Abstract
Mesenchymal stem cells (MSCs), also known as multipotent mesenchymal stromal stem cells, are found in the perivascular space of several tissues. These cells have been subject of intense research in the last decade due to their low teratogenicity, as well as their ability to differentiate into mature cells and to secrete immunomodulatory and trophic factors. However, they usually promote only a modest benefit when transplanted in experimental disease models, one of the limitations for their clinical application. The CRISPR-Cas system, in turn, is highlighted as a simple and effective tool for genetic engineering. This system was tested in clinical trials over a relatively short period of time after establishing its applicability to the edition of the mammalian cell genome. Similar to the research evolution in MSCs, the CRISPR-Cas system demonstrated inconsistencies that limited its clinical application. In this review, we outline the evolution of MSC research and its applicability, and the progress of the CRISPR-Cas system from its discovery to the most recent clinical trials. We also propose perspectives on how the CRISPR-Cas system may improve the therapeutic potential of MSCs, making it more beneficial and long lasting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mesenchymal Stem Cells and their Therapeutic Potential
Mesenchymal stem cells (MSCs), also called mesenchymal stromal cells, are multipotent cells located throughout the body and coupled to the blood vessels walls [1] . These medicinal signaling cells [2] can be found in almost all organism tissue types, such as bone marrow, adipose tissue, dental pulp, among other sources [3,4,5,6]. According to The International Society for Cellular Therapy, a cell defined as a MSC must show the ability to adhere to the plastic under standard culture conditions, capacity for differentiation into osteoblasts, chondrocytes and adipocytes, and it must be positive for the surface markers CD105, CD73 and CD90, and negative for CD45, CD34, CD14 or CD11b, CD79 or CD19, and HLA-DR [7,8,9] .
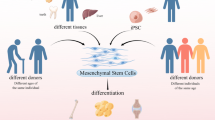
MSCs have an important differentiating capacity [3,4,5,6]. Also, they secrete a large set of paracrine chemical factors (secretome) [10] and have a remarkable homing ability, i.e., the ability to migrate to sites of tissue injury when systemically infused [11, 12] (Fig. 1). All these properties make MSCs excellent candidates for cell therapy, justifying preclinical and clinical studies involving the treatment with such cells for different types of cardiac, neurological, and bone dysfunctions, among others [13,14,15,16,17,18,19].
Mesenquimal stem-cells (MSCs): functions and differentiation process. Transdifferentiation is the ability of MSCs to differentiate in various types of cells. Stem cells respond to stimulatory and inhibitory factors which they are subjected to, and by the inhibition and/or activation of certain molecular pathways, MSCs give rise to new tissue-specific cells. Homing happens when the MSCs moves to an injured tissue endothelium atracted by the chemoattractant effect from the citokynes released by the tissue injuries. The release of paracrine factors are the main mechanisms for the MSCs therapeutic action, since these factors contribute for pro-angiogenic, anti-fibrotic, anti-apoptotic, and immunomodulatory functions. Starting from the isolation of a tissue specific MSCs – from skin, lungs, liver, placenta or adipose tissue – from mouse or human, this cells can be expanded and enriched by various methods in vitro. bFGF promotes the expansion of MSCs. During the MSCs expansion, positive markers (CD105, CD73, CD90 and others) and negative markers (CD45, CD34, CD14 ou CD11b, CD79 ou CD19, HLA-DR and others) are used to classify this cell population. In addiction to self renewel in vitro, the MSCs can differentiate between osteoblasts, chondrocytes, adipocytes and other cell types. MSCs differentiation can be enhanced by specific-lineage factors such as Sox-9 VEGF, TGF-β1, insulin, dexamethasone, acid ascorbic, β-catenin and others factors
MSCs are multipotent cells, which means they have the ability to differentiate both in vitro and in vivo into several specific cell types. In addition to their typical ability to differentiate into chondrogenic, adipogenic and osteogenic lineages, studies have shown they also can differentiate into cardiomyocytes [20], hepatocytes [21], steroidogenic cells [22], skeletal muscle cells [23] smooth muscle cells [24], motor neurons [25], endothelial cells [26], among other cell types. These transdifferentiation ability is highly controversial both in vitro and in vivo, with several studies suggesting that the phenomenon occurs, whereas many others demonstrate it does not [3, 4]. As examples of well-succeeded transdifferentiations, the work of Mendivil-Perez et al. [5] can be highlighted, where human Wharton’s jelly MSCs were differentiated into cholinergic-like neurons after incubation with Cholinergic-N-Run medium for 4 days [5]. These controversies with regard to MSCs transdifferentiation will be clarified when the cell transdifferentiation phenomenon, as well as the intermediate differentiation states, the epigenetic factors and the signaling pathways of this process are better understood.
The differentiation capacity of MSCs depends both on intrinsic cell conditions and on the microenvironment to which they belong. Therefore, variations in differentiation rates may be observed between MSCs from different tissue sources [27]. During the differentiation process, stem cells respond to stimulatory and inhibitory factors to which they are subjected, and through the inhibition and/or activation of certain molecular pathways, MSCs give rise to new tissue-specific cells [28]. The comprehension of the precise differentiation process allows the manipulation and control of important molecular pathways, which may be relevant for cell therapy.
One of the main properties of MSCs regarding their use for cell therapy is their ability to secrete a wide range of bioactive factors [29, 30]. This paracrine function would represent the main mechanism for the therapeutic action of the MSCs. The released factors, acting in a paracrine way on the microenvironment, would contribute to pro-angiogenic, anti-fibrotic, anti-apoptotic, and immunomodulatory functions [27, 29, 31, 32].
When systemically injected, MSCs respond to the cytokines secreted by injured tissues. As a consequence of the chemoattractant effect, MSCs move to these sites [33, 34]. When in the injured tissue, the cells make connections through their receptors for chemokines, extracellular matrix proteins and endothelial cells ligands [35, 36]. These connections contribute to MSCs transmigration through the endothelium, characterizing their homing ability [35, 36]. When stablished in the tissue, the MScs could interact with the resident cells, promoting or intensifying therapeutic effects, and this idea is reinforced by the ability of these cells to maintain the tissue stem cell stemness, as already described [6].
