Abstract
Purpose
It has become increasingly clear in cancer treatment that radiotherapy can be enhanced by immunotherapy. In the present study, we evaluated a novel triple combination therapy consisting of local radiotherapy, intratumoral CpG, and systemic PD-1 blockade in lung cancer models.
Methods
The efficacy of a novel triple therapy was examined by recording tumor volume and survival time. The immunologic effects of this novel triple therapy were evaluated by the frequency and percentage of immune cells and cytokines using flow cytometry.
Results
This triple combination proved more effective than its subcomponents and its positive antitumor effects included reducing tumor growth and improving host survival. The antitumor effect was not only observed in directly irradiated tumors but also in at distant tumor sites in a CD8+ T-cell-dependent fashion. Phenotypic analyses of CD8+ T cells revealed that the triple combination therapy increased the percentage of effector memory T cells in the spleen. Furthermore, the combination therapy significantly increased the frequency of IFN-γ and TNF-α-positive-CD8+ tumor-infiltrating lymphocytes (TIL) and mature-activated dendritic cells (DCs) within treated tumors, indicating that the antitumor effects likely depend on the activation of a DC subset specialized in antigen crosspriming to induce cytotoxic lymphocyte (CTLs). In addition, the triple therapy reduced immunosuppressive factors, like regulatory T cells (Tregs) in the spleen and tumor microenvironment while inducing the robust systemic antitumor effect. Finally, the triple treatment was, indeed, well tolerated and had a little effect on the hemogram and lung.
Conclusions
These results suggest that this triple therapy promotes a local antitumor immune response with systemic consequences. The efficacy and limited toxicity of this strategy are attractive for clinical translation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence in oncology indicates that radiotherapy (RT) is effective on several different levels including by directly shrinking tumors and releasing tumor antigens. In addition, RT is well established to have immunomodulatory effects that aid in therapeutic outcome. For instance, local irradiation of a tumor nodule can induce immunogenic forms of tumor-cell death (ICD) and liberation of tumor-cell-derived antigens (Golden and Apetoh 2015; Golden et al. 2014; Rodriguez-Ruiz et al. 2016; Sharabi et al. 2015; Vandenabeele et al. 2016). These tumor-cell-derived antigens can be recognized and then processed by antigen-presenting cells (dendritic cells) within the tumor (Golden and Apetoh 2015; Golden et al. 2014; Rodriguez-Ruiz et al. 2016; Sharabi et al. 2015; Vandenabeele et al. 2016). Cytotoxic T cells which recognize these tumor antigens may, in turn, be primed by the tumor antigen-presenting cells (Golden and Apetoh 2015; Golden et al. 2014; Rodriguez-Ruiz et al. 2016; Sharabi et al. 2015; Vandenabeele et al. 2016). In contrast to the local effect of irradiation on the tumor cells, cytotoxic T cells will circulate via the blood stream throughout the body and are thereby able to destroy tumor cells in distant parts of the organism which were not part of the primary, irradiated cancer (Golden and Apetoh 2015; Golden et al. 2014; Rodriguez-Ruiz et al. 2016; Sharabi et al. 2015; Vandenabeele et al. 2016). Indeed, increases in tumor-specific cytotoxic T cells have been shown to correlate with the abscopal antitumor response in patients (Postow et al. 2012). Thus, local RT clearly can stimulate a systemic antitumor effect through the immune system. Therefore, RT would seem an ideal option for combined cancer therapy with immunotherapy.
CpG oligodeoxynucleotides are ligands of Toll-like receptor (TLR)-9, and show promise when combined with RT by inducing synergistic tumor suppressive effects (Gholizadeh et al. 2018; Moreno Ayala et al. 2017). Dendritic cells (DCs) specifically express Toll-like receptor (TLR) 9 and efficiently cross-present exogenous antigens after CpG activation. Thus, CpG oligodeoxynucleotides (CpG-ODN) are always regarded as an immune adjuvant and used along with DC vaccines (Moreno Ayala et al. 2017). The previous studies show that the combination of radiation and CpG has superior tumor suppression than either treatment alone (Appelbe et al. 2017). In fact, the combination often shows a significant synergy in preclinical models (Mason et al. 2006; Yuan et al. 2011) and the ability to induce regression of systemic cancers (lymphoma) in clinical trial (Brody et al. 2010; Kim et al. 2012). Furthermore, CpG-ODN can protect from irradiation-induced injury. The mechanism here might be that CpG-ODN inhibits proliferating cell apoptosis through regulating the expression of apoptosis-related protein, involving that up-regulating Bcl-2 protein and down-regulating Bax protein and caspase-3 protein (Zhang et al. 2013). CpG-ODN mediated the activation of nuclear factor κB (NF-κB) and minimized bone marrow damage which contributed to the amelioration of hematopoiesis radiation injury (Zhang et al. 2011). Therefore, it appears RT in combination with CpG-ODN which could be a promising strategy for cancer therapy. In addition, CpG can reduce regulatory T cells (Tregs) by converting them to a T-helper phenotype via IL-6 production and can also directly reverse Treg function (Baban et al. 2009; Monjazeb et al. 2016).
