathway (Park and Han 2009).
From these studies, we found that Cav1 can improve proto-oncogene transcription, pluripotent effector expressions, cell cycle regulator expressions, and F-actin reorganization in ESCs. Taking as a whole, Cav1 can promote ESC self-renewal, proliferation, and migration (Fig. 1).
The positive role of Cav1 in stem cells and tumor-initiating cells (TICs). Cav1 can regulate related signaling pathways to promote: a self-renewal, proliferation, and migration in ESCs; b migration, differentiation, motility, and proliferation in SSCs, and stem cell-based wound healing and angiogenesis; c CSC subpopulation, CSC formation, self-renewal, and aggressive behaviors in CSCs; d invasion and chemo-resistance in TICs
Somatic stem cells
Hematopoietic stem cells
HSCs, a very rare cell population with self-renewal and multi-potency, are critical for hematopoiesis. HSCs can be detected in bone marrow (BM), peripheral blood, umbilical cord blood (UCB), and placenta (Yadav et al. 2020). Young HSCs can remain balanced in myeloid and lymphoid differentiation. While elderly HSCs are capable of enhanced myeloid-biased differentiation and declined lymphoid-biased differentiation (Akunuru and Geiger 2016). The HSC transplantation can reconstitute the whole-blood system and maintain long-term hematopoiesis in a recipient, which can be a therapy for hematological diseases (Khaddour et al. 2020). Cav1 deletion can not only impair HSC self-renewal, but also abolish the differentiation of HSCs into mature blood cells. Cav1 deficiency can also accelerate cellular senescence in HSCs. Besides, Cav1 silence in mice can decrease the percentage of HSCs in BM. And the transplantation of HSCs from Cav1-deficient mice can’t reconstitute hematopoiesis in irradiated mice (Bai et al. 2014). PTRF, one putative caveolar coat protein, plays a vital role in caveolae formation at the plasma membrane (Hill et al. 2008). In addition to destroying self-renewal, over-expressed PTRF can also induce cellular senescence and skewed myeloid differentiation in HSCs. Cav1 levels in HSCs are increased by PTRF. Knocking out Cav1 in HSCs can restore self-renewal and inhibit cell senescence in response to up-regulated PTRF (Bai et al. 2020). In sum, these studies indicate that Cav1 is necessary to maintain HSC physiological functions. And the interaction of Cav1 with caveolar protein PTRF impairs self-renewal and stimulates cell senescence in HSCs.
Mesenchymal stem cells
MSCs can exist in BM, adipose tissue, UCB, placenta, peripheral blood, and gingiva (Fan et al. 2020; Jayaramayya et al. 2020). Notably, MSCs can differentiate into several tissue-specific cell lineages, including osteocytes, chondrocytes, cardiomyocytes, adipocytes, neurocytes, and endothelial cells (Ramazzotti et al. 2019; Ma et al. 2020).
Motility
In human UCB-derived MSCs, Cav1 can be activated via galectin-1 through c-Src. The Smad2/3 phosphorylation can be reduced by galectin-1 through Cav1, resulting in down-regulating collagen III and V. Eventually, decreased collagen III and V can increase MSC motility (Yun et al. 2014).
Differentiation
In human BM-derived MSCs (BMSCs), Cav1 is up-regulated during osteogenesis progression. Cav1 can dampen the PI3K/AKT signaling pathway in lipid rafts to inhibit BMSC osteogenesis (Baker et al. 2015). Decreased Cav1 expression can facilitate BMSC proliferation. And Cav1 depletion can also improve BMSC matrix mineralization and the expressions of osteogenic regulators (e.g., osteocalcin, bone sialoprotein 2, Col1a2, and Runx2). Cav1 can function as an antagonist of BMSC proliferation and osteogenic differentiation (Baker et al. 2012). Further, one study demonstrated that Cav1 absence is failed to express bone sialoprotein or form mineralized nodules of murine BMSCs, which suggests that Cav1 knockout can lead to dysfunctional osteogenic differentiation (Case et al. 2010). Cav1 is highly expressed in rat BMSCs undergoing cardiomyocyte differentiation. Cav1 silence can potentiate BMSC differentiation into cardiomyocytes by reducing the STAT3 pathway (Chen et al. 2017). Cav1 up-regulation can attenuate insulin signaling and decline peroxisome proliferator-activated receptor gamma 2 (PPARγ2, one positive effector for adipocyte development) expression. Up-regulated Cav1 eventually represses the adipogenic differentiation of human BMSCs (Park et al. 2005). Cav1 is decreased during neuronal differentiation in human adipose-derived MSCs. Cav1 down-regulation can potentiate MSC differentiation into dopaminergic-like neurons through increasing dopamine levels and dopaminergic neuronal marker (e.g., tyrosine hydroxylase, Lmx1a, and Nurr1) expressions (Han et al. 2021). Additionally, the combination of reduced Cav1 with increased ATP6AP2 (a key component of the renin-angiotensin system) in lipid raft micro-domains can facilitate MSC neurogenesis (Makdissy et al. 2018). Once cleaved after interacting with Notch ligands (Delta and Jagged), Notch can release Notch intracellular domain (NICD) into the cell nucleus, thereby, to induce the transcription of its target genes, for example, the hairy enhancer of split (Hes) family (Zanotti and Canalis 2016). In rat BMSCs, silencing Cav1 can abrogate the Notch-1, NICD, and Hes5 expressions. The impaired Notch signaling pathway ultimately stimulates BMSC neuronal differentiation (Wang et al. 2013).
Therapy
Wound healing is a complex process in humans and abnormal wound healing leads to high healthcare costs (Rodrigues et al. 2019). Extracellular vesicles (EVs) are cargo-carrying vesicles that can affect cell communication (Teng and Fussenegger 2020). The gingiva-derived MSCs (GMSCs) can produce and secrete small EVs (sEVs) expressing interleukin-1 receptor antagonist (IL-1RA). Cav1 membrane translocation in GMSCs can be increased via tumor necrosis factor-alpha (TNF-α) through up-regulating Fas and Fas-associated phosphatase-1 (Fap-1). Increased membrane translocation of Cav1 can promote IL-RA-sEVs release in GMSCs, which can ultimately accelerate cutaneous wound healing in mouse gingiva (Kou et al. 2018).
Taken together, activated Cav1 promotes MSC motility, while increased Cav1 inhibits osteogenic differentiation, cardiomyocyte differentiation, adipogenic differentiation, and neuronal differentiation in MSCs. And enhanced Cav1 membrane translocation in MSCs enhances skin wound healing.
Neural stem cells
NSCs can proliferate, self-renew and give rise to neurons and glial cells (e.g., astrocytes and oligodendrocytes) (Kobayashi and Kageyama 2020). In the adult brain, NSCs reside in the subventricular zone (SVZ) and subgranular zone (SGZ). When the brain gets injured, the endogenous quiescent NSCs become active and migrate to the damaged area, subsequently, repair the brain (Othman and Tan 2020). In the ischemic brain of rats, Cav1 and vascular endothelial growth factor (VEGF) protein levels are significantly increased by treadmill exercise. Down-regulated Cav1 can reduce VEGF expression, thereby, to abolish exercise-induced NSC migration and neural differentiation in the ischemic brain. Cav1/VEGF pathway can contribute to the exercise-promoted recovery of brain function after middle cerebral artery occlusion through improving neurogenesis (Zhao et al. 2017). In short, there is a positive relationship between Cav1 and NSC neurogenesis, which are beneficial for functional recovery in the ischemic brain.
Adipose-derived stem cells
ADSCs, which are abundant in adipose tissues, display multi-potency and have the potential to differentiate into hepatocytes, osteocytes, adipocytes, and so on (Megaloikonomos et al. 2018). Cav1 protein expression and phosphorylated MAPK are increased during human ADSC hepatocyte differentiation. Cav1 suppression can abrogate the MAPK pathway to decrease the levels of hepatic marker proteins, including albumin and hepatocyte nuclear factor‑1‑α, resulting in repressing ADSC hepatic differentiation (Guan et al. 2016). The phenylalanine at position 92 (F92) in the Cav1 scaffolding region is responsible for eNOS suppression. The mutation of F92 to alanine can result in mutant Cav1 (F92A-Cav1), which reverses the impact on eNOS and thereby increases nitric oxide (NO) synthesis (Bernatchez et al. 2011). In equine ADSCs, the interaction of F92A-Cav1 with eNOS can augment NO production, which can promote the activation of the Wnt3a/β-catenin signaling pathway. Finally, the activated Wnt3a/β-catenin signal can facilitate osteogenesis of equine ADSCs (Bandara et al. 2016). In summary, in ADSCs, Cav1 positively regulates hepatic differentiation and the mutation in Cav1 at position 92 reduces Cav1 inhibition on osteogenesis differentiation.