Although encouraging results have been observed when using MSCs to treat several diseases [13, 15, 17], some studies have still shown a few frustrating data. Considering the MSCs notorious therapeutic potential, some alternatives can be explored to optimize their capacity of generating beneficial effects in the treatment of many dysfunction types. However, what alternative could be used to intensify the differentiation capacity, the effects of the secretome and the homing ability of such cells? Until recently, this question would be lost in many different answers which would not lead to significant results. Nonetheless, biomedical science has acquired a valuable genetic editing tool that certainly applies to MSCs: the CRISPR technique.
CRISPR: The Discovery
Although originally discovered in 1993 [7], the CRISPR function took a long time to be fully understood. In 2007, researchers from the dairy food company Danisco (based in Copenhagen and now owned by DuPont) studying the bacteria Streptococcus thermophilus found that CRISPR groups observed in the DNA of these bacteria yield their immunity against attacks of certain bacteriophages [8]. Testing the S. thermophilus strain DGCC7710 against Phage 858 and Phage 2972 bacteriophage strains (simultaneously or separately), BARRANGOU et al. noted that the bacteria integrated new spacers into CRISPR1 locus derived from the phages DNA [8].
This fragments addition of viral DNA in the spacer regions made the bacteria resistant to one or both viral strains. In addition, bacteriophage attack resistance has been shown to be altered by adding or deleting spacer sequences containing Phage 858 and Phage 2972 DNAs.
In 2008, it was found that the transcription of CRISPR-containing loci, as well as the maturation of the CRISPR RNA (crRNA) fragment, depends on the Cas proteins (CRISPR-associated endonucleases) [5]. Therefore, this bacterial “adaptive immune system” has been called CRISPR-Cas System [9]. The CRISPR-Cas System operates in 3 phases [10].
Every time a bacteriophage DNA infects a bacterial cell, part of the viral genetic material is integrated into CRISPR sequences in the form of spacers. The spacers remain interspersed with the short repeat sequences of bacterial DNA in the CRISPR regions (adaptation phase).
The crRNA transcribed from these CRISPR loci (biogenesis phase) associates with Cas9 protein, a nuclease enzyme capable of cleaving DNA [11]. After that, the CrRNA-Cas9 associates with the trans-activating CRISPR RNA (tracrRNA), transcribed from an sequence adjacent to CRISPR. The tracrRNA and crRNA are combined to form a single strand called the guide RNA (gRNA) during the assembly of CRISPR-Cas system [12]. Due to gRNA complementarity to the sequence containing viral genome in the DNA, CRISPR-Cas is accurately bound to the target (targeting phase). The Cas enzyme opens the double strand of the DNA in the region where gRNA is bound and extracts the invading viral DNA [13]. (Fig. 2).
CRISPR immunity: acquisition, crDNA biogenesis and targeting. CRISPR loci’s clusters of repeats (black diamonds) and spacers (colored boxes) flanked by L (leader sequence) and CRISPR associated genes (cas). Following a unknown mechanism, in adaptation phase, the virus incorpores new spacers from his genome on the CRISPR array. The synthesis of a new repeat is also required. The crRNA transcribed from these CRISPR loci (biogenesis phase) associates with the Cas9 protein, a nuclease enzyme capable of cleaving DNA, and then the CrRNA-Cas9 associates to the trans-activating CRISPR RNA (tracrRNA), transcribed from an adjacent sequence to CRISPR. During the assembly of the CRISPR-Cas system, the tracrRNA and the crRNA combine to form a single strand called the guiding RNA (gRNA). Due to gRNA complementarity to the sequence containing the viral genome in the DNA, CRISPR-Cas binds accurately to the target (targeting phase)
The CRISPR system is divided into two major classes (which in turn present subclasses): class I, which contains multiple effector subunits; and class II, which depends on single effector proteins [14]. The class II mechanism was the one previously described in this article. The CRISPR-Cas9 system, for instance, belongs to class II, and it is the most studied in CRISPR genetic engineering. This class II system was developed from S. thermophilus [15] and S. pyogenes [12].
Genetic Engineering through the CRISPR-Cas System
Based on an article of Marraffini and Sontheimer [16] – who clarified that CRISPR-Cas system includes a programmable restriction enzyme – it was possible to observe that such system might be the most efficient tool for gene editing [17]. Certainly, this finding explains the boom in publications involving CRISPR from 2014 to 2016 [18]. However, the genome editing of human and mammalian cells with CRISPR was only achieved in 2013 [19,20,21,22].
Unlike other genetic engineering technologies, such as site-directed zinc finger nucleases (ZFNs) and transcription activated-like effector nucleases (TALENS), CRISPR technology is affordable, efficient and can efficiently act on multiple gene targets (80% or more) [11, 23,24,25]. Therefore, the CRISPR-Cas system would be safer for future clinical applications and it can present several other advantages when compared to the other techniques [26, 27].
In order to use this new technology, a guide RNA (gRNA) – complementary to the DNA sequence to be modified – must be designed a priori. This gRNA will direct the gRNA-Cas complex to the DNA target region. The gRNA-Cas causes a double-strand DNA break in the locus to be modified. After this break, the genome edition can possibly occur through the non-homologous end joining (NHEJ) or through the homology directed repair (HDR). The NHEJ pathway usually leads to gene silencing by the deletion of a target gene. On the other hand, the HDR pathway occurs in the presence of a repair model and it results in the insertion/correction of a gene of interest [11, 25]. As noted by SANDER and JOUNG [25], HDR mediated repair can be used to introduce specific point mutations or to insert desired sequences by recombining the target locus with an exogenous model strand. The homologous pathway usually occurs naturally in cells that are being divided, and the efficiency of this pathway a lot depending on the cellular type and conditions [23].