Studies have demonstrated that tumors will escape the attack of a host’s immune system through programmed death-1 (PD-1) pathway. In brief, PD-1 expressed on the surface of activated T cells will bind to the programmed death-ligand 1(PD-L1) expressed on the surface of tumor cells, and this can result in the depletion of the T cells, and eventual suppression of T-cell function and immunological escape of tumor cells (Zhu and Lang 2017). Local high-dose radiotherapy can induce tumor-cell surface PD-L1 up-regulation (Oweida et al. 2017). This enhances T-cell death by tumor cells and provides strong support for combining RT with PD-1 blockade. This synergistic effect has been demonstrated in multiple murine models, such as renal cortical adenocarcinoma (Park et al. 2015), breast cancer, colon adenocarcinoma, melanoma, and bladder cancer (Walshaw et al. 2018) models. Clinical trials involving combination therapy are currently recruiting patients. Interestingly, CpG reduced PD-1 expression by effector CD8+ T cells via the IL-12 pathway. The combination of CpG and PD-1 blockade also shows a synergistic effect in the generation of systemic antitumor immunity (Yin et al. 2016).
Thus, in the present study, we examined the antitumor effect of a novel triple therapy consisting of local RT, intratumoral CpG, and systemic PD-1 blockade in a murine lung cancer models. We hypothesized that RT releases tumor antigens while killing tumors cells and reducing tumor size. Tumor antigens are thus cross-presented to tumor antigen-specific T lymphocytes by activated dendritic cells (DCs). Then, effector T cells elicit the antitumor immune response. CpG-ODN further promotes dendritic cell maturation and activation and PD-1 inhibitors reverse T-cell depletion. We observed a significant tumor mitigation in the mice given the triple therapy, while the size of the non-irradiated tumors was also reduced with just the activated systemic immune response. These notable results may be due to an increase in activated dendritic cells within spleen and tumor microenvironment resulting in an increase in the percentage of CD8+ T cells and an increase in CTL-producing TNF-α and IFN-γ. In addition, the proportion of regulatory T cells decreased significantly. The side effects of the triple therapy were found to be negligible. Given the efficacy and limited toxicity of this strategy, it is attractive for rapid clinical translation.
Materials and methods
Mice
Female 8–12-week-old C57BL/6 mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. Mice were housed in a controlled environment (12 h light/dark cycle, 22 ± 1 °C, 60–70% humidity) and fed a standard chow diet ad libitum. All animal work was performed in compliance with a research protocol approved by the Ethics Committee of the China Medical University.
Cell lines and reagents
The mouse Lewis lung cell (LLC) line was obtained from ATCC and maintained at 37 °C with 5% CO2. The following antibodies were used: Antimouse PD-1 (clone J43, BioXcell, West Lebanon, NH); CD8-depleting antibody (Clone 2.43,BioXcell); CD4-depleting antibody (Clone GK1.5, BioXcell); CpG-ODN 1826 (Invivogen, San Diego, CA).
Tumor-cell inoculation and host treatments
The mice were injected s.c. with 1 × 106 LLCs in the right hindlimb (primary tumor) on day 0 and with 5 × 105 in the left back (secondary tumor) on day 2. Tumor diameters were estimated externally with a vernier caliper every 2–3 days, and tumor volumes were calculated as length × width2/2 (mm3). On day 10, when the diameter of primary tumor is about 5 cm, mice were randomly assigned to six treatment groups including: Control, RT, RT + PD-1 blockade, RT + CpG-ODN, PD-1 blockade + CpG-ODN, and triple combination (RT + PD-1 blockade + CpG-ODN). RT (8 Gy × 3 fractions) was administered locally to the primary tumors on days 11, 13, and 15 post-inoculation. If appropriate, each fraction of RT was accompanied by intratumoral injection of 25ug of CpG-ODN 1826. For PD-1 blockade, PD-1 blocking mAb was administered as 200 μg/mouse i.p. injected on days 12, 14, and 16. To determine the impact of depleted CD8+/CD4+ T cells on RT, 250 ug antiCD8/antiCD4 was given i.p. three times for 3 days, starting 1 day before RT on day 10, and continuing until 1 day after RT. Tumor growth was evaluated until day 24 after inoculation when the mice were euthanized. In separate sets of mice, the impact of the various treatments on survival time was assessed until 80th day after tumor-cell inoculation. Mouse tumor studies were performed as outlined in Fig. S1.