Epidermal stem cells
EpiSCs preserve self-renewal and proliferative properties. And they are enriched in hair follicles, sebaceous glands, and inter-follicular epidermis. Importantly, EpiSCs can migrate to wound sites and promote wound healing (Yang et al. 2019a). It is showed that Cav1 can potentiate EpiSC proliferation. And the treatment of EpiSCs over-expressing Cav1 can accelerate angiogenesis and skin wound healing in rats. Additionally, the conditioned medium of EpiSCs with over-expressed Cav1 can increase endothelial cell tube formation in human umbilical vein endothelial cells (HUVECs) (Yang et al. 2019b). Curcumin isolated from turmeric belongs to traditional Chinese medicine and it can act as treatment methods for skin diseases, obesity, and cancer (Kocaadam and Şanlier 2017). Cav1 can be elevated via curcumin in mouse EpiSCs. And suppressing Cav1 can attenuate EpiSC proliferation, HUVEC tube formation, and EpiSC-based wound healing under curcumin (Yang et al. 2019c). As mentioned above, Cav1 improves EpiSC proliferation and is necessary for EpiSC-related HUVEC tube formation and wound healing.
Mammary stem cells
MaSCs mainly reside in mammary glands and normally keep quiescent. Upon certain stimuli, for example, the steroid hormones, multipotent MaSCs become active and then give rise to both luminal and basal progenitor cells, which can promote mammary gland development (Lee et al. 2019). It is indicated that Cav1 deficiency can enhance the expressions of MaSC markers stem cell antigen-1 (Sca-1), keratin 5 (K5), and K6 in mammary glands. Cav1 deletion can also significantly increase β-catenin expression. Sotgia et al. suggested that Cav1 silence can increase MaSC populations through activating the β-catenin pathway (Sotgia et al. 2005). As mentioned above, it can be seen that Cav1 can be considered to be an inhibitor for MaSC populations.
Intestinal stem cells
ISCs are highly proliferative cell populations mainly located at the intestinal crypts. They are beneficial for epithelial renewal and injury-related regeneration in the intestine (Bankaitis et al. 2018). Cav1 attenuation can enhance proliferation via promoting the Wnt/β-catenin pathway in intestinal crypt stem cells. Moreover, Cav1 abrogation can increase apoptosis of crypt stem cells in response to radiation, indicating the increased sensitivity to radiation (Li et al. 2005). In summary, Cav1 displays negative effects on ISC proliferation and radiation sensitivity.
Neural progenitor cells
Neural progenitor cells (NPCs) can be produced by NSCs and have the potential to differentiate into neurons, astrocytes, and oligodendrocytes (Beattie and Hippenmeyer 2017). Besides, NPCs can also be generated by ESCs or iPSCs (Martínez-Cerdeño and Noctor 2018). In rat NPCs, Cav1 can accelerate astroglial differentiation via up-regulating NICD and Hes1 expressions (Li et al. 2011a). Another study proved that Cav1 can attenuate oligodendroglial differentiation of rat NPCs through increasing β-catenin expression (Li et al. 2011b). The Ras and Rho GTPases (i.e., cell division cycle 42 (Cdc42), Rac1, etc.) can improve axonal growth and guidance. And it is said that appropriate axonal growth and guidance are essential for functional neuronal network development (Hall and Lalli 2010). In NPCs derived from human iPSCs, both Cav1 expression and its phosphorylation levels are elevated by constitutively active Cdc42 and Rac1. Phosphorylated Cav1 can facilitate axonal growth during neuronal differentiation in NPCs (Wang et al. 2019b). In normoxic conditions, Cav1 is increased. Cav1 can abrogate VEGF signals, which can reduce neuronal differentiation in NPCs. However, when exposed to hypoxia, the Cav1 expression is decreased, resulting in enhancing NPC differentiation into neurons. These results can explain the reason why hypoxia can promote neuronal differentiation (Li et al. 2011c). Exosomes, a kind of extracellular vesicles with a diameter of ~ 40 to 160 nm, can deliver many cell constituents (DNA, RNA, proteins, etc.) and mediate intercellular communication (Kalluri and LeBleu 2020). Cav1 is rich in exosomes originated from brain microvascular endothelial cells. Exosomal Cav1 can repress NeuroD1 expression and VEGF signal, leading to inhibiting neuronal differentiation of NPCs in post-ischemic brains (Li et al. 2020). It is can be seen that Cav1 promotes astroglial differentiation and suppresses oligodendroglial differentiation and neuronal differentiation in NPCs.
Endothelial progenitor cells
Endothelial progenitor cells (EPCs) are undifferentiated cells with the potential to proliferate and differentiate into endothelial cells. EPCs can be discovered in peripheral blood, BM, UCB, and adipose tissues (Kaushik and Das 2019). In response to several stimuli (e.g., hypoxia, injury, etc.), EPCs can migrate to the peripheral circulation from BM, therefore, to form or repair blood vessels. EPCs are critical for vascularization and angiogenesis, and EPC-associated therapies are helpful for diabetes and diabetes‑related diseases (Ambasta et al. 2017). Cav1 expression is increased by aerobic and resistance training in EPCs obtained from the mice with type 2 diabetes mellitus. Aerobic and resistance training can improve EPC proliferation, migration, and angiogenesis (Dai et al. 2020). Cav1 knockdown can impair EPC proliferation and adherent ability upon aerobic and resistance training (Zhai et al. 2020). In rat BM EPCs, Cav1 expression can be up-regulated via estrogen through suppressing the lysosomal degradation pathway. And increased Cav1 can contribute to EPC proliferation (Tan et al. 2012). Under high glucose stimuli, Cav1 mRNA and protein levels are increased, while the eNOS protein expression is declined. Impaired Cav1–eNOS complex can decrease NO synthesis, resulting in reduced EPC proliferation, migration, and angiogenesis (Cao et al. 2012). The urokinase-type plasminogen activator receptor (uPAR) is linked to the caveolae organization. In UCB-derived EPCs, Cav1 depletion can cause the absence of full-length uPAR in the caveolae, which can abrogate EPC angiogenesis (Margheri et al. 2011). Taken together, Cav1 can promote EPC proliferation, migration, and angiogenesis. However, the impaired Cav1–eNOS complex reverses Cav1 effects on EPC biology.
As mentioned above, in addition to showing positive roles in SSCs, Cav1 also displays negative effects. The realization of these influences mostly depends on the Cav1-related signaling pathway (Figs. 1 and 2).
Cav1 and cancer stem cells
Breast cancer stem cells
Breast cancer is the most frequently diagnosed malignancy and the second leading cause of cancer-related death among female populations (Siegel et al. 2020). According to the protein expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), breast cancer can be divided into luminal A (ER+, PR+, HER2−), luminal B (ER+, PR+, HER2+), basal-like (triple-negative breast cancer) (ER−, PR−, HER2−), and HER2-enriched (ER−, PR−, HER2+) subtypes (Testa et al. 2020). Breast cancer stem cells (BCSCs), a subpopulation of cells within the breast tumor bulk, are more frequently observed in luminal and triple-negative breast cancer tissues than in HER2-enriched ones. BCSCs possess self-renewal properties and can repopulate the entire tumor through differentiation (Urueña et al. 2020). The mutations of breast tumor cells, mammary stem cells, mammary progenitor cells, or differentiated breast tissue cells can contribute to BCSC formation. Importantly, CD24, CD44, CD133, aldehyde dehydrogenase (ALDH), ATP-binding cassette subfamily G member 2 (ABCG2), and epithelial cell adhesion molecule (EpCAM) are commonly considered to be surface markers to identify BCSCs (Bai et al. 2018). Cav1 levels are lower in BCSCs than those in normal breast stem cells. Cav1 up-regulation can reduce BCSC subpopulation. Cav1 can accelerate c-Myc ubiquitination and degradation via the proteasome pathway. When Cav1 is lost, c-Myc is up-regulated. c-Myc destroys mitochondrial respiration and elevates aerobic glycolysis activity, leading to promoted BCSC self-renewal and mammary tumor formation. As a result, Cav1 can attenuate c-Myc-induced BCSC metabolic reprogramming and self-renewal. And low Cav1 levels can predict poor overall survival in breast cancer patients (Wang et al. 2020b). In BCSCs sorted from MDA-MB-231 and MCF-7 cells, Cav1 can improve self-renewal but limit their differentiation into basal-like and luminal-like breast cancer cells. Additionally, Cav1 depletion can suppress the β-catenin/ABCG2 signaling pathway via activating glycogen synthase kinase 3 beta (GSK3β) and repressing AKT phosphorylation. Removing Cav1 can inhibit drug efflux ability via β-catenin/ABCG2 signal, which can contribute to enhanced chemo-sensitivity in BCSCs (Wang et al. 2014a). Hsu et al. revealed that Cav1 knockdown can reduce γ-secretase activity. The interaction of Cav1 with γ-secretase can be potentiated by the interleukin-6/ILK signal, resulting in Notch-1 cleavage to release NICD into the cell nucleus. NICD can increase CSC-related marker (Hes1, c-Myc, Nestin, and ABCG2) expressions. Eventually, BCSC subpopulations are increased (Hsu et al. 2015). As mentioned above, Cav1 plays dual roles in BCSC self-renewal and BCSC subpopulations. Cav1 acts as a suppressor for BCSC differentiation and chemo-sensitivity.