Finally, it is observed that the ability to make highly accurate modifications in the DNA (involving sequence change, deletion or insertion) makes possible to use CRISPR-Cas system for therapeutic purposes in many levels, from a cell to an entire organism [22, 28].
Therapeutic Applications of the CRISPR-Cas System
Gene therapy represents an important and promising tool for the effective treatment and cure of monogenic diseases by correcting the underlying genetic cause. The CRISPR-Cas system has emerged as a very promising tool for gene therapy by enabling accurate genome editing. The use of such methodology could include, for example, removing cells from an unhealthy individual, editing the genome of injured cells through the CRISPR-Cas system, and reintroducing the edited cells back into the body via autologous transplantation. This would make possible to replace injured cells and correct the disease. In addition to its use in gene therapy, CRISPR-Cas system may be used for disease modeling, drug screening studies, and specific diseases diagnosis [29].
Several studies have been carried out on the use of the CRISPR-Cas system for the treatment of different diseases, both in animal and human models (Table 1) [27, 29, 30]. Such studies have shown great progress in the application of CRISPR-Cas system in preclinical disease models and in vitro culture of human cells. This is the reason for their transition to therapeutic use and the development of clinical trials (Table 2). However, evidence from animal models is not fully sufficient to ensure that patients undergoing this approach are not exposed to risks without potential benefit [27, 30].
The genome editing through the CRISPR-Cas system shows great promise for therapeutic applications, although several questions still need to be answered before its use in clinical practice. First of all, some ethical issues could be raised regarding the genetic manipulation of cells, such as the extent to which CRISPR use should be permitted, the establishment of a regulatory policy for clinical research involving human subjects in order to accommodate this modality of genome editing, and the issue of genetic editing of germline cells [31]. Some of the technical issues to be addressed include events possibly occurring outside the target sequences [27] and the effective in vivo distribution of the genome-editing enzymes to the cells in need of correction [29]. The most common strategies for this distribution include the use of viral vectors and lipid nanoparticles, among others. Also, the choice of the best method should consider the tissue that needs correction, the route of administration, and the type of editing needed [29]. Thus, there is a need of optimizing this technology prior to its use in clinical routine, especially in relation to its effectiveness, safety and specificity [32].
Genetic Engineering of MSCs through CRISPR-Cas 9 System
Osteoblasts, chondrocytes, and adipocytes are just some of the cell types in which MSCs have proven to be able to be differentiated [45]. Among the many cell types originated from MSCs, myocardiocytes [46], hepatocytes [47, 48] pancreatic cells [49] and neurons and glial cells [50, 51] have already been described. For the occurrence of the differentiation process, MSCs need to be maintained in a suitable culture medium and be subjected to the addition of certain hormones and growth factors according to the cell type to be reached.
Due to the diversity of cell types already described and originated by MSCs, they have been employed in a large number of therapeutic studies involving a wide range of diseases. Some of them include muscular dystrophy [52,52,54], bone defects [55,55,57], myocardial infarction [58,58,60], liver diseases [61,61,63], diabetes mellitus type 1 [64,64,66], spinal cord injuries [67,67,68,70], Parkinson’s disease [71,71,73], cancer [74,74,75,77], and many other conditions. The therapeutic effect of MSCs, within the scope of their multipotentiality, is based on their integration into the target tissue by replacing the diseased cells, with no generation of an immune response [78]. Apart from the therapeutic potential and the progress in the clinical employment of the MSCs, the poor engraftment and the low survival rate of the MSCs in the organ receiving the transplant are among the main obstacles to be surpassed so that the cellular therapy with MSCs brings significant benefits [79].
Works using genome-editing in human cells with CRISPR/Cas9 suggest how this technology can be applied to the MSCs, for instance, interrupting the self-renewal and committing these cells with certain lineage [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,83] – which would contribute to increase the engraftment. In order to increase therapeutic capacity through MSCs differentiation using the CRISPR-Cas9 system, it is necessary to interfere in certain points of the differentiation process by directing them according to the therapeutic objective. PAM (Protospacer Adjacent Motifs) sequences are DNA sequences containing on average 4 base pairs followed by two guanosines and located at the 5 ‘end of the CRISPR-Cas system target sequence. Apparently, almost all loci adjacent to the PAM sequences can be edited by this system, provided that a specific gRNA is present. Therefore, it is reasonable to believe that virtually all genes are editable by CRISPR-Cas, given the frequency of PAMs in the genomes of several species – including the human species [80].
As previously discussed in this article, the genetic editing through class II of CRISPR-Cas (in which Cas9 acts) has been the most used. This edition occurs with the intracellular insertion and/or expression of Cas9 and the gRNA designed according to the target sequence – jointly called RGN (RNA-guided nucleases). This insertion/expression occurs through electroporation, nucleofection, lentiviral vectors or by lipofectamine mediated transfection [21, 24, 25, 79, 81]. In addition, so the RGN can perform the editing, the MSC can be subjected to plasmids viral transfection containing the coding sequence of the CRISPR-Cas9 system, or the gRNA and Cas9 can be singly inserted [18].
Once expressed within the MSC, the RGN can act in several ways. According to the purpose of the genetic engineering, in addition to deletion (knockout via the NHEJ path) and insertion (knock-in, via HDR having an exogenous template tape), the CRISPR-Cas system can be used to promote rearrangements, gene expression activation/increment, or even histones modification [25]. Consequently, it also can influence the transcription speed/rate of a given locus (Fig. 3).