Flow cytometric analysis
Single-cell suspensions were prepared from spleens and tumors. To obtain tumor-cell suspensions, solid tumors were removed completely; single-cell suspensions prepared by digestion with collagenase and hyaluronidase solution (STEMCELL, Cambridge, MA). Cells were stained with fluorescent-labeled antibodies (BioLegend, San Diego, CA) and analyzed by BD celesta flow cytometer. The following antibodies were used: CD4-FITC, CD8a-PerCP, CD3-PE-Cy7, CD45-PE-Cy7, CD11c-APC, I-A/I-E-PerCP, CD25-PE, Foxp3-APC, IFN-γ-PE, TNF-α-APC, CD70-PE, CD40-FITC, CD62L-APC, and CD44-PE. For intracellular cytokine staining, cells were activated with Cell Stimulation Cocktail plus protein transport inhibitors (eBioscience, Thermo Fisher Scientific) for 4 h and then stained as indicated. Gates and quadrants were set based on negative control staining. Data were analyzed using FlowJo v10.
Lung tissue H&E staining
Lungs were removed, fixed by standard protocol (4% paraformaldehyde, etc.), embedded in paraffin, made into sections, stained with H&E, and assessed for pathological lesions by light microscopy. In particular, the extent of inflammatory infiltrates was calculated.
Blood cell count
On the 23th day post-inoculation, 1 ml of retro-orbital blood was taken from all mice using a microcentrifuge tube with EDTA-2Na. Blood cell count (leucocytes—WBC, erythrocytes—RBC, and thrombocytes—PLT) was determined by use of a hemocounter.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software 7.0. Data were expressed as mean ± SD. Statistical differences between survival curves were analyzed with log-rank test. The statistical differences between specific groups were determined either by Student’s t test or ANOVA followed by Tukey’s multiple comparison tests. A two-sided p value < 0.05 was considered significant.
Results
PD-1 blockade enhances the local antitumor efficacy of RT + CpG, while the effect of the triple therapy is systemic
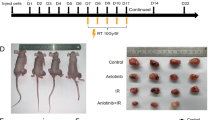
Mice-bearing two tumors derived from lung cancer cells were used as a model to monitor the antitumor effects of a novel triple therapeutic strategy, namely RT + CpG + PD-1 blockade. RT in fractionated external beam doses (8 Gy) were selectively directed only to the primary tumors originating from the cells inoculated into the leg area. We ensure that secondary tumors, arising from cells inoculated into the dorsal thoracic area, were outside the irradiation field. With RT, estimated tumor volume plateaued during the irradiation sessions, and as expected, there was a growth suppression of nearly 65% after irradiation based on tumor volume by day 23 compared to no treatment. The tumor suppressive effects of combined CpG + PD-1 were similar to RT alone. However, when either one was singly combined with RT, they significantly inhibited tumor growth even further than the individual treatments alone. It is highly noteworthy that the triple combination (i.e; RT + CpG + PD-1) showed significantly more suppression of tumor growth than any other groups singly or in binary combination. In fact, some mice appeared to be completely free of primary tumor upon autopsy (Fig. 1a, b). The triple therapy also showed systemic antitumor effects (Fig. 1d, e). This was apparently by concurrently stimulating the immune response towards these non-irradiated secondary tumors. Clearly, the volume of secondary tumors in triple therapy group was markedly reduced. Through gross examination, the differences of tumor volume among each treatment group were obvious (Fig. 1c, f).
Local RT + CpG antitumor efficacy is enhanced by PD-1 blockade and the antitumor effects of this triple therapy are systemic. Tumor size was measured as estimated volume in the primary, directly irradiated tumor (leg; a) and in the secondary, non-irradiated tumor (back; d). The primary tumor volume (b) and the secondary tumor volume (e) in the various treatment groups were recorded on day 23. The picture excised gross tumor (c, f) shows the obvious changes in mass with treatments. n = 6–10 mice per group for tumor growth studies. Bar graphs represent mean volume ± SD. Results analyzed by two-way ANOVA followed by Tukey’s multiple comparison tests. (*p < 0.05 vs Control; #p < 0.05 vs RT; &p < 0.05 vs RT + CpG; $p < 0.05 vs RT + PD-1; +p < 0.05 vs PD-1 + CpG at the same time)
Triple therapy prolongs survival with minimal toxicity
Starting with the inoculation of tumor cells, the survival of mice was monitored. The control group of tumor-bearing mice began to die on the 32nd day. However, mice in the triple therapy group showed complete long-term survival, and 100% of tumor-cell inoculated mice in this group survived until day 80 (Fig. 2).