Lung cancer stem cells
Lung cancer is the most common malignancy around the world and is histologically classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). NSCLC includes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (Olbromski et al. 2020). Increasing evidence reported that lung CSCs can arise from differentiated lung tissue cells, normal lung stem cells, or lung progenitor cells. And lung CSCs can form tumorspheres and possess multipotent differentiation and tumorigenic potential (Rivera et al. 2011). Recently, several biomarkers can be used to label lung CSCs for further experiments, such as CD44, CD24, CD133, ABCG2, and ALDH (Wang et al. 2014b). Cav1 is strongly up-regulated under ciprofloxacin stimuli in lung CSC populations. Activated Cav1 can phosphorylate ERK and AKT to increase CSC marker (CD44, CD133, ABCG2, and ALDH) expressions. Activated Cav1 finally promotes the induction of lung CSCs (Phiboonchaiyanan et al. 2016). Carbon nanotubes are nanomaterials that are known to be attributed to inflammation, fibrosis, and cancer under certain conditions (Dong and Ma 2019). Cav1 expression is increased in lung CSCs induced by the exposure of chronic single-walled carbon nanotubes. Cav1 can promote lung CSC induction via reducing p53 (Luanpitpong et al. 2014). One study reported that Cav1 can be up-regulated by NO. Increased Cav1 can stimulate aggressive behaviors of lung CSCs, including migration, colony-forming activity, and resistance to anoikis (Yongsanguanchai et al. 2015). When NO is absent, Cav1 down-regulates Oct4 expression through ubiquitin-proteasomal degradation, resulting in diminishing CSC formation. In the existence of NO, the phosphorylation of Cav1 on the tyrosine14 is induced by the NO/AKT signal, which can facilitate Oct4 dissociation from the Cav1-Oct4 degradation complex. Oct4 accumulation in the cell nucleus increases stemness-related gene expressions. Ultimately, lung CSC formation is enhanced (Maiuthed et al. 2018). Taken together, in response to certain conditions, Cav1 enhances or reduces lung CSC formation. And the Cav1 phosphorylation on tyrosine 14 can inhibit the negative effects of Cav1 on lung CSC formation.
Liver cancer stem cells
Liver cancer, a malignant tumor with high incidence and mortality, can be divided into primary liver cancer (i.e., hepatocellular carcinoma (HCC, major type), liver angiosarcoma, and hepatoblastoma) and secondary liver cancer (Liu et al. 2020a). Liver CSCs are a subpopulation of cancer cells within liver cancer. On the one hand, liver CSCs can promote tumor growth in primary liver cancers. On the other hand, liver CSCs can also enhance distant metastasis, which can cause liver cancer recurrence (Fan et al. 2011). MicroRNAs (miRNAs), a class of small non-coding RNAs with 18 – 25 nucleotides in length, can bind to mRNA 3’ untranslated regions (3’UTRs) of their targets and reduce target gene expressions (de Sousa et al. 2019). Cav1 is increased in liver CSCs derived from primary HCC patients. MiR-124 is down-regulated in liver CSCs and can suppress liver CSC expansion. As a target miR-124, Cav1 can be inhibited by miR-124. In the presence of up-regulated miR-124, over-expressed Cav1 can suppress miR-124 effects on liver CSCs and promote liver CSC self-renewal and tumorigenic ability (Feng et al. 2020). As mentioned above, it can be seen that Cav1 can be repressed by miRNAs. And Cav1 serves as a promoter of liver CSC properties.
Prostate cancer stem cells
Prostate cancer (PCa), the second-most common cancer and the sixth in cancer mortality among men globally (Bray et al. 2018), develops from the prostate gland due to many risk factors (e.g., age, high-fat diet, family history, and obesity) (Kaler et al. 2020). Prostate CSCs can come from normal prostate stem cells, differentiated prostate tissue cells, or PCa cells. Further, prostate CSCs are responsible for prostate tumor initiation, metastasis, colonization, and therapeutic resistance (Qin et al. 2017). Cav1 mRNA and protein expression levels are elevated in prostate CSCs. Exogenous Cav1 protein can stimulate prostate sphere formation. Additionally, Cav1 can be delivered into PCa cells by tumor-derived exosomes. Exosomal Cav1 can promote prostate CSC formation via activating the nuclear factor kappa B (NF-κB) signaling pathway (Lin et al. 2019). Altogether, exosomal Cav1 can regulate the cell–cell communication between PCa cells and prostate CSCs. Cav1 plays a positive role in prostate CSC formation.
As mentioned above, Cav1 serves as a promoter or inhibitor in CSC self-renewal and CSC formation (Figs. 1 and 2). Cav1 can improve CSC aggressive behaviors (Fig. 1) and suppress chemo-sensitivity (Fig. 2).
Cav1 and tumor-initiating cells
TICs can exist in various solid cancers, like breast, colon, liver, lung, pancreas, prostate, etc. Similar to ESCs and HSCs, TICs possess the capacity to survive under the stimuli of genotoxic stress and injury. TICs can also self-renew and produce differentiated tumor cells (Mathews et al. 2011). It is said that the cell of origin is the normal cell with the first cancer-promoting mutations. And the cell of origin is distinct from the CSC. While the TIC can be referred to as the cell of origin (Visvader 2011). The tumor transplantation assay and lineage-tracing assay are commonly used to identify CSCs and determine the cell of origin, respectively (Rycaj and Tang 2015). It is proved that CSCs can derive from TICs. TICs and CSCs have distinct markers. TICs are related to tumor initiation. CSCs are responsible for tumor maintenance and tumor growth (Chatterji et al. 2018). Pancreatic cancer is one of the most aggressive malignancies with a poor prognosis and a low five-year survival rate despite decades of unremitting efforts. Among pancreatic cancer cases, pancreatic ductal adenocarcinoma accounts for more than 90% (Paternoster and Falasca 2020). Characterized by the capacity to self-renew, differentiate and form colonies, pancreatic TICs can be separated from pancreatic cancer tissues and cell lines based on the expression of surface markers CD44, CD24, CD133, and EpCAM (Cabarcas et al. 2013; Shimizu et al. 2013). Cav1 is higher in CD133-positive pancreatic TICs compared with human pancreatic cancer cell lines. Cav1 depletion abolishes the activation of FAK and NF-κB to suppress the invasion of pancreatic TICs. Besides, diminishing Cav1 reduces drug transporter (ABCG2 and ATP-binding cassette subfamily C member 4 (ABCC4)) activity and therefore destroys chemo-resistance in pancreatic TICs. Additionally, high Cav1 levels can also indicate poor survival in pancreatic cancer patients (Gupta et al. 2018). Taken together, Cav1 plays a positive role in pancreatic TIC invasion and drug resistance (Fig. 1).
Discussion and conclusion
Both normal stem cells and CSCs possess two basic stemness signatures (self-renewal and differentiation) and use common stemness-associated signaling pathways. Normal stem cells are essential for tissue repair and regeneration. CSCs are related to tumor initiation, metastasis, and therapy resistance. Cav1, a scaffolding protein in the caveolae, can regulate cell lipid transport, cell migration, cell proliferation, cell signal transduction, etc. Recently, the rational design of therapies based on the Cav1-stem cell axis for human diseases has attracted widespread attention. In this review, we have collected evidence to reveal the involvement of Cav1 in stem cells (Fig. 3). We hope that this review will provide new sights for more clinical therapies, thereby allowing us to treat human diseases more effectively.
In the current review, we discussed how Cav1 can exert positive or negative roles in stem cells (Tables 1 and 2). In normal stem cells, Cav1 can play dual roles in HSC self-renewal and senescence (Bai et al. 2014, 2020), MSC neuronal differentiation (Wang et al. 2013; Du et al. 2011), and EPC proliferation (Tan et al. 2012; Cao et al. 2012). Cav1 displays a positive role in ESCs (migration (Park and Han 2009; Park et al. 2012), proliferation (Park et al. 2009, 2011; Lee et al. 2009, 2011) and self-renewal (Lee et al. 2010; Sasaki et al. 2011)), NSC neural differentiation (Zhao et al. 2017), ADSC hepatic differentiation (Guan et al. 2016), EpiSC proliferation (Yang et al. 2019b, c), and NPC astroglial differentiation (Li et al. 2011a). Cav1 exhibits a negative role in MSC osteogenic differentiation (Baker et al. 2012, 2015), MSC cardiomyocyte differentiation (Chen et al. 2017), MSC adipogenic differentiation (Park et al. 2005), NSC proliferation (Peffer et al. 2014; Jasmin et al. 2009), MaSC populations (Sotgia et al. 2005), ISC proliferation (Li et al. 2005), NPC oligodendroglial (Li et al. 2011b) and neuronal differentiation (Li et al. 2011c, 2020). In response to high glucose, Cav1 promotes ESC proliferation (Lee et al. 2011), however, inhibits EPC proliferation, migration, and angiogenesis (Cao et al. 2012). F92A-Cav1 can increase NO production to improve MSC endothelial differentiation (Bandara et al. 2019) and ADSC osteogenesis (Bandara et al. 2016). In CSCs, Cav1 exerts dual roles in BCSC self-renewal (Wang et al. 2014a, 2020b), lung CSC formation (Phiboonchaiyanan et al. 2016; Maiuthed et al. 2018). Cav1 plays a positive role in self-renewal, CSC formation, and cell invasion in liver CSCs (Feng et al. 2020), prostate CSCs (Lin et al. 2019), and pancreatic TICs (Gupta et al. 2018), respectively. Furthermore, normal stem cells and CSCs can share stemness effectors, for example, the Oct4 (Lee et al. 2010; Maiuthed et al. 2018), and use the same stem cell-related signals, like Notch (Wang et al. 2013; Li et al. 2011a; Hsu et al. 2015), AKT (Lee et al. 2009; Park and Han 2009; Park et al. 2011; Baker et al. 2015; Phiboonchaiyanan et al. 2016) and NO (Cao et al. 2012; Yongsanguanchai et al. 2015; Maiuthed et al. 2018) signals. Interestingly, in our study, we found that the mutation of phenylalanine to alanine at position 92 in Cav1 suppresses Cav1 repression on ADSC osteogenesis differentiation (Bandara et al. 2016). In lung CSCs, the Cav1 phosphorylation on tyrosine 14 inhibits Cav1 suppression on CSC formation (Maiuthed et al. 2018). Taken together, it can be seen that the effects of Cav1 on stem cells mostly depend on cell types, related signal transduction (Figs. 1 and 2; Tables 1 and 2), Cav1 phosphorylation, and Cav1 mutation (Williams and Lisanti 2005).