Genetic engineering through the CRISPR-Cas system and Cas applications. (A) First of all, a guiding RNA (gRNA), complementary to the DNA sequence to be modified, must be designed. This gRNA will direct the gRNA-Cas complex to the DNA target region, complementary to its sequence of nitrogenous base pairs. The gRNA-Cas causes a double-strand DNA break. The genomic edition can occur through the non homologous end joining (NHEJ), producing variable length mutation (insertion or deletion); or through the homology directed repair (HDR), promoting precise length mutation. Cas applications: (B) Indel mutations. (C) Specific sequence insertion or replacement. (D) Large deletions or rearrangements. (E) Gene activation (reversible activation of coding/non-coding genes). (F) Histone modifications (transcription speed/rate of a given locus)
For the occurrence of the differentiation, MSCs must commit to a specific lineage: by transforming themselves into progenitor cells of that lineage. This commitment depends on physical (extracellular matrix components and mechanical forces, for example); chemical (such as signaling molecules); and biological factors (such as cellular metabolism) [82, 83].
After being committed with a lineage, progenitor cells originated in MSCs must undergo a maturation process which results in differentiated cells and therefore adapted to a specific histological niche. During this differentiation process, several genes, micro RNAs, transcription factors and signaling pathways are activated or repressed according to the lineage to which MSCs have committed [83].
Once the differentiation starts, transcription factors necessary for the process are expressed [84]. For example, Sox9 is the main transcription factor involved in the MSCs differentiating ability into chondrocytes [85], although other genes also play important roles. Regarding the generation of osteoblasts, several transcription factors have been described as differentiation regulators, such as Runx2, osterix, and β-catenin [84]. Likewise, many transcription factors have already been studied and reported as fundamental for adipogenic differentiation, and the PPARy is among them [86].
The CRISPR-Cas9 system has already been used in association with stem cells, with the purpose of treating several dysfunctions [87,87,89]. For β-thalassemia, the use of the CRISPR-Cas9 system in induced pluripotent stem cells (iPS) caused a correction of the gene involved in the disease, the β-globin gene (HBB) [83]. Also, it led to an increase in the rate of hematopoietic differentiation [89]. Thus, the study of the genes involved in the MSCs (multipotent stem cells and therefore therapeutically safer than the iPS) differentiation into specific cells and the genetic manipulation through the CRISPR-Cas9 technique represent a way of magnifying its potential as a cell therapy.
Regarding the homing and the differentiation ability, it is well documented in the literature that most of the therapeutic effects of MSCs comes from their secretome [90,90,92]. Therefore, studies aiming to enhance the MSCs therapeutic potential should always consider the crucial role of bioactive molecules secreted by such cells.
Any modifications via CRISPR-Cas9 to increase/diversify the production of the secretome constituents – pro-angiogenic, anti-fibrotic, anti-apoptotic, and immunomodulatory factors – should be adapted to each study model and clinical case. In doing so, the RGN – active enzyme of the CRISPR-Cas9 system, as already described – can be directed towards the increase of the expression of an anti-inflammatory factor gene – in the case of an arthritis, as instance [93], while in a case of acute myocardial infarction or stroke, the genes responsible for the production of antiapoptotic molecules and the angiogenic factor VEGF, among others, may have the expression increased [94,94,96].
The CRISPR technique made the secretome modulation possible. For example, one of the consequences of a stroke is the local inflammatory process with microglial activation – this neuroinflammation, along with tissue loss, is responsible for the damage of this ischemia [97]. Therefore, the secretome in a stroke should, among other effects, promote neurogenesis, angiogenesis and contain the inflammatory process [98,98,100]. This can be achieved through the secretion of factors such as VEGF, which promotes vascularization, stimulates cell survival and proliferation [101]; IGF-1, which has anti-inflammatory and antiapoptotic action [102]; and neurotrophic factors, which promote neuronal survival and neurogenesis [103, 104].
The increase on the production of some secretome components for a therapeutic effect on a stroke has already been described. A wider therapeutic efficiency was observed when submitting MSCs to a previous treatment with granulocyte colony stimulating factor (G-CSF) [105, 106]. Certainly, with the CRISPR-Cas9 technology, this increase in therapeutic effectiveness would be further enhanced, taking into account the possibilities of RGN-mediated editing.
In the end, the CRISPR-Cas may delay these cells senescence process previously to the transfusion, which besides prolonging its therapeutic effect, would improve its feasibility, also contributing to a better engraftment to the target-tissue. For this purpose, the telomere shortening can be delayed, reducing histones deacetylation, as well as the DNA methylation – alterations characteristic of the cell senescence process (111). Additionally, this genetic engineering technology can be used to favor the expression of receptor to chemokines and pro-inflammatory factors, increasing the homing and the MSCs adhesion to the target-tissue, making the intravenous administration as efficient as the intraarterial or in situ one (112) – which would prevent invasive and potentially harmful procedures with the in situ injection to the heart or brain, for instance.
Conclusion
Despite the positive results observed in several studies, the therapeutic potential of MSCs has been minimized by the relatively modest benefits they demonstrate in vivo. This fact has been attributed to the difficulty of survival and integration of the MSCs in their target-tissue [36, 107], as well as their relative low secretome production and their difficulty to adapt to certain inflammatory conditions [108]. Despite the limitations, in an advanced clinical trial, Panés et al. (2016; 2018) observed a significant improvement in perianal fistulas related to Crohn’s Disease treated with MCSs arising from adipose tissue, and this positive effect lasted 1 year after the allogeneic transplantation (115, 116). Besides, in March, 2018, the European Commission approved a drug containing MSCs, called Alofisel©, for the treatment of enterocutaneous fistula arising from Crohn’s Disease (117). Such examples show how robust is the therapeutic potential, which can be performed through genetic engineering with the CRISPR-Cas system. In turn, the paradoxical combination of simplicity and efficiency of the CRISPR-Cas9 system in genetic engineering promises to keep this technology on focus for many years, despite its limitations [25, 109]. There are several gene targets in the MSCs in which the CRISPR-Cas system can be used to enhance the therapeutic potential of these cells, for example, by enhancing their secretome and/or the survival and their migration capacity [110]. Thus, a therapeutic application of CRISPR-Cas could be carried out through MSCs. In this way, these cells would retake a prominent role in the field of regenerative medicine when compared to other types of stem cells [107].