Of note was the minimal toxicity of the triple therapy. The triple treatment was, indeed, well tolerated and had a little effect on the hemogram. The peripheral WBC count of the triple therapy group was slightly lower than that in the control group (P < 0.05), but was still considered within the normal range. There were no differences among the control and treatment groups in the RBC count and PLT count (n = 3, Table S1). According to the Szapiel’s grading method (Yavas et al. 2013), alveolitis of the six groups of tumor-bearing mice all belonged to grade 1 (i.e., mild, the alveolar septa widened due to inflammatory cell infiltration and the involved area was less than 20%, Fig. S2).
The therapeutic effect of triple therapy is CD8+ T-cell-dependent
To explore the main T-cell subsets involved in tumor regression induced by triple combination therapy, the changes in the proportions of CD8+ T cell and CD4+ T cell in different treatment groups were examined. It was determined that triple treatment mainly caused a splenic (Fig. 3a, c) and intratumoral (Fig. 3b, d) CD8+ T-cell increase. To further quantify these changes, we calculated the number of CD8+ T cells per 10^6 gated events. As shown in Fig. S3a and b, triple treatment significantly increased CD8 T-cell infiltration.
Therapeutic effect of triple therapy is CD8+ T-cell-dependent. Representative flow cytometry contour plots demonstrate staining of splenic CD3+ cells for CD8 and CD4 (a) and tumor-infiltrating CD45+ cells staining for CD8 and CD4 (b). Flow cytometry data of tumor-infiltrating CD8+ T cells are represented as a bar graph expressed as % CD8+ cells of all CD3+ cells (c) and as % of CD8+ cells of all CD45+ cells (d). n = 4–5 mice per group for Flow cytometry tests. Results analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests (*p < 0.05 vs Control; #p < 0.05 vs RT; &p < 0.05 vs RT + PD-1; $p < 0.05 vs RT + CpG; +p < 0.05 vs PD-1 + CpG). Growth of tumors following CD4 and CD8 lymphocyte depletion including the primary tumors (e) and the secondary tumors (f). Overall survival of the various groups (g). n = 4–5 mice per group for tumor volume studies. Results analyzed by two-way ANOVA followed by Tukey’s multiple comparison tests (*p < 0.05 vs Control; #p < 0.05 vs triple at the same time). n = 3–4 mice per group for survival studies (#p < 0.05 vs triple via log-rank test)
We also studied the depletion of CD8+ T cells/CD4+ T cells by intraperitoneal administration of antiCD8/CD4 Mab. With depletion of CD8+T cells, we observed that the antitumor effects of triple therapy were significantly diminished (Fig. 3e, f). The survival benefit of the triple therapy was also abolished by CD8 depletion (Fig. 3g), indicating the critical role of CD8+ T cells in the antitumor effects. However, when CD4+ T cells were deleted, the antitumor effect and the enhanced survival induced by triple therapy were unaltered. This may be related to a reduction of Tregs.
Tumor regression with triple therapy may be due to enhanced activation of cytotoxic CD8+ T cell
If CD8+ T cells are critical to efficacy of triple therapy, they should show activation and functionality by producing IFN-γ and TNF-α. Interestingly, IFN-γ and TNF-α production by CD8+ T cells was enhanced by PD-1 blockade in the presence of RT + CpG, suggesting that improved priming of T cells may contribute to the therapeutic effect of triple therapy. Indeed, there was a twofold increase in IFN-γ+ CD8+ T cells (31.1 ± 1.9% vs 14 ± 1.9%), a threefold increase in TNF-α+ CD8+ T cells (44.4 ± 5.0% vs 13.7 ± 0.6%), and more than threefold increase in IFN-γ+ TNF-α+ cells (20.8 ± 1.1% vs 6.5 ± 1.0%) within spleen after the triple combination therapy (Fig. 4a–c; Fig. S4a–c).
Marked tumor regression with triple therapy may be due to enhanced activation of cytotoxic CD8+ T cell. Intracellular IFN-γ or TNF-α immunostaining in the gated CD8+ splenic and tumor-infiltrating T lymphocytes as indicated following a 4-h re-stimulation with PMA + ionomycin. Bar graph showing the percentages of IFN-γ+(a), TNF-α+ (b) and IFN-γ+ TNF-α+ (e) within splenic CD8+T cells. Bar graph showing the percentages of IFN-γ+ (b), TNF-α+ (d) and IFN-γ+ TNF-α+ (f) within tumor-infiltrating CD8+T cells. n = 4–5 mice per group. Results analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests (*p < 0.05 vs Control; #p < 0.05 vs RT; &p < 0.05 vs RT + PD-1; $p < 0.05 vs RT + CpG; +p < 0.05 vs PD-1 + CpG). Results analyzed by log-rank test. (#p < 0.05 vs triple)
Furthermore, there was approximately a threefold increase both in the percentage of IFN-γ+ CD8+T cells (34.1 ± 4.4% vs 13.5 ± 0.8%), TNF-α+ CD8+T cells (31.4 ± 3.6% vs 11.8 ± 0.9%), and more than fourfold increase in the percentage of IFN-γ+ TNF-α+ cells (22.8 ± 5.2% vs 5.6 ± 0.4%) within the tumor microenvironment compared with the control group (Fig. 4d–f). These data show that the triple therapy causes CD8+ T-cell activation and these cells killing capability increases dramatically, causing a significant tumor mass reduction.