Interestingly, there is evidence that Cav1 has dual roles in the development of some cancers. At early tumor stages, Cav1 acts as a tumor suppressor. While in the late stages of cancer, Cav1 serves as a tumor promoter (Simón et al. 2020). In breast cancer cells, Cav1 can activate Rab5 by recruiting p85α, subsequently, to increase Rac1 activity. The Cav1/Rab5/Rac1 signal finally potentiates cell migration and cell invasion (Díaz et al. 2014). In BT-474 cells, Cav1 promotes cell proliferation via activating the ERK1/2 pathway and increasing the expression of cell cycle-associated proteins (Wang et al. 2014c). In MCF-7 cells, the interaction of Cav1 with CD95 can be enhanced by Antarctic krill docosahexaenoic acid, which can repress cell migration and cell invasion (Zheng et al. 2018). Moreover, increased Cav1 represses Ca2+-activated potassium channel, resulting in suppressing cell proliferation and invasion in breast cancer (Du et al. 2014). In lung cancer, Cav1 up-regulates Snail protein and mRNA expressions, which can accelerate NSCLC brain metastasis (Kim et al. 2019). Volonte et al. indicated that Cav1 can enhance oncogenic K-Ras-induced lung cancer cell senescence. And low Cav1 can predict poor survival of lung cancer patients (Volonte et al. 2018). It is suggested that the communication between cells and their microenvironment is essential for normal tissue homeostasis and tumor growth (Quail and Joyce 2013). In recent years, the roles of the tumor microenvironment in cancer development have received a lot of attention. The tumor microenvironment encompasses immune cells, blood vessels, extracellular matrix, fibroblasts, lymphocytes, inflammatory cells, and signaling molecules (Spill et al. 2016; Prete et al. 2017). Cancer-associated fibroblasts (CAFs) are the most abundant stromal cells in the tumor microenvironment. CAFs repress cancer cell apoptosis and promote tumor cell proliferation, tumor metastasis, and tumor angiogenesis (Jang and Beningo 2019; Chen and Che 2014). It is demonstrated that Cav1 down-regulation stimulates the transformation of fibroblasts into CAFs (Martinez-Outschoorn et al. 2010). SHI et al. demonstrated that Cav1 depletion in CAFs increases the expression and secretion of stromal cell-derived factor-1, EGF, and fibroblast-specific protein-1, and all of them enhance breast cancer cell proliferation (Shi et al. 2016). And down-regulation and up-regulation of Cav1 in CAFs can predict poor (Ren et al. 2014) and good (Shan-Wei et al. 2012) outcomes in breast cancer patients, respectively. In summary, it is suggested that decreased stromal Cav1 is always beneficial for tumor progression (Chen and Che 2014; Martinez-Outschoorn et al. 2010; Shi et al. 2016; Ren et al. 2014; Shan-Wei et al. 2012).
In the current review, we found that treadmill exercise increases Cav1 and VEGF levels in NSCs, which can improve neurological recovery of the brain after ischemic injury (Zhao et al. 2017). Curcumin, the traditional Chinese medicine used to treat skin diseases, can elevate Cav1 expression in EpiSCs. Inhibiting Cav1 in the curcumin-treated EpiSCs can significantly repress wound healing (Yang et al. 2019c). In various cancer types, Cav1 is related to chemo-drug resistance. Taking lung cancer as an example, it is reported that Cav1 attenuation reduces NSCLC sensitivity to albumin-bound paclitaxel (Chatterjee et al. 2017). Liu et al. suggested that Cav1 silence increases lung cancer sensitivity to cisplatin (Liu et al. 2020b). Furthermore, suppressing Cav1 can sensitize BCSCs and pancreatic TICs to epirubicin (Wang et al. 2014a) and paclitaxel (Gupta et al. 2018), respectively. These results indicate potential treatment strategies associated with the combination of Cav1 and stem cells for ischemic injury, wound healing, and cancer drug resistance. Increasing evidence suggests that nano-drugs are widely used for anti-tumor drug delivery, gene therapy, and imaging diagnosis. Cav1-mediated endocytosis pathway can increase the entrance of nano-drugs into tumor cells and promote antitumor response. The strategies for Cav1 targeting nano-drugs will be a new way to achieve antitumor efficacy (Yang et al. 2021). Presently, great efforts have been made in stem cell-based therapies for human tissue repair and cancer treatment resistance. As mentioned above, Cav1 is widely involved in stem cell biology. Targeting Cav1 will strongly influence stem cell properties, which might provide new insights to more effectively treat human tissue repair and cancer drug resistance. However, these studies previously mentioned are performed mainly on stem cells, and studies on tissues are still lacking. Therefore, further explorations in these microenvironments are required to elucidate the roles of Cav1 in stem cells more completely.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ahmed SA, Parama D, Daimari E, Girisa S, Banik K, Harsha C, Dutta U, Kunnumakkara AB (2020) Rationalizing the therapeutic potential of apigenin against cancer. Life Sci. https://doi.org/10.1016/j.lfs.2020.118814
Akunuru S, Geiger H (2016) Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol Med 22(8):701–712. https://doi.org/10.1016/j.molmed.2016.06.003
Ambasta RK, Kohli H, Kumar P (2017) Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder. J Transl Med 15(1):185. https://doi.org/10.1186/s12967-017-1280-y
Bai L, Shi G, Zhang L, Guan F, Ma Y, Li Q, Cong YS, Zhang L (2014) Cav-1 deletion impaired hematopoietic stem cell function. Cell Death Dis 5(3):e1140. https://doi.org/10.1038/cddis.2014.105
Bai X, Ni J, Beretov J, Graham P, Li Y (2018) Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev 69:152–163. https://doi.org/10.1016/j.ctrv.2018.07.004
Bai L, Lyu Y, Shi G, Li K, Huang Y, Ma Y, Cong YS, Zhang L, Qin C (2020) Polymerase I and transcript release factor transgenic mice show impaired function of hematopoietic stem cells. Aging 12(20):20152–20162. https://doi.org/10.18632/aging.103729
Baker N, Tuan RS (2013) The less-often-traveled surface of stem cells: caveolin-1 and caveolae in stem cells, tissue repair and regeneration. Stem Cell Res Ther 4(4):90. https://doi.org/10.1186/scrt276
Baker N, Zhang G, You Y, Tuan RS (2012) Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem 113(12):3773–3787. https://doi.org/10.1002/jcb.24252
Baker N, Sohn J, Tuan RS (2015) Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther 6:238. https://doi.org/10.1186/s13287-015-0225-8
Bandara N, Gurusinghe S, Lim SY, Chen H, Chen S, Wang D, Hilbert B, Wang LX, Strappe P (2016) Molecular control of nitric oxide synthesis through eNOS and caveolin-1 interaction regulates osteogenic differentiation of adipose-derived stem cells by modulation of Wnt/β-catenin signaling. Stem Cell Res Ther 7(1):182. https://doi.org/10.1186/s13287-016-0442-9
Bandara N, Gurusinghe S, Kong A, Mitchell G, Wang LX, Lim SY, Strappe P (2019) Generation of a nitric oxide signaling pathway in mesenchymal stem cells promotes endothelial lineage commitment. J Cell Physiol 234(11):20392–20407. https://doi.org/10.1002/jcp.28640
Bankaitis ED, Ha A, Kuo CJ, Magness ST (2018) Reserve stem cells in intestinal homeostasis and injury. Gastroenterology 155(5):1348–1361. https://doi.org/10.1053/j.gastro.2018.08.016
Beattie R, Hippenmeyer S (2017) Mechanisms of radial glia progenitor cell lineage progression. FEBS Lett 591(24):3993–4008. https://doi.org/10.1002/1873-3468.12906
Belaadi N, Aureille J, Guilluy C (2016) Under pressure: mechanical stress management in the nucleus. Cells. https://doi.org/10.3390/cells5020027
Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC (2011) A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Investig 121(9):3747–3755. https://doi.org/10.1172/jci44778