References
da Silva Meirelles, L., Caplan, A. I., & Nardi, N. B. (2008). In search of the in vivo identity of mesenchymal stem cells. Stem Cells, 26(9), 2287–2299.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name. Stem Cells Translational Medicine., 6(6), 1445–1451.
Rose, R. A., Jiang, H., Wang, X., Helke, S., Tsoporis, J. N., Gong, N., et al. (2008). Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells, 26(11), 2884–2892.
Pijnappels, D. A., Schalij, M. J., Ramkisoensing, A. A., van Tuyn, J., de Vries, A. A., van der Laarse, A., et al. (2008). Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circulation Research., 103(2), 167–176.
Mendivil-Perez, M., Velez-Pardo, C., & Jimenez-Del-Rio, M. (2019). Direct transdifferentiation of human Wharton's jelly mesenchymal stromal cells into cholinergic-like neurons. Journal of Neuroscience Methods., 312, 126–138.
Haragopal, H., Yu, D., Zeng, X., Kim, S. W., Han, I. B., Ropper, A. E., et al. (2015). Stemness enhancement of human neural stem cells following bone marrow MSC coculture. Cell Transplantation., 24(4), 645–659.
Mojica, F. J., Juez, G., & Rodriguez-Valera, F. (1993). Transcription at different salinities of Haloferax mediterranei sequences adjacent to partially modified PstI sites. Molecular Microbiology., 9(3), 613–621.
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science., 315(5819), 1709–1712.
Brouns, S. J., Jore, M. M., Lundgren, M., Westra, E. R., Slijkhuis, R. J., Snijders, A. P., et al. (2008). Small CRISPR RNAs guide antiviral defense in prokaryotes. Science., 321(5891), 960–964.
Barrangou, R., & Marraffini, L. A. (2014). CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Molecular Cell, 54(2), 234–244.
Doudna, J. A., & Charpentier, E. (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science., 346(6213), 1258096.
Deltcheva, E., Chylinski, K., Sharma, C. M., Gonzales, K., Chao, Y., Pirzada, Z. A., et al. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature., 471(7340), 602–607.
Pennisi, E. (2013). The CRISPR craze. Science., 341(6148), 833–836.
Makarova, K. S., Wolf, Y. I., Alkhnbashi, O. S., Costa, F., Shah, S. A., Saunders, S. J., et al. (2015). An updated evolutionary classification of CRISPR-Cas systems. Nature Reviews Microbiology., 13(11), 722–736.
Bolotin, A., Quinquis, B., Sorokin, A., & Ehrlich, S. D. (2005). Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology., 151(Pt 8, 2551–2561.
Marraffini, L. A., & Sontheimer, E. J. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science., 322(5909), 1843–1845.
Lander, E. S. (2016). The heroes of CRISPR. Cell., 164(1–2), 18–28.
Chen, K. Y., & Knoepfler, P. S. (2016). To CRISPR and beyond: The evolution of genome editing in stem cells. Regenerative Medicine., 11(8), 801–816.
Cho, S. W., Kim, S., Kim, J. M., & Kim, J. S. (2013). Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnology., 31(3), 230–232.
Cong, L., Ran, F. A., Cox, D., Lin, S., Barretto, R., Habib, N., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science., 339(6121), 819–823.
Fu, Y., Foden, J. A., Khayter, C., Maeder, M. L., Reyon, D., Joung, J. K., et al. (2013). High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature Biotechnology., 31(9), 822–826.
Jinek, M., East, A., Cheng, A., Lin, S., Ma, E., & Doudna, J. (2013). RNA-programmed genome editing in human cells. eLife., 2, e00471.
Cong, L., & Zhang, F. (2015). Genome engineering using CRISPR-Cas9 system. Methods in Molecular Biology., 1239, 197–217.
Kim, S., Kim, D., Cho, S. W., Kim, J., & Kim, J. S. (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Research, 24(6), 1012–1019.
Sander, J. D., & Joung, J. K. (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology., 32(4), 347–355.
Maeder, M. L., & Gersbach, C. A. (2016). Genome-editing Technologies for Gene and Cell Therapy. Molecular Therapy : the Journal of the American Society of Gene Therapy., 24(3), 430–446.
Mollanoori, H., & Teimourian, S. (2018). Therapeutic applications of CRISPR/Cas9 system in gene therapy. Biotechnology Letters, 40(6), 907–914.
Jiang, F., & Doudna, J. A. (2017). CRISPR-Cas9 structures and mechanisms. Annual Review of Biophysics, 46, 505–529.
Foss, D. V., Hochstrasser, M. L., & Wilson, R. C. (2019). Clinical applications of CRISPR-based genome editing and diagnostics. Transfusion., 59, 1389–1399.
Baylis, F., & McLeod, M. (2017). First-in-human phase 1 CRISPR gene editing Cancer trials: Are we ready? Current Gene Therapy., 17(4), 309–319.
Brokowski, C., & Adli, M. (2019). CRISPR ethics: Moral considerations for applications of a powerful tool. Journal of Molecular Biology., 431(1), 88–101.
Martinez-Lage, M., Puig-Serra, P., Menendez, P., Torres-Ruiz, R., & Rodriguez-Perales, S. (2018). CRISPR/Cas9 for Cancer therapy: Hopes and challenges. Biomedicines., 6(4).
Soppe, J. A., & Lebbink, R. J. (2017). Antiviral Goes viral: Harnessing CRISPR/Cas9 to combat viruses in humans. Trends in Microbiology., 25(10), 833–850.
Xie, C., Zhang, Y. P., Song, L., Luo, J., Qi, W., Hu, J., et al. (2016). Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Research., 26(10), 1099–1111.
Liu, Y., Yang, Y., Kang, X., Lin, B., Yu, Q., Song, B., et al. (2017). One-step Biallelic and Scarless correction of a beta-thalassemia mutation in patient-specific iPSCs without drug selection. Molecular Therapy Nucleic acids., 6, 57–67.