Triple therapy increases effector memory cells adding an immunological memory to therapeutic impact
The phenotype of expanded CD8 T-cell population induced by triple therapy was analyzed in depth. For these studies, we defined naïve cells as CD62L+ and CD44−. In a similar manner, the effector memory phenotype was defined as CD44+ CD62L−, and central memory cells as CD44+ CD62L+ (Silaeva et al. 2013). In this Lewis lung cancer model, the triple therapy increased the relative percentage of CD8+ T cells with the effector memory phenotype. RT alone and PD-1 + CpG increased the proportion of effector memory CD8+ T cells about 1.5-fold compared to control, while the combination of RT and either immunotherapy further increased the proportion of effector memory. However, with the triple therapy, a sharp increase in the portion of effector memory cells occurred with more than twice that of control. (68.8 ± 5.6% vs 29.4 ± 0.8%, Fig. 5a; Fig. S5).
Triple therapy increased effector memory cells creating therapeutic immunological memory effect. The naïve cells were defined as CD62L+ and CD44−. The effector memory phenotype was defined as CD44+ CD62L−, and central memory cells as CD44+ CD62L+. Bar graph showing the percentages of effector memory populations in treated groups (a). n = 4–5 mice per group. Results analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests (*p < 0.05 vs Control; #p < 0.05 vs RT; &p < 0.05 vs RT + PD-1; $p < 0.05 vs RT + CpG; +p < 0.05 vs PD-1 + CpG). Tumor growth was measured in the re-challenged tumor (b). n = 4 mice per group for tumor growth studies (*p < 0.05 vs control via Student’s t test)
Some of the mice in triple therapy group appeared to be completely cured in that there were no signs of tumor present at either site. To study if these “tumor free” mice had an effective immunological memory, in that they had an ability to prevent tumor recurrence based in the immune system, we used a high-level tumor-cell re-challenge experiment to simulate a cancer recurrence event. These cancer-free mice were re-challenged with 1 × 107 LLCs inoculated (s.c.) in the left hind limb and showed clear evidence of tumor immunity 2 months later. Similarly, inoculated naive mice (n = 3) developed palpable tumors by day 4 after inoculation and died on day 26, 26, and 28 post-inoculation, while the “tumor free” mice survived and remained essentially caner free (Fig. 5b). These results suggest that triple combination therapy produces an effective immunological memory that is protective against tumor recurrence, an added benefit of this therapeutic strategy.
Triple therapy promotes maturation and activation of dendritic cells and decreases regulatory T cells in the spleen and local tumor microenvironment, possibly related to antitumor effects
The effects of the triple therapy on DCs were examined. The previous studies have demonstrated that intratumoral DCs, although a minor population, play a critical role in controlling priming vs tolerance induction of CD8+ T cells (Keller et al. 2008; Ruffell et al. 2014). TIDC were defined as CD45+ CD11c+ MHC-II+ cells and activation markers were defined as CD40 and CD70. There were an increased number of intratumoral dendritic cells after triple treatment (three- to fourfold vs control) (Fig. S6a). The RT alone promoted the activation of DC to a certain extent, and the activation was further enhanced after combination with CpG. An increased number of activated DCs within triple-treated tumors occurred, with a 12-fold increase in CD40 and CD70-expressing TIDC compared with control mice (47.3 ± 2.3% vs 3.6 ± 1.8%) (Fig. 6a; Fig. S6b).
Triple therapy promotes maturity and activation of dendritic cells which then activate T-cell priming and decreases regulatory T cells within spleen and local tumor microenvironment, possibly enhancing antitumor effects. TIDC were gated on CD45+ CD11c+ IAd+ and analyzed for the expression of activation markers CD40 and CD70. Bar graphs showing a significant increase in mean percentage of TIDC expressing CD40 and CD70 in tumors of mice treated with triple therapy (a). Day 24 post-inoculation levels of Tregs as assessed by flow cytometry in LLC tumor-bearing mice treated with triple therapy, etc. Flow cytometry data represented as a bar graph expressed as % Treg (CD4+, CD25+, Foxp3+) of CD4+ cells within spleen (b) and local tumor microenvironment (c). n = 4–5 mice per group. Results analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests for a, b (*p < 0.05 vs Control; #p < 0.05 vs RT; &p < 0.05 vs RT + PD-1; $p < 0.05 vs RT + CpG; +p < 0.05 vs PD-1 + CpG). Results analyzed by Student’s t test for c and a (*) indicates a significant difference
Regulatory T cells (Tregs) play a critical suppressive role in the tumor microenvironment. The destiny of Tregs in the tumors and spleen was examined. Tregs showed clear reductions both in immune organ and in the tumor microenvironments. Tregs levels were maintained within the spleen after RT. Compared to control, two-agent therapy alone slightly reduced the Tregs subset of CD4+ T cells in spleen, while triple therapy resulted in a robust 3.5-fold decrease in splenic Tregs (Fig. 6b; Fig. S7a). In tumors, the Tregs subset of CD4+T cells showed a greater than twofold reduction compared with control (Fig. 6c; Fig. S7b).