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Cabarcas SM, Sun L, Mathews L, Thomas S, Zhang X, Farrar WL (2013) The differentiation of pancreatic tumor-initiating cells by vitronectin can be blocked by cilengitide. Pancreas 42(5):861–870. https://doi.org/10.1097/MPA.0b013e318279d568
Campos A, Burgos-Ravanal R, González MF, Huilcaman R, Lobos González L, Quest AFG (2019) Cell intrinsic and extrinsic mechanisms of caveolin-1-enhanced metastasis. Biomolecules. https://doi.org/10.3390/biom9080314
Cao H, Courchesne WE, Mastick CC (2002) A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem 277(11):8771–8774. https://doi.org/10.1074/jbc.C100661200
Cao C, Zhang H, Gong L, He Y, Zhang N (2012) High glucose conditions suppress the function of bone marrow-derived endothelial progenitor cells via inhibition of the eNOS-caveolin-1 complex. Mol Med Rep 5(2):341–346. https://doi.org/10.3892/mmr.2011.644
Case N, Xie Z, Sen B, Styner M, Zou M, O’Conor C, Horowitz M, Rubin J (2010) Mechanical activation of β-catenin regulates phenotype in adult murine marrow-derived mesenchymal stem cells. J Orthop Res 28(11):1531–1538. https://doi.org/10.1002/jor.21156
Chatterjee M, Ben-Josef E, Robb R, Vedaie M, Seum S, Thirumoorthy K, Palanichamy K, Harbrecht M, Chakravarti A, Williams TM (2017) Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res 77(21):5925–5937. https://doi.org/10.1158/0008-5472.can-17-0604
Chatterji P, Douchin J, Giroux V (2018) Demystifying the differences between tumor-initiating cells and cancer stem cells in colon cancer. Curr Colorectal Cancer Rep 14(4):242–250
Chen D, Che G (2014) Value of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (review). Oncol Lett 8(4):1409–1421. https://doi.org/10.3892/ol.2014.2385
Chen Y, Wang C, Huang Q, Wu D, Cao J, Xu X, Yang C, Li X (2017) Caveolin-1 plays an important role in the differentiation of bone marrow-derived mesenchymal stem cells into cardiomyocytes. Cardiology 136(1):40–48. https://doi.org/10.1159/000446869
Cheng JPX, Nichols BJ (2016) Caveolae: one function or many? Trends Cell Biol 26(3):177–189. https://doi.org/10.1016/j.tcb.2015.10.010
Chidlow JH Jr, Sessa WC (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86(2):219–225. https://doi.org/10.1093/cvr/cvq075
Chiodi I, Mondello C (2020) Life style factors, tumor cell plasticity and cancer stem cells. Mutat Res 784:108308. https://doi.org/10.1016/j.mrrev.2020.108308
Clara JA, Monge C, Yang Y, Takebe N (2020) Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol 17(4):204–232. https://doi.org/10.1038/s41571-019-0293-2
Dai X, Zhai L, Su Q, Luo B, Wei C, Liu Y, Huang Y, Ma C, Ying Y (2020) Effect of aerobic and resistance training on endothelial progenitor cells in mice with type 2 diabetes. Cell Reprogram 22(4):189–197. https://doi.org/10.1089/cell.2019.0063
de Sousa MC, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M (2019) Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci. https://doi.org/10.3390/ijms20246249
Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S (2017) Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol 35:40–47. https://doi.org/10.1016/j.coph.2017.05.004
Díaz J, Mendoza P, Ortiz R, Díaz N, Leyton L, Stupack D, Quest AFG, Torres VA (2014) Rab5 is required in metastatic cancer cells for caveolin-1-enhanced Rac1 activation, migration and invasion. J Cell Sci 127(Pt 11):2401–2406. https://doi.org/10.1242/jcs.141689
Dong J, Ma Q (2019) Integration of inflammation, fibrosis, and cancer induced by carbon nanotubes. Nanotoxicology 13(9):1244–1274. https://doi.org/10.1080/17435390.2019.1651920
Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C (2011) Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci USA 108(23):9466–9471. https://doi.org/10.1073/pnas.1106467108
Du C, Chen L, Zhang H, Wang Z, Liu W, Xie X, Xie M (2014) Caveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasion. Int J Mol Sci 15(11):20706–20722. https://doi.org/10.3390/ijms151120706
Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J (2011) Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg 254(4):569–576. https://doi.org/10.1097/SLA.0b013e3182300a1d
Fan XL, Zhang Y, Li X, Fu QL (2020) Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci 77(14):2771–2794. https://doi.org/10.1007/s00018-020-03454-6
Feng Y, Jiang W, Zhao W, Lu Z, Gu Y, Dong Y (2020) miR-124 regulates liver cancer stem cells expansion and sorafenib resistance. Exp Cell Res 394(2):112162. https://doi.org/10.1016/j.yexcr.2020.112162
Filippini A, D’Alessio A (2020) Caveolae and lipid rafts in endothelium: valuable organelles for multiple functions. Biomolecules. https://doi.org/10.3390/biom10091218
Fong JT, Nimlamool W, Falk MM (2014) EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Lett 588(5):836–844. https://doi.org/10.1016/j.febslet.2014.01.048
Guan X, Wang N, Cui F, Liu Y, Liu P, Zhao J, Han C, Li X, Leng Z, Li Y, Ji X, Zou W, Liu J (2016) Caveolin-1 is essential in the differentiation of human adipose-derived stem cells into hepatocyte-like cells via an MAPK pathway-dependent mechanism. Mol Med Rep 13(2):1487–1494. https://doi.org/10.3892/mmr.2015.4743
Gupta VK, Sharma NS, Kesh K, Dauer P, Nomura A, Giri B, Dudeja V, Banerjee S, Bhattacharya S, Saluja A, Banerjee S (2018) Metastasis and chemoresistance in CD133 expressing pancreatic cancer cells are dependent on their lipid raft integrity. Cancer Lett 439:101–112. https://doi.org/10.1016/j.canlet.2018.09.028
Hall A, Lalli G (2010) Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol 2(2):a001818. https://doi.org/10.1101/cshperspect.a001818
Han C, Wang YJ, Wang YC, Guan X, Wang L, Shen LM, Zou W, Liu J (2021) Caveolin-1 downregulation promotes the dopaminergic neuron-like differentiation of human adipose-derived mesenchymal stem cells. Neural Regen Res 16(4):714–720. https://doi.org/10.4103/1673-5374.295342
Hansen CG, Nichols BJ (2010) Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20(4):177–186. https://doi.org/10.1016/j.tcb.2010.01.005
Harvey A, Caretti G, Moresi V, Renzini A, Adamo S (2019) Interplay between metabolites and the epigenome in regulating embryonic and adult stem cell potency and maintenance. Stem Cell Rep 13(4):573–589. https://doi.org/10.1016/j.stemcr.2019.09.003
Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG (2008) PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132(1):113–124. https://doi.org/10.1016/j.cell.2007.11.042
Hsu EC, Kulp SK, Huang HL, Tu HJ, Salunke SB, Sullivan NJ, Sun D, Wicha MS, Shapiro CL, Chen CS (2015) Function of integrin-linked kinase in modulating the stemness of IL-6-abundant breast cancer cells by regulating γ-secretase-mediated Notch1 activation in caveolae. Neoplasia 17(6):497–508. https://doi.org/10.1016/j.neo.2015.06.001
Jang I, Beningo KA (2019) Integrins CAFs and mechanical forces in the progression of cancer. Cancers. https://doi.org/10.3390/cancers11050721
Jasmin JF, Yang M, Iacovitti L, Lisanti MP (2009) Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle 8(23):3978–3983. https://doi.org/10.4161/cc.8.23.10206
Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, Kim SJ, An JY, Lee Y, Choi Y, Raj A, Cho SG, Vellingiri B (2020) Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB Rep 53(8):400–412. https://doi.org/10.5483/BMBRep.2020.53.8.121
Kaler J, Hussain A, Haque A, Naveed H, Patel S (2020) A comprehensive review of pharmaceutical and surgical interventions of prostate cancer. Cureus 12(11):e11617. https://doi.org/10.7759/cureus.11617
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science. https://doi.org/10.1126/science.aau6977
Kaushik K, Das A (2019) Endothelial progenitor cell therapy for chronic wound tissue regeneration. Cytotherapy 21(11):1137–1150. https://doi.org/10.1016/j.jcyt.2019.09.002
Khaddour K, Hana CK, Mewawalla P (2020) Hematopoietic stem cell transplantation. StatPearls Publishing LLC, Treasure Island