Park, C. Y., Kim, D. H., Son, J. S., Sung, J. J., Lee, J., Bae, S., et al. (2015). Functional correction of large factor VIII gene chromosomal inversions in hemophilia a patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell, 17(2), 213–220.
Chang, C. W., Lai, Y. S., Westin, E., Khodadadi-Jamayran, A., Pawlik, K. M., Lamb, L. S., Jr., et al. (2015). Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting. Cell Reports, 12(10), 1668–1677.
Pankowicz, F. P., Barzi, M., Legras, X., Hubert, L., Mi, T., Tomolonis, J. A., et al. (2016). Reprogramming metabolic pathways in vivo with CRISPR/Cas9 genome editing to treat hereditary tyrosinaemia. Nature Communications, 7, 12642.
Koo, T., Yoon, A. R., Cho, H. Y., Bae, S., Yun, C. O., & Kim, J. S. (2017). Selective disruption of an oncogenic mutant allele by CRISPR/Cas9 induces efficient tumor regression. Nucleic Acids Research., 45(13), 7897–7908.
Li, H. L., Fujimoto, N., Sasakawa, N., Shirai, S., Ohkame, T., Sakuma, T., et al. (2015). Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports., 4(1), 143–154.
Wang, L., Yi, F., Fu, L., Yang, J., Wang, S., Wang, Z., et al. (2017). CRISPR/Cas9-mediated targeted gene correction in amyotrophic lateral sclerosis patient iPSCs. Protein & Cell., 8(5), 365–378.
Firth, A. L., Menon, T., Parker, G. S., Qualls, S. J., Lewis, B. M., Ke, E., et al. (2015). Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs. Cell Reports, 12(9), 1385–1390.
Hainzl, S., Peking, P., Kocher, T., Murauer, E. M., Larcher, F., Del Rio, M., et al. (2017). COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Molecular Therapy : the Journal of the American Society of Gene Therapy., 25(11), 2573–2584.
Yu W, Mookherjee S, Chaitankar V, Hiriyanna S, Kim JW, Brooks M, et al. (2017) Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun [Internet], 8, 1–15. Available from: https://doi.org/10.1038/ncomms14716.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science., 284(5411), 143–147.
Lu, D. F., Yao, Y., Su, Z. Z., Zeng, Z. H., Xing, X. W., He, Z. Y., et al. (2014). Downregulation of HDAC1 is involved in the cardiomyocyte differentiation from mesenchymal stem cells in a myocardial microenvironment. PLoS One, 9(4), e93222.
Khanjani, S., Khanmohammadi, M., Zarnani, A. H., Talebi, S., Edalatkhah, H., Eghtesad, S., et al. (2015). Efficient generation of functional hepatocyte-like cells from menstrual blood-derived stem cells. Journal of Tissue Engineering and Regenerative Medicine., 9(11), E124–E134.
Lee, K. D., Kuo, T. K., Whang-Peng, J., Chung, Y. F., Lin, C. T., Chou, S. H., et al. (2004). In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology., 40(6), 1275–1284.
Zanini, C., Bruno, S., Mandili, G., Baci, D., Cerutti, F., Cenacchi, G., et al. (2011). Differentiation of mesenchymal stem cells derived from pancreatic islets and bone marrow into islet-like cell phenotype. PLoS One, 6(12), e28175.
Tohill, M., Mantovani, C., Wiberg, M., & Terenghi, G. (2004). Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neuroscience Letters, 362(3), 200–203.
Tropel, P., Platet, N., Platel, J. C., Noel, D., Albrieux, M., Benabid, A. L., et al. (2006). Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells, 24(12), 2868–2876.
Di Rocco, G., Iachininoto, M. G., Tritarelli, A., Straino, S., Zacheo, A., Germani, A., et al. (2006). Myogenic potential of adipose-tissue-derived cells. Journal of cell science., 119(Pt 14), 2945–2952.
Goudenege, S., Pisani, D. F., Wdziekonski, B., Di Santo, J. P., Bagnis, C., Dani, C., et al. (2009). Enhancement of myogenic and muscle repair capacities of human adipose-derived stem cells with forced expression of MyoD. Molecular therapy : the Journal of the American Society of Gene Therapy., 17(6), 1064–1072.
Rajput, B. S., Chakrabarti, S. K., Dongare, V. S., Ramirez, C. M., & Deb, K. D. (2015). Human umbilical cord mesenchymal stem cells in the treatment of Duchenne muscular dystrophy: Safety and feasibility study in India. Journal of Stem Cells., 10(2), 141–156.
Hattori, H., Sato, M., Masuoka, K., Ishihara, M., Kikuchi, T., Matsui, T., et al. (2004). Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells, Tissues, Organs., 178(1), 2–12.
Morrison, D. A., Kop, A. M., Nilasaroya, A., Sturm, M., Shaw, K., & Honeybul, S. (2018). Cranial reconstruction using allogeneic mesenchymal stromal cells: A phase 1 first-in-human trial. Journal of Tissue Engineering and Regenerative Medicine., 12(2), 341–348.
Thesleff, T., Lehtimaki, K., Niskakangas, T., Mannerstrom, B., Miettinen, S., Suuronen, R., et al. (2011). Cranioplasty with adipose-derived stem cells and biomaterial: A novel method for cranial reconstruction. Neurosurgery., 68(6), 1535–1540.
Bel, A., Planat-Bernard, V., Saito, A., Bonnevie, L., Bellamy, V., Sabbah, L., et al. (2010). Composite cell sheets: A further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation., 122(11 Suppl), S118–S123.
Cai, M., Shen, R., Song, L., Lu, M., Wang, J., Zhao, S., et al. (2016). Bone marrow mesenchymal stem cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Scientific Reports, 6, 28250.