Discussion
In recent years, the notion that cancer therapeutic strategies are likely to attain a higher rate of success in many cases by the combination of radiotherapy and immunotherapy has become firmly entrenched (Formenti and Demaria 2013). In the present report, we examine such combination strategy and study the therapeutic impact of combining local radiotherapy, intratumoral CpG, and systemic PD-1 blockade using a mouse model and aggressive tumors formed by implanted lung cancer cells. The triple therapy showed excellent anticancer efficacy and was able to essentially destroy otherwise fatal tumors, allowing complete survival, leaving the host in many cases tumor free. This tumor remarkable suppressive effect was not only in the irradiated/injected primary inoculation site tumors, but also in distant inoculation site tumors that had not been irradiated or injected with CpG. Again, these distant site tumors were markedly diminished by the triple therapy, even though much of the impact was clearly indirect. Depletion experiments showed that CD8+ T cells were crucial to triple therapy activity. Indeed, CD8+ T cells in the spleen and tumor microenvironment were clearly increased, as were activation and functioning. These factors brought an increased number of mature and activated intratumoral DCs, indicating that the enhanced antitumor response may be activated by the increased mature-activated dendritic cells. In addition, this novel combination also decreased percentages of CD4+ T-regulatory cells, further enhancing the antitumor effects.
Abscopal effects of ionizing radiation, where localized tumor irradiation also suppresses distant un-irradiated tumors, often are blocked by the immunosuppressive micromilieu inside the irradiated tumor, since it can prevent effective T-cell priming. This provides an explanation for why an abscopal effect is so rarely seen in patients receiving RT alone. In contrast, the combination of immunomodulatory drugs such as ipilimumab can stimulate systemic antitumor immune reactions induced after local tumor RT (Twyman-Saint Victor et al. 2015). In addition, the previous studies show that different radiation doses, fractions, and intervals of exposure have markedly different impact on the immune function. The optimal combination of radiation dose and fractionation together with immunomodulatory drugs is currently under intensive investigation. For instance, a recent study suggests that radiation doses above 10–12 Gy might be ineffective in inducing immunogenic forms of cell death (Vanpouille-Box et al. 2017). In our study, we chose 8 Gy doses three times on the basis of the published evidence, suggesting that this regimen yielded better results from an immunological perspective when combined with RT and antiCTLA-4 mAb (Dewan et al. 2009; Ruocco et al. 2012). In addition in an experiment of local RT combined with PD-1 antibody and CD137 antibody injections, 8 Gy × 3 RT achieved very positive antitumor effects(Rodriguez-Ruiz et al. 2016), similar to our present work.
Accumulating data show that intratumoral DCs, although a minor population, play a critical role in antitumor T-cell responses (Monjazeb et al. 2016; Sharabi et al. 2015). In addition, this critical role is to cross-present tumor antigens to tumor antigen-specific T lymphocytes (Broz et al. 2014; Ruffell et al. 2014). In addition, the activation of tumor-associated DCs is the mechanism through which local high-dose radiotherapy supports the function of local, tumor-specific CD8+ effector T cells (Gupta et al. 2012; Sharabi et al. 2015). Recent evidences showed that radiotherapy combined with PD-1 blockade and CD137 blockade augmented antigen-specific PD-1-mediated T-cell antitumor immune responses via activated DC (Rodriguez-Ruiz et al. 2016; Sanchez-Paulete et al. 2016). In our study, it is speculated that the enhanced antitumor response via CD8+ cells could be activated by the increased frequency of mature DCs. However, to prove this speculation, the experiments should be repeated with a DC depletion regimen.
Given the major effects of dendritic cells, we added CPG-ODN to the treatment regimen. The previous study suggests that CpG-ODN can increase the radiosensitivity of Lewis lung cancer, which may be associated with stimulation of immune system and enhanced cell apoptosis (Yuan et al. 2011). Recent data showed that CpG-mediated antitumor immunity induces an increase of IFN-γ secretion by CD8+T cells via IL-12 and down-regulates the expression of PD-1. Therefore, the addition of CpG can not only induce tumor apoptosis and enhance the ability of radiation to kill tumor cells, but also appears to enhance the ability of CTL to kill tumors by promoting the maturation and activity of dendritic cells.