Kim YJ, Kim JH, Kim O, Ahn EJ, Oh SJ, Akanda MR, Oh IJ, Jung S, Kim KK, Lee JH, Kim HS, Kim H, Lee KH, Moon KS (2019) Caveolin-1 enhances brain metastasis of non-small cell lung cancer, potentially in association with the epithelial-mesenchymal transition marker SNAIL. Cancer Cell Int 19:171. https://doi.org/10.1186/s12935-019-0892-0
Kobayashi T, Kageyama R (2020) Lysosomes and signaling pathways for maintenance of quiescence in adult neural stem cells. FEBS J. https://doi.org/10.1111/febs.15555
Kocaadam B, Şanlier N (2017) Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr 57(13):2889–2895. https://doi.org/10.1080/10408398.2015.1077195
Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, Giraudo C, Le A, Shi S (2018) The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aai8524
Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM (2015) Cavin family proteins and the assembly of caveolae. J Cell Sci 128(7):1269–1278. https://doi.org/10.1242/jcs.167866
Lajoie P, Nabi IR (2010) Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol 282:135–163. https://doi.org/10.1016/s1937-6448(10)82003-9
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648. https://doi.org/10.1038/367645a0
Lee MY, Lee SH, Park JH, Han HJ (2009) Interaction of galectin-1 with caveolae induces mouse embryonic stem cell proliferation through the Src, ERas, Akt and mTOR signaling pathways. Cell Mol Life Sci 66(8):1467–1478. https://doi.org/10.1007/s00018-009-8691-8
Lee MY, Ryu JM, Lee SH, Park JH, Han HJ (2010) Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res 51(8):2082–2089. https://doi.org/10.1194/jlr.M001545
Lee SH, Lee YJ, Park SW, Kim HS, Han HJ (2011) Caveolin-1 and integrin β1 regulate embryonic stem cell proliferation via p38 MAPK and FAK in high glucose. J Cell Physiol 226(7):1850–1859. https://doi.org/10.1002/jcp.22510
Lee E, Piranlioglu R, Wicha MS, Korkaya H (2019) Plasticity and potency of mammary stem cell subsets during mammary gland development. Int J Mol Sci. https://doi.org/10.3390/ijms20092357
Li J, Hassan GS, Williams TM, Minetti C, Pestell RG, Tanowitz HB, Frank PG, Sotgia F, Lisanti MP (2005) Loss of caveolin-1 causes the hyper-proliferation of intestinal crypt stem cells, with increased sensitivity to whole body gamma-radiation. Cell Cycle 4(12):1817–1825. https://doi.org/10.4161/cc.4.12.2199
Li Y, Lau WM, So KF, Tong Y, Shen J (2011a) Caveolin-1 promote astroglial differentiation of neural stem/progenitor cells through modulating Notch1/NICD and Hes1 expressions. Biochem Biophys Res Commun 407(3):517–524. https://doi.org/10.1016/j.bbrc.2011.03.050
Li Y, Lau WM, So KF, Tong Y, Shen J (2011b) Caveolin-1 inhibits oligodendroglial differentiation of neural stem/progenitor cells through modulating β-catenin expression. Neurochem Int 59(2):114–121. https://doi.org/10.1016/j.neuint.2011.05.019
Li Y, Luo J, Lau WM, Zheng G, Fu S, Wang TT, Zeng HP, So KF, Chung SK, Tong Y, Liu K, Shen J (2011c) Caveolin-1 plays a crucial role in inhibiting neuronal differentiation of neural stem/progenitor cells via VEGF signaling-dependent pathway. PLoS ONE 6(8):e22901. https://doi.org/10.1371/journal.pone.0022901
Li Y, Zhao Y, Gao C, Wu M, So KF, Tong Y, Shen J (2020) Caveolin-1 derived from brain microvascular endothelial cells inhibits neuronal differentiation of neural stem/progenitor cells in vivo and in vitro. Neuroscience 448:172–190. https://doi.org/10.1016/j.neuroscience.2020.09.031
Lin CJ, Yun EJ, Lo UG, Tai YL, Deng S, Hernandez E, Dang A, Chen YA, Saha D, Mu P, Lin H, Li TK, Shen TL, Lai CH, Hsieh JT (2019) The paracrine induction of prostate cancer progression by caveolin-1. Cell Death Dis 10(11):834. https://doi.org/10.1038/s41419-019-2066-3
Liu YC, Yeh CT, Lin KH (2020a) Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. https://doi.org/10.3390/cells9061331
Liu Y, Fu Y, Hu X, Chen S, Miao J, Wang Y, Zhou Y, Zhang Y (2020b) Caveolin-1 knockdown increases the therapeutic sensitivity of lung cancer to cisplatin-induced apoptosis by repressing Parkin-related mitophagy and activating the ROCK1 pathway. J Cell Physiol 235(2):1197–1208. https://doi.org/10.1002/jcp.29033
Luanpitpong S, Wang L, Stueckle TA, Tse W, Chen YC, Rojanasakul Y (2014) Caveolin-1 regulates lung cancer stem-like cell induction and p53 inactivation in carbon nanotube-driven tumorigenesis. Oncotarget 5(11):3541–3554. https://doi.org/10.18632/oncotarget.1956
Ly CH, Lynch GS, Ryall JG (2020) A metabolic roadmap for somatic stem cell fate. Cell Metab 31(6):1052–1067. https://doi.org/10.1016/j.cmet.2020.04.022
Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX (2020) Mesenchymal stem cell-derived exosomes: toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells 12(8):814–840. https://doi.org/10.4252/wjsc.v12.i8.814
Maiuthed A, Bhummaphan N, Luanpitpong S, Mutirangura A, Aporntewan C, Meeprasert A, Rungrotmongkol T, Rojanasakul Y, Chanvorachote P (2018) Nitric oxide promotes cancer cell dedifferentiation by disrupting an Oct4:caveolin-1 complex: a new regulatory mechanism for cancer stem cell formation. J Biol Chem 293(35):13534–13552. https://doi.org/10.1074/jbc.RA117.000287
Makdissy N, Haddad K, AlBacha JD, Chaker D, Ismail B, Azar A, Oreibi G, Ayoub D, Achkar I, Quilliot D, Fajloun Z (2018) Essential role of ATP6AP2 enrichment in caveolae/lipid raft microdomains for the induction of neuronal differentiation of stem cells. Stem Cell Res Ther 9(1):132. https://doi.org/10.1186/s13287-018-0862-9
Margheri F, Chillà A, Laurenzana A, Serratì S, Mazzanti B, Saccardi R, Santosuosso M, Danza G, Sturli N, Rosati F, Magnelli L, Papucci L, Calorini L, Bianchini F, Del Rosso M, Fibbi G (2011) Endothelial progenitor cell-dependent angiogenesis requires localization of the full-length form of uPAR in caveolae. Blood 118(13):3743–3755. https://doi.org/10.1182/blood-2011-02-338681
Martínez-Cerdeño V, Noctor SC (2018) Neural progenitor cell terminology. Front Neuroanat 12:104. https://doi.org/10.3389/fnana.2018.00104
Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F (2010) Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle 9(12):2423–2433. https://doi.org/10.4161/cc.9.12.12048
Mathews LA, Cabarcas SM, Farrar WL (2011) DNA repair: the culprit for tumor-initiating cell survival? Cancer Metastasis Rev 30(2):185–197. https://doi.org/10.1007/s10555-011-9277-0
Megaloikonomos PD, Panagopoulos GN, Bami M, Igoumenou VG, Dimopoulos L, Milonaki A, Kyriakidou M, Mitsiokapa E, Anastassopoulou J, Mavrogenis AF (2018) Harvesting, isolation and differentiation of rat adipose-derived stem cells. Curr Pharm Biotechnol 19(1):19–29. https://doi.org/10.2174/1389201019666180418101323
Nassoy P, Lamaze C (2012) Stressing caveolae new role in cell mechanics. Trends Cell Biol 22(7):381–389. https://doi.org/10.1016/j.tcb.2012.04.007
Nimmakayala RK, Batra SK, Ponnusamy MP (2019) Unraveling the journey of cancer stem cells from origin to metastasis. Biochim Biophys Acta Rev Cancer 1871(1):50–63. https://doi.org/10.1016/j.bbcan.2018.10.006
Nogueira ML, da Veiga MJ, Baronzio GF, Dubois B, Steyaert JM, Schwartz L (2015) Mechanical stress as the common denominator between chronic inflammation, cancer, and Alzheimer’s disease. Front Oncol 5:197. https://doi.org/10.3389/fonc.2015.00197
Olbromski M, Podhorska-Okołów M, Dzięgiel P (2020) Role of SOX protein groups F and H in lung cancer progression. Cancers. https://doi.org/10.3390/cancers12113235
Othman FA, Tan SC (2020) Preconditioning strategies to enhance neural stem cell-based therapy for ischemic stroke. Brain Sci. https://doi.org/10.3390/brainsci10110893
Park JH, Han HJ (2009) Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol 297(4):C935-944. https://doi.org/10.1152/ajpcell.00121.2009
Park JS, Kim HY, Kim HW, Chae GN, Oh HT, Park JY, Shim H, Seo M, Shin EY, Kim EG, Park SC, Kwak SJ (2005) Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech Ageing Dev 126(5):551–559. https://doi.org/10.1016/j.mad.2004.11.014