Valina, C., Pinkernell, K., Song, Y. H., Bai, X., Sadat, S., Campeau, R. J., et al. (2007). Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. European Heart Journal., 28(21), 2667–2677.
Kholodenko, I. V., & Yarygin, K. N. (2017). Cellular mechanisms of liver regeneration and cell-based therapies of liver diseases. BioMed Research International., 2017, 8910821.
Liang, J., Zhang, H., Zhao, C., Wang, D., Ma, X., Zhao, S., et al. (2017). Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. International Journal of Rheumatic Diseases., 20(9), 1219–1226.
Zhang, Y., Li, Y., Zhang, L., Li, J., & Zhu, C. (2018). Mesenchymal stem cells: Potential application for the treatment of hepatic cirrhosis. Stem Cell Research & Therapy., 9(1), 59.
Kajiyama, H., Hamazaki, T. S., Tokuhara, M., Masui, S., Okabayashi, K., Ohnuma, K., et al. (2010). Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. The International Journal of Developmental Biology., 54(4), 699–705.
Lin, G., Wang, G., Liu, G., Yang, L. J., Chang, L. J., Lue, T. F., et al. (2009). Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells and Development., 18(10), 1399–1406.
Moreira, A., Kahlenberg, S., & Hornsby, P. (2017). Therapeutic potential of mesenchymal stem cells for diabetes. Journal of Molecular Endocrinology., 59(3), R109–RR20.
Okuda, A., Horii-Hayashi, N., Sasagawa, T., Shimizu, T., Shigematsu, H., Iwata, E., et al. (2017). Bone marrow stromal cell sheets may promote axonal regeneration and functional recovery with suppression of glial scar formation after spinal cord transection injury in rats. Journal of Neurosurgery SPINE., 26(3), 388–395.
Ryu, H. H., Lim, J. H., Byeon, Y. E., Park, J. R., Seo, M. S., Lee, Y. W., et al. (2009). Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. Journal of Veterinary Science., 10(4), 273–284.
Shende, P., & Subedi, M. (2017). Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie., 91, 693–706.
Ropper, A. E., Thakor, D. K., Han, I., Yu, D., Zeng, X., Anderson, J. E., et al. (2017). Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proceedings of the National Academy of Sciences of the United States of America., 114(5), E820–E8E9.
Capitelli, C. S., Lopes, C. S., Alves, A. C., Barbiero, J., Oliveira, L. F., da Silva, V. J., et al. (2014). Opposite effects of bone marrow-derived cells transplantation in MPTP-rat model of Parkinson's disease: A comparison study of mononuclear and mesenchymal stem cells. International journal of medical sciences., 11(10), 1049–1064.
Jinfeng, L., Yunliang, W., Xinshan, L., Yutong, W., Shanshan, W., Peng, X., et al. (2016). Therapeutic effects of CUR-activated human umbilical cord mesenchymal stem cells on 1-Methyl-4-phenylpyridine-induced Parkinson's disease cell model. BioMed research international., 2016, 9140541.
Mendes Filho, D., Ribeiro, P. D. C., Oliveira, L. F., de Paula, D. R. M., Capuano, V., de Assuncao, T. S. F., et al. (2018). Therapy with mesenchymal stem cells in Parkinson disease: History and perspectives. The Neurologist., 23(4), 141–147.
Chulpanova, D. S., Kitaeva, K. V., Tazetdinova, L. G., James, V., Rizvanov, A. A., & Solovyeva, V. V. (2018). Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Frontiers in Pharmacology., 9, 259.
Francois S, Usunier B, Forgue-Lafitte ME, L'Homme B, Benderitter M, Douay L, et al. (2018) Mesenchymal stem cell administration attenuates Colon Cancer progression by modulating the immune component within the colorectal tumor microenvironment. Stem cells translational medicine.
Kalimuthu, S., Zhu, L., Oh, J. M., Lee, H. W., Gangadaran, P., Rajendran, R. L., et al. (2018). Regulated mesenchymal stem cells mediated Colon Cancer therapy assessed by reporter gene based optical imaging. International journal of molecular sciences., 19(4).
Nakamura, K., Ito, Y., Kawano, Y., Kurozumi, K., Kobune, M., Tsuda, H., et al. (2004). Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Therapy., 11(14), 1155–1164.
Bartholomew, A., Sturgeon, C., Siatskas, M., Ferrer, K., McIntosh, K., Patil, S., et al. (2002). Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology., 30(1), 42–48.
Ding, Q., Regan, S. N., Xia, Y., Oostrom, L. A., Cowan, C. A., & Musunuru, K. (2013). Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell, 12(4), 393–394.
Ding, Y., Li, H., Chen, L. L., & Xie, K. (2016). Recent advances in genome editing using CRISPR/Cas9. Frontiers in Plant Science, 7, 703.
Mali, P., Yang, L., Esvelt, K. M., Aach, J., Guell, M., DiCarlo, J. E., et al. (2013). RNA-guided human genome engineering via Cas9. Science., 339(6121), 823–826.
Barrero, M. J., Boue, S., & Izpisua Belmonte, J. C. (2010). Epigenetic mechanisms that regulate cell identity. Cell Stem Cell, 7(5), 565–570.
Chen, Q., Shou, P., Zheng, C., Jiang, M., Cao, G., Yang, Q., et al. (2016). Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death and Differentiation., 23(7), 1128–1139.
Almalki, S. G., & Agrawal, D. K. (2016). Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation; Research in Biological Diversity., 92(1–2), 41–51.
Augello, A., & De Bari, C. (2010). The regulation of differentiation in mesenchymal stem cells. Human Gene Therapy., 21(10), 1226–1238.
Bionaz, M., Monaco, E., & Wheeler, M. B. (2015). Transcription adaptation during in vitro Adipogenesis and osteogenesis of porcine mesenchymal stem cells: Dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS One, 10(9), e0137644.
Kang, H., Minder, P., Park, M. A., Mesquitta, W. T., Torbett, B. E., & Slukvin, I. I. (2015). CCR5 disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 virus. Molecular Therapy Nucleic Acids., 4, e268.