The regulatory T cells (Tregs), formerly known as suppressor T cells, maintain tolerance to self-antigens, and prevent autoimmune diseases. Tregs are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells (Bettelli et al. 2006). The effect of CpG on Treg-mediated immunosuppression may be contradictory. The CpG can reduce Tregs by converting Treg into Th, or directly reverse Treg functions. On the other hand, CpG can directly induce the expression of Foxp3 and raise indopamine-2, 3-dioxidase (IDO), which is known to induce and maintain the Tregs (Monjazeb et al. 2016). In our study, we found that the addition of PD-1 blockade to RT + CpG transforms the immune-suppressive tumor microenvironment, strongly indicating the reduction of Tregs.
In addition to its effectiveness, the toxicity of the triple therapy used in the present study was minimal. However, only Lewis lung cancer cells (LLCs) were used in the present study to inoculate mice to form our tumor model. It would be of great interest to repeat these experiments on other cancer cell lines to see if the success of triple therapy seen with these lung cancer cells is broadly applicable to other types of cancers.
Overall, our data provide a strong rationale for rapid clinical translation of this novel triple combination strategy. Its use could substantially improve the clinical efficacy of the existing cancer treatment protocols.
Abbreviations
- CpG-ODN:
-
CpG oligodeoxynucleotides
- CTL:
-
Cytotoxic lymphocyte
- CTLA-4:
-
Cytotoxic T lymphocyte-associated antigen-4
- DC:
-
Dendritic cell
- Foxp3:
-
Forkhead box P3
- ICD:
-
Immunogenic cell death
- IDO:
-
Indolamine-2,3-dioxygenase
- IFN-γ:
-
Interferon-γ
- IL-12:
-
Interleukin 12
- LLC:
-
Lewis lung cells
- NF-κB:
-
Nuclear factor κB
- PD-1:
-
Programmed death-1
- PD-L1:
-
Programmed death-ligand 1
- PLT:
-
Platelet
- RBC:
-
Red blood cell
- RT:
-
Radiotherapy
- TIDC:
-
Tumor-infiltrating dendritic cell
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor
- Tregs:
-
Regulatory T cells
- WBC:
-
White blood cell
References
Appelbe OK, Moynihan KD, Flor A, Rymut N, Irvine DJ, Kron SJ (2017) Radiation-enhanced delivery of systemically administered amphiphilic-CpG oligodeoxynucleotide. J Control Release 266:248–255. https://doi.org/10.1016/j.jconrel.2017.09.043
Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL (2009) IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol (Baltimore, Md: 1950) 183:2475–2483. https://doi.org/10.4049/jimmunol.0900986
Bettelli E et al (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238. https://doi.org/10.1038/nature04753
Brody JD et al (2010) In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 28:4324–4332. https://doi.org/10.1200/jco.2010.28.9793
Broz ML et al (2014) Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer cell 26:938. https://doi.org/10.1016/j.ccell.2014.11.010
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388. https://doi.org/10.1158/1078-0432.ccr-09-0265
Formenti SC, Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 105:256–265. https://doi.org/10.1093/jnci/djs629
Gholizadeh Z, Tavakkol-Afshari J, Nikpoor AR, Jalali SA, Jaafari MR (2018) Enhanced immune response induced by P5 HER2/neu-derived peptide-pulsed dendritic cells as a preventive cancer vaccine. J Cell Mol Med 22:558–567. https://doi.org/10.1111/jcmm.13343
Golden EB, Apetoh L (2015) Radiotherapy and immunogenic cell death. Semin Radiation Oncol 25:11–17. https://doi.org/10.1016/j.semradonc.2014.07.005
Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC (2014) Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3:e28518. https://doi.org/10.4161/onci.28518
Gupta A et al (2012) Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol (Baltimore Md: 1950) 189:558–566. https://doi.org/10.4049/jimmunol.1200563
Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J (2008) Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity 29:934–946. https://doi.org/10.1016/j.immuni.2008.10.009
Kim YH et al (2012) In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 119:355–363. https://doi.org/10.1182/blood-2011-05-355222
Mason KA, Neal R, Hunter N, Ariga H, Ang K, Milas L (2006) CpG oligodeoxynucleotides are potent enhancers of radio- and chemoresponses of murine tumors. Radiother Oncol 80:192–198. https://doi.org/10.1016/j.radonc.2006.07.024
Monjazeb AM et al (2016) Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res 22:4328–4340. https://doi.org/10.1158/1078-0432.ccr-15-3026
Moreno Ayala MA et al (2017) Dual activation of Toll-like receptors 7 and 9 impairs the efficacy of antitumor vaccines in murine models of metastatic breast cancer. J Cancer Res Clin Oncol 143:1713–1732. https://doi.org/10.1007/s00432-017-2421-7