Park JH, Lee MY, Han HJ (2009) A potential role for caveolin-1 in estradiol-17beta-induced proliferation of mouse embryonic stem cells: involvement of Src, PI3K/Akt, and MAPKs pathways. Int J Biochem Cell Biol 41(3):659–665. https://doi.org/10.1016/j.biocel.2008.07.010
Park JH, Ryu JM, Han HJ (2011) Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol 226(1):267–275. https://doi.org/10.1002/jcp.22338
Park JH, Ryu JM, Yun SP, Kim MO, Han HJ (2012) Fibronectin stimulates migration through lipid raft dependent NHE-1 activation in mouse embryonic stem cells: involvement of RhoA, Ca(2+)/CaM, and ERK. Biochim Biophys Acta 1820(10):1618–1627. https://doi.org/10.1016/j.bbagen.2012.05.013
Patel HH, Murray F, Insel PA (2008) Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48:359–391. https://doi.org/10.1146/annurev.pharmtox.48.121506.124841
Paternoster S, Falasca M (2020) The intricate relationship between diabetes, obesity and pancreatic cancer. Biochim Biophys Acta Rev Cancer 1873(1):188326. https://doi.org/10.1016/j.bbcan.2019.188326
Peffer ME, Chandran UR, Luthra S, Volonte D, Galbiati F, Garabedian MJ, Monaghan AP, DeFranco DB (2014) Caveolin-1 regulates genomic action of the glucocorticoid receptor in neural stem cells. Mol Cell Biol 34(14):2611–2623. https://doi.org/10.1128/mcb.01121-13
Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P (2016) Ciprofloxacin mediates cancer stem cell phenotypes in lung cancer cells through caveolin-1-dependent mechanism. Chem Biol Interact 250:1–11. https://doi.org/10.1016/j.cbi.2016.03.005
Qian XL, Pan YH, Huang QY, Shi YB, Huang QY, Hu ZZ, Xiong LX (2019) Caveolin-1: a multifaceted driver of breast cancer progression and its application in clinical treatment. Onco Targets Ther 12:1539–1552. https://doi.org/10.2147/ott.s191317
Qin W, Zheng Y, Qian BZ, Zhao M (2017) Prostate cancer stem cells and nanotechnology: a focus on Wnt signaling. Front Pharmacol 8:153. https://doi.org/10.3389/fphar.2017.00153
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437. https://doi.org/10.1038/nm.3394
Ramazzotti G, Ratti S, Fiume R, Follo MY, Billi AM, Rusciano I, Obeng EO, Manzoli L, Cocco L, Faenza I (2019) Phosphoinositide 3 kinase signaling in human stem cells from reprogramming to differentiation: a tale in cytoplasmic and nuclear compartments. Int J Mol Sci. https://doi.org/10.3390/ijms20082026
Rebuzzini P, Zuccotti M, Redi CA, Garagna S (2015) Chromosomal abnormalities in embryonic and somatic stem cells. Cytogenet Genome Res 147(1):1–9. https://doi.org/10.1159/000441645
Ren M, Liu F, Zhu Y, Li Y, Lang R, Fan Y, Gu F, Zhang X, Fu L (2014) Absence of caveolin-1 expression in carcinoma-associated fibroblasts of invasive micropapillary carcinoma of the breast predicts poor patient outcome. Virchows Arch 465(3):291–298. https://doi.org/10.1007/s00428-014-1614-6
Ren K, Tang J, Jiang X, Sun H, Nong L, Shen N, Gu Y (2018) Periodic mechanical stress stimulates Cav-1-dependent IGF-1R mitogenic signals in rat chondrocytes through ERK1/2. Cell Physiol Biochem 48(4):1652–1663. https://doi.org/10.1159/000492288
Rigaud VOC, Hoy R, Mohsin S, Khan M (2020) Stem cell metabolism: powering cell-based therapeutics. Cells. https://doi.org/10.3390/cells9112490
Rivera C, Rivera S, Loriot Y, Vozenin MC, Deutsch E (2011) Lung cancer stem cell: new insights on experimental models and preclinical data. J Oncol 2011:549181. https://doi.org/10.1155/2011/549181
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC (2019) Wound healing: a cellular perspective. Physiol Rev 99(1):665–706. https://doi.org/10.1152/physrev.00067.2017
Romeo F, Costanzo F, Agostini M (2012) Embryonic stem cells and inducible pluripotent stem cells: two faces of the same coin? Aging 4(12):878–886. https://doi.org/10.18632/aging.100513
Root KT, Plucinsky SM, Glover KJ (2015) Recent progress in the topology, structure, and oligomerization of caveolin: a building block of caveolae. Curr Top Membr 75:305–336. https://doi.org/10.1016/bs.ctm.2015.03.007
Rycaj K, Tang DG (2015) Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res 75(19):4003–4011. https://doi.org/10.1158/0008-5472.can-15-0798
Sasaki N, Shinomi M, Hirano K, Ui-Tei K, Nishihara S (2011) LacdiNAc (GalNAcβ1-4GlcNAc) contributes to self-renewal of mouse embryonic stem cells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells 29(4):641–650. https://doi.org/10.1002/stem.615
Semba T, Sammons R, Wang X, Xie X, Dalby KN, Ueno NT (2020) JNK signaling in stem cell self-renewal and differentiation. Int J Mol Sci. https://doi.org/10.3390/ijms21072613
Shan-Wei W, Kan-Lun X, Shu-Qin R, Li-Li Z, Li-Rong C (2012) Overexpression of caveolin-1 in cancer-associated fibroblasts predicts good outcome in breast cancer. Breast Care 7(6):477–483. https://doi.org/10.1159/000345464
Shi XY, Xiong LX, Xiao L, Meng C, Qi GY, Li WL (2016) Downregulation of caveolin-1 upregulates the expression of growth factors and regulators in co-culture of fibroblasts with cancer cells. Mol Med Rep 13(1):744–752. https://doi.org/10.3892/mmr.2015.4610
Shimizu K, Chiba S, Hori Y (2013) Identification of a novel subpopulation of tumor-initiating cells from gemcitabine-resistant pancreatic ductal adenocarcinoma patients. PLoS ONE 8(11):e81283. https://doi.org/10.1371/journal.pone.0081283
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics. CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Simón L, Campos A, Leyton L, Quest AFG (2020) Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer. Cancer Metastasis Rev 39(2):435–453. https://doi.org/10.1007/s10555-020-09890-x
Singh V, Lamaze C (2020) Membrane tension buffering by caveolae: a role in cancer? Cancer Metastasis Rev 39(2):505–517. https://doi.org/10.1007/s10555-020-09899-2
Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144(3):402–413. https://doi.org/10.1016/j.cell.2010.12.031
Skotland T, Kavaliauskiene S, Sandvig K (2020) The role of lipid species in membranes and cancer-related changes. Cancer Metastasis Rev 39(2):343–360. https://doi.org/10.1007/s10555-020-09872-z
Soleimani F, Babaei E, Feizi MAH, Fathi F (2020) CRISPR-Cas9-mediated knockout of the Prkdc in mouse embryonic stem cells leads to the modulation of the expression of pluripotency genes. J Cell Physiol 235(4):3994–4000. https://doi.org/10.1002/jcp.29295
Soman SS, Vijayavenkataraman S (2020) Applications of 3D bioprinted-induced pluripotent stem cells in healthcare. Int J Bioprint 6(4):280. https://doi.org/10.18063/ijb.v6i4.280
Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP (2005) Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell Cycle 4(12):1808–1816. https://doi.org/10.4161/cc.4.12.2198
Spill F, Reynolds DS, Kamm RD, Zaman MH (2016) Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol 40:41–48. https://doi.org/10.1016/j.copbio.2016.02.007
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Tan Z, Zhou LJ, Li Y, Cui YH, Xiang QL, Lin GP, Wang TH (2012) E2-BSA activates caveolin-1 via PI3K/ERK1/2 and lysosomal degradation pathway and contributes to EPC proliferation. Int J Cardiol 158(1):46–53. https://doi.org/10.1016/j.ijcard.2010.12.106
Teng F, Fussenegger M (2020) Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci 8(1):2003505. https://doi.org/10.1002/advs.202003505
Teo JL, Gomez GA, Weeratunga S, Davies EM, Noordstra I, Budnar S, Katsuno-Kambe H, McGrath MJ, Verma S, Tomatis V, Acharya BR, Balasubramaniam L, Templin RM, McMahon KA, Lee YS, Ju RJ, Stebhens SJ, Ladoux B, Mitchell CA, Collins BM, Parton RG, Yap AS (2020) Caveolae control contractile tension for epithelia to eliminate tumor cells. Dev Cell 54(1):75-91.e77. https://doi.org/10.1016/j.devcel.2020.05.002
Testa U, Castelli G, Pelosi E (2020) Breast cancer: a molecularly heterogenous disease needing subtype-specific treatments. Med Sci. https://doi.org/10.3390/medsci8010018
Thankamony AP, Saxena K, Murali R, Jolly MK, Nair R (2020) Cancer stem cell plasticity—a deadly deal. Front Mol Biosci 7:79. https://doi.org/10.3389/fmolb.2020.00079