Lai, F. P., Lau, S. T., Wong, J. K., Gui, H., Wang, R. X., Zhou, T., et al. (2017). Correction of Hirschsprung-associated mutations in human induced pluripotent stem cells via clustered regularly interspaced short palindromic repeats/Cas9, restores neural crest cell function. Gastroenterology., 153(1), 139–53 e8.
Song, B., Fan, Y., He, W., Zhu, D., Niu, X., Wang, D., et al. (2015). Improved hematopoietic differentiation efficiency of gene-corrected beta-thalassemia induced pluripotent stem cells by CRISPR/Cas9 system. Stem Cells and Development., 24(9), 1053–1065.
Caplan, A. I., & Dennis, J. E. (2006). Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry, 98(5), 1076–1084.
Caplan, A. I., & Sorrell, J. M. (2015). The MSC curtain that stops the immune system. Immunology Letters., 168(2), 136–139.
Yao, Y., Huang, J., Geng, Y., Qian, H., Wang, F., Liu, X., et al. (2015). Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One, 10(6), e0129164.
Wu, C. C., Liu, F. L., Sytwu, H. K., Tsai, C. Y., & Chang, D. M. (2016). CD146+ mesenchymal stem cells display greater therapeutic potential than CD146- cells for treating collagen-induced arthritis in mice. Stem Cell Research & Therapy., 7, 23.
Butler, J., Epstein, S. E., Greene, S. J., Quyyumi, A. A., Sikora, S., Kim, R. J., et al. (2017). Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: Safety and efficacy results of a phase II-A randomized trial. Circulation Research., 120(2), 332–340.
Caplan, A. I., & Correa, D. (2011). The MSC: An injury drugstore. Cell Stem Cell, 9(1), 11–15.
Ye, X., Hu, J., & Cui, G. (2016). Therapy effects of bone marrow stromal cells on ischemic stroke. Oxidative medicine and cellular longevity., 2016, 7682960.
Yan, T., Chopp, M., & Chen, J. (2015). Experimental animal models and inflammatory cellular changes in cerebral ischemic and hemorrhagic stroke. Neuroscience Bulletin., 31(6), 717–734.
Diez-Tejedor, E., Gutierrez-Fernandez, M., Martinez-Sanchez, P., Rodriguez-Frutos, B., Ruiz-Ares, G., Lara, M. L., et al. (2014). Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: A safety assessment: A phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. Journal of Stroke and Cerebrovascular Diseases : the Official Journal of National Stroke Association., 23(10), 2694–2700.
Locatelli, F., Bersano, A., Ballabio, E., Lanfranconi, S., Papadimitriou, D., Strazzer, S., et al. (2009). Stem cell therapy in stroke. Cellular and Molecular Life Sciences : CMLS., 66(5), 757–772.
Zheng, H., Zhang, B., Chhatbar, P. Y., Dong, Y., Alawieh, A., Lowe, F., et al. (2018). Mesenchymal stem cell therapy in stroke: A systematic review of literature in pre-clinical and clinical research. Cell Transplantation., 27(12), 1723–1730.
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V., & Ferrara, N. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science., 246(4935), 1306–1309.
Conti, E., Carrozza, C., Capoluongo, E., Volpe, M., Crea, F., Zuppi, C., et al. (2004). Insulin-like growth factor-1 as a vascular protective factor. Circulation., 110(15), 2260–2265.
Abe, K., Yamashita, T., Takizawa, S., Kuroda, S., Kinouchi, H., & Kawahara, N. (2012). Stem cell therapy for cerebral ischemia: From basic science to clinical applications. Journal of Cerebral Blood Flow and Metabolism : Official Journal of the International Society of Cerebral Blood Flow and Metabolism., 32(7), 1317–1331.
Zhong, C., Qin, Z., Zhong, C. J., Wang, Y., & Shen, X. Y. (2003). Neuroprotective effects of bone marrow stromal cells on rat organotypic hippocampal slice culture model of cerebral ischemia. Neuroscience Letters, 342(1–2), 93–96.
Chiba, Y., Kuroda, S., Osanai, T., Shichinohe, H., Houkin, K., & Iwasaki, Y. (2012). Impact of ageing on biological features of bone marrow stromal cells (BMSC) in cell transplantation therapy for CNS disorders: Functional enhancement by granulocyte-colony stimulating factor (G-CSF). Neuropathology : official journal of the Japanese Society of Neuropathology., 32(2), 139–148.
Hokari, M., Kuroda, S., Chiba, Y., Maruichi, K., & Iwasaki, Y. (2009). Synergistic effects of granulocyte-colony stimulating factor on bone marrow stromal cell transplantation for mice cerebral infarct. Cytokine., 46(2), 260–266.
Kim, H. J., & Park, J. S. (2017). Usage of human mesenchymal stem cells in cell-based therapy: Advantages and disadvantages. Development & reproduction., 21(1), 1–10.
Kim, N., & Cho, S. G. (2015). New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. International Journal of Stem Cells., 8(1), 54–68.
Conboy, I., Murthy, N., Etienne, J., & Robinson, Z. (2018). Making gene editing a therapeutic reality. F1000Research., 7.
Wang, W., Huang, X., Lin, W., Qiu, Y., He, Y., Yu, J., et al. (2018). Hypoxic preconditioned bone mesenchymal stem cells ameliorate spinal cord injury in rats via improved survival and migration. International Journal of Molecular Medicine., 42(5), 2538–2550.
Funding
This work was supported by National Council for Scientific and Technological Development (CNPq, Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Filho, D.M., de Carvalho Ribeiro, P., Oliveira, L.F. et al. Enhancing the Therapeutic Potential of Mesenchymal Stem Cells with the CRISPR-Cas System. Stem Cell Rev and Rep 15, 463–473 (2019). https://doi.org/10.1007/s12015-019-09897-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09897-0