Oweida A et al (2017) Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology 6:e1356153. https://doi.org/10.1080/2162402x.2017.1356153
Park SS et al (2015) PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 3:610–619. https://doi.org/10.1158/2326-6066.cir-14-0138
Postow MA et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366:925–931. https://doi.org/10.1056/NEJMoa1112824
Rodriguez-Ruiz ME et al (2016) Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and Are dependent on CD8 T cells and. Cross Priming Cancer Res 76:5994–6005. https://doi.org/10.1158/0008-5472.can-16-0549
Ruffell B et al (2014) Macrophage IL-10 blocks CD8 + T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26:623–637. https://doi.org/10.1016/j.ccell.2014.09.006
Ruocco MG et al (2012) Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Investig 122:3718–3730. https://doi.org/10.1172/jci61931
Sanchez-Paulete AR et al (2016) Cancer immunotherapy with immunomodulatory anti-CD137 and Anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic. Cells Cancer Discov 6:71–79. https://doi.org/10.1158/2159-8290.cd-15-0510
Sharabi AB et al (2015) Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 3:345–355. https://doi.org/10.1158/2326-6066.cir-14-0196
Silaeva YY et al (2013) Decrease in pool of T lymphocytes with surface phenotypes of effector and central memory cells under influence of TCR transgenic beta-chain expression. Biochem Biokhimiia 78:549–559. https://doi.org/10.1134/s0006297913050143
Twyman-Saint Victor C et al (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377. https://doi.org/10.1038/nature14292
Vandenabeele P, Vandecasteele K, Bachert C, Krysko O, Krysko DV (2016) Immunogenic apoptotic cell death and anticancer immunity advances. Exp Med Biol 930:133–149. https://doi.org/10.1007/978-3-319-39406-0_6
Vanpouille-Box C et al (2017) DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 8:15618. https://doi.org/10.1038/ncomms15618
Walshaw RC, Honeychurch J, Illidge TM, Choudhury A (2018) The anti-PD-1 era—an opportunity to enhance radiotherapy for patients with bladder cancer. Nat Rev Urol 15:251–259. https://doi.org/10.1038/nrurol.2017.172
Yavas G, Yavas C, Acar H, Toy H, Yuce D, Ata O (2013) Comparison of the effects of aromatase inhibitors and tamoxifen on radiation-induced lung toxicity: results of an experimental study. Support Care Cancer 21:811–817. https://doi.org/10.1007/s00520-012-1584-7
Yin P, Liu X, Mansfield AS, Harrington SM, Li Y, Yan Y, Dong H (2016) CpG-induced antitumor immunity requires IL-12 in expansion of effector cells and down-regulation of PD-1. Oncotarget 7:70223–70231. https://doi.org/10.18632/oncotarget.11833
Yuan S, Qiao T, Chen W (2011) CpG oligodeoxynucleotide 1826 enhances the Lewis lung cancer response to radiotherapy in murine tumor. Cancer Biother Radiopharm 26:203–208. https://doi.org/10.1089/cbr.2010.0871
Zhang C et al (2011) The mechanism for the ameliorative effect of CpG-oligodeoxynucleotides on bone marrow hemopoiesis radiation injury. Basic Clin Pharmacol Toxicol 109:11–16. https://doi.org/10.1111/j.1742-7843.2011.00695.x
Zhang C et al (2013) CpG-oligodeoxynucleotide treatment protects against ionizing radiation-induced intestine. Injury PloS One 8:e66586. https://doi.org/10.1371/journal.pone.0066586
Zhu X, Lang J (2017) Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer. J Gynecol Oncol 28:e64. https://doi.org/10.3802/jgo.2017.28.e64
Acknowledgements
This work was supported by the Natural Science Foundation of Liaoning Province.
Funding
This study was supported by funding from the Natural Science Foundation of Liaoning Province (No. 2013225021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Guang Li declares that he has no conflict of interest. Author Yuan Zhuang declares that she has no conflict of interest. Author Sihan Li declares that she has no conflict of interest. Author Jingbo Pi declares that he has no conflict of interest. Author Huihui Wang declares that she has no conflict of interest. Author Yuhui Xing declares that he has no conflict of interest.
Ethical approval
All animal work was performed in compliance with a research protocol approved by the Ethics Committee of the China Medical University (IACUC Issue No. 2017016).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhuang, Y., Li, S., Wang, H. et al. PD-1 blockade enhances radio-immunotherapy efficacy in murine tumor models. J Cancer Res Clin Oncol 144, 1909–1920 (2018). https://doi.org/10.1007/s00432-018-2723-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2723-4