Tweedell KS (2017) The adaptability of somatic stem cells: a review. J Stem Cells Regener Med 13(1):3–13. https://doi.org/10.46582/jsrm.1301002
Urueña C, Sandoval TA, Lasso P, Tawil M, Barreto A, Torregrosa L, Fiorentino S (2020) Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci Rep 10(1):19639. https://doi.org/10.1038/s41598-020-76619-9
Vainonen JP, Aboulaich N, Turkina MV, Strålfors P, Vener AV (2004) N-terminal processing and modifications of caveolin-1 in caveolae from human adipocytes. Biochem Biophys Res Commun 320(2):480–486. https://doi.org/10.1016/j.bbrc.2004.05.196
Visvader JE (2011) Cells of origin in cancer. Nature 469(7330):314–322. https://doi.org/10.1038/nature09781
Volonte D, Vyas AR, Chen C, Dacic S, Stabile LP, Kurland BF, Abberbock SR, Burns TF, Herman JG, Di YP, Galbiati F (2018) Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J Biol Chem 293(5):1794–1809. https://doi.org/10.1074/jbc.M117.815902
Wang S, Kan Q, Sun Y, Han R, Zhang G, Peng T, Jia Y (2013) Caveolin-1 regulates neural differentiation of rat bone mesenchymal stem cells into neurons by modulating notch signaling. Int J Dev Neurosci 31(1):30–35. https://doi.org/10.1016/j.ijdevneu.2012.09.004
Wang Z, Wang N, Li W, Liu P, Chen Q, Situ H, Zhong S, Guo L, Lin Y, Shen J, Chen J (2014a) Caveolin-1 mediates chemoresistance in breast cancer stem cells via β-catenin/ABCG2 signaling pathway. Carcinogenesis 35(10):2346–2356. https://doi.org/10.1093/carcin/bgu155
Wang J, Li ZH, White J, Zhang LB (2014b) Lung cancer stem cells and implications for future therapeutics. Cell Biochem Biophys 69(3):389–398. https://doi.org/10.1007/s12013-014-9844-4
Wang R, Li Z, Guo H, Shi W, Xin Y, Chang W, Huang T (2014c) Caveolin 1 knockdown inhibits the proliferation, migration and invasion of human breast cancer BT474 cells. Mol Med Rep 9(5):1723–1728. https://doi.org/10.3892/mmr.2014.2018
Wang D, Bu F, Zhang W (2019a) The role of ubiquitination in regulating embryonic stem cell maintenance and cancer development. Int J Mol Sci. https://doi.org/10.3390/ijms20112667
Wang S, Zhang Z, Almenar-Queralt A, Leem J, DerMardirossian C, Roth DM, Patel PM, Patel HH, Head BP (2019b) Caveolin-1 phosphorylation is essential for axonal growth of human neurons derived from iPSCs. Front Cell Neurosci 13:324. https://doi.org/10.3389/fncel.2019.00324
Wang Y, Jiang Z, Yu M, Yang G (2020a) Roles of circular RNAs in regulating the self-renewal and differentiation of adult stem cells. Differentiation 113:10–18. https://doi.org/10.1016/j.diff.2020.03.001
Wang S, Wang N, Zheng Y, Yang B, Liu P, Zhang F, Li M, Song J, Chang X, Wang Z (2020b) Caveolin-1 inhibits breast cancer stem cells via c-Myc-mediated metabolic reprogramming. Cell Death Dis 11(6):450. https://doi.org/10.1038/s41419-020-2667-x
Williams TM, Lisanti MP (2004) The caveolin proteins. Genome Biol 5(3):214. https://doi.org/10.1186/gb-2004-5-3-214
Williams TM, Lisanti MP (2005) Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol 288(3):C494-506. https://doi.org/10.1152/ajpcell.00458.2004
Wong TH, Dickson FH, Timmins LR, Nabi IR (2020) Tyrosine phosphorylation of tumor cell caveolin-1: impact on cancer progression. Cancer Metastasis Rev 39(2):455–469. https://doi.org/10.1007/s10555-020-09892-9
Yadav P, Vats R, Bano A, Bhardwaj R (2020) Hematopoietic stem cells culture, expansion and differentiation: an insight into variable and available media. Int J Stem Cells. https://doi.org/10.15283/ijsc19157
Yamaguchi T, Lu C, Ida L, Yanagisawa K, Usukura J, Cheng J, Hotta N, Shimada Y, Isomura H, Suzuki M, Fujimoto T, Takahashi T (2016) ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nat Commun 7:10060. https://doi.org/10.1038/ncomms10060
Yan F, Su L, Chen X, Wang X, Gao H, Zeng Y (2020) Molecular regulation and clinical significance of caveolin-1 methylation in chronic lung diseases. Clin Transl Med 10(1):151–160. https://doi.org/10.1002/ctm2.2
Yanagisawa M, Yu RK (2007) The expression and functions of glycoconjugates in neural stem cells. Glycobiology 17(7):57r–74r. https://doi.org/10.1093/glycob/cwm018
Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K (2019a) Epidermal stem cells in wound healing and their clinical applications. Stem Cell Res Ther 10(1):229. https://doi.org/10.1186/s13287-019-1312-z
Yang R, Wang J, Zhou Z, Qi S, Ruan S, Lin Z, Xin Q, Lin Y, Chen X, Xie J (2019b) Role of caveolin-1 in epidermal stem cells during burn wound healing in rats. Dev Biol 445(2):271–279. https://doi.org/10.1016/j.ydbio.2018.11.015
Yang R, Wang J, Zhou Z, Qi S, Ruan S, Lin Z, Xin Q, Lin Y, Chen X, Xie J (2019c) Curcumin promotes burn wound healing in mice by upregulating caveolin-1 in epidermal stem cells. Phytother Res 33(2):422–430. https://doi.org/10.1002/ptr.6238
Yang C, He B, Dai W, Zhang H, Zheng Y, Wang X, Zhang Q (2021) The role of caveolin-1 in the biofate and efficacy of anti-tumor drugs and their nano-drug delivery systems. Acta Pharm Sin B 11(4):961–977. https://doi.org/10.1016/j.apsb.2020.11.020
Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P (2015) Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am J Physiol Cell Physiol 308(2):C89-100. https://doi.org/10.1152/ajpcell.00187.2014
Yun SP, Ryu JM, Kim MO, Park JH, Han HJ (2012) Rapid actions of plasma membrane estrogen receptors regulate motility of mouse embryonic stem cells through a profilin-1/cofilin-1-directed kinase signaling pathway. Mol Endocrinol 26(8):1291–1303. https://doi.org/10.1210/me.2012-1002
Yun SP, Lee SJ, Jung YH, Han HJ (2014) Galectin-1 stimulates motility of human umbilical cord blood-derived mesenchymal stem cells by downregulation of smad2/3-dependent collagen 3/5 and upregulation of NF-κB-dependent fibronectin/laminin 5 expression. Cell Death Dis 5(2):e1049. https://doi.org/10.1038/cddis.2014.3
Zanotti S, Canalis E (2016) Notch signaling and the skeleton. Endocr Rev 37(3):223–253. https://doi.org/10.1210/er.2016-1002
Zhai L, Liu Y, Zhao W, Chen Q, Guo T, Wei W, Luo Z, Huang Y, Ma C, Huang F, Dai X (2020) Aerobic and resistance training enhances endothelial progenitor cell function via upregulation of caveolin-1 in mice with type 2 diabetes. Stem Cell Res Ther 11(1):10. https://doi.org/10.1186/s13287-019-1527-z
Zhao Y, Pang Q, Liu M, Pan J, Xiang B, Huang T, Tu F, Liu C, Chen X (2017) Treadmill exercise promotes neurogenesis in ischemic rat brains via caveolin-1/VEGF signaling pathways. Neurochem Res 42(2):389–397. https://doi.org/10.1007/s11064-016-2081-z
Zheng W, Li J, Wang X, Yuan Y, Zhang J, Xiu Z (2018) Effects of Antarctic krill docosahexaenoic acid on MCF-7 cell migration and invasion induced by the interaction of CD95 with caveolin-1. Life Sci 192:270–277. https://doi.org/10.1016/j.lfs.2017.11.011
Zhu J, Wang S, Chen Y, Li X, Jiang Y, Yang X, Li Y, Wang X, Meng Y, Zhu M, Ma X, Huang C, Wu R, Xie C, Geng S, Wu J, Zhong C, Han H (2017) miR-19 targeting of GSK3β mediates sulforaphane suppression of lung cancer stem cells. J Nutr Biochem 44:80–91. https://doi.org/10.1016/j.jnutbio.2017.02.020
Acknowledgements
We would like to thank the English editing support by Wanting Yi and Yubo Shi.
Funding
This work was supported by the National Natural Science Foundation of China, Major Research Plan (NSFC No. 31860317) and the National natural science foundation of Jiangxi province (No. 20202BAB206056), as well as the college students innovation training program of Nanchang University (No. 20190402024, No. 20190402025).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Yes.
Consent for publication
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, X., Guo, Y., Chen, M. et al. Caveolin1: its roles in normal and cancer stem cells. J Cancer Res Clin Oncol 147, 3459–3475 (2021). https://doi.org/10.1007/s00432-021-03793-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03793-2