Abstract
The aim was to elude differences in published paediatric reference intervals (RIs) and the implementations hereof in terms of classification of samples. Predicaments associated with transferring RIs published elsewhere are addressed. A local paediatric (aged 0 days to < 18 years) population of platelet count, haemoglobin level and white blood cell count, based on first draw samples from general practitioners was established. PubMed was used to identify studies with transferable RIs. The classification of local samples by the individual RIs was evaluated. Transference was done in accordance with the Clinical and Laboratory Standards Institute EP28-A3C guideline. Validation of transference was done using a quality demand based on biological variance. Twelve studies with a combined 28 RIs were transferred onto the local population, which was derived from 20,597 children. Studies varied considerably in methodology and results. In terms of classification, up to 63% of the samples would change classification from normal to diseased, depending on which RI was applied. When validating the transferred RIs, one RI was implementable in the local population. Conclusion: Published paediatric RIs are heterogeneous, making assessment of transferability problematic and resulting in marked differences in classification of paediatric samples, thereby potentially affecting diagnosis and treatment of children.
What is Known: • Reference intervals (RIs) are fundamental for the interpretation of paediatric samples and thus correct diagnosis and treatment of the individual child. • Guidelines for the establishment of adult RIs exist, but there are no specific recommendations for establishing paediatric RIs, which is problematic, and laboratories often implement RIs published elsewhere as a consequence. | |
What is New: • Paediatric RIs published in peer-reviewed scientific journals differ considerably in methodology applied for the establishment of the RI. • The RIs show marked divergence in the classification of local samples from healthy children. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reference intervals (RIs) are essential for the interpretation of paediatric biochemical results, enabling distinction between healthy and diseased children. It is thus fundamental that the RIs are representative of the paediatric population, and providing age- and gender-specific RIs is considered an important responsibility of clinical laboratories [20].
The Clinical Laboratory Standards Institute (CLSI) and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have created a guideline for the establishment of RIs (EP28-A3C), in which a direct sampling approach using representative, healthy individuals is recommended [5]. However, this approach is demanding, costly and time-consuming; particularly in a paediatric population, requiring large populations to create multiple age and gender partitions. Sampling from healthy children, especially very young children, is furthermore technically difficult and has considerable ethical implications [20, 25].
According to the CLSI guideline, RIs might also be transferred from other populations, e.g. from kit inserts or the literature [5]. Subsequently, transference of the RI has to be validated. Validation of a transferred RI often relies on subjective assessment, as the recommended method of validation also requires direct sampling. Assessment of RI applicability is however crucial, as misclassification of samples as either healthy or diseases can result in erroneous diagnosis and treatment. Choosing which RI to implement can therefore be precarious.
The aim of this study is to evaluate differences in published paediatric RIs for haemoglobin (hgb), platelet count (plt) and white blood cell count (WBC) by assessing how the RIs classify local laboratory results stemming from healthy children. The significance of implementing different RIs will thus be highlighted. Means of validating transferred RIs by using locally generated laboratory data will further be illustrated.
Materials and methods
Selection of RIs
A search on PubMed (17th of March, 2018) on existing paediatric plt count, hgb and WBC count RIs was conducted. We included studies which made assessment of the RI according to the topics listed in Table 1 possible. Studies were excluded if they were conducted on a study population substantially different from Southern Denmark in terms of ethnicity (i.e., Caucasian) and overall children health as this could influence the complete blood count (CBC). Physician density [32] and under-five mortality rate [31] were used to assess conformity of reference populations. Thus, we only included studies conducted on European and North American populations. Studies on neonates were also excluded.
Assessment of RIs
Studies were evaluated according to the criteria listed in Table 1.
To standardise for comparison, we converted hgb results in g/dL and g/L to mmol/L by multiplying by 0.6206 and 0.06206, respectively [30]. Plt and WBC counts were declared in × 109/L.
The paediatric population
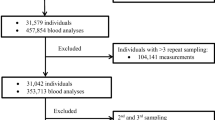
We defined a dataset of paediatric (age 0 days to < 18 years) haematological parameters from general practitioners (GPs) analysed at Lillebaelt Hospital (LH) and Odense University Hospital (OUH) over a 5-year period (13 September 2012–4 August 2017), which constituted our LH/OUH population. The local population was used to assess classification of samples and validate transference of RIs. We exclusively used samples originating from GPs for our paediatric population, as both hospitals manage specialised paediatric functions (i.e. paediatric emergency wards, neonatological wards and paediatric oncology wards). To ensure a healthy population, children with > 3 repeat measurements, or samples taken at hospital, were excluded, and only the first samples of each child was included in the final dataset. Both LH and OUH are situated in the region of Southern Denmark. Plt, hgb and WBC counts were analysed using Sysmex XN-9000 (Sysmex, Kobe, Japan).
Statistical validation of the transferred RIs
The LH/OUH dataset was transformed to a Gaussian distribution using Box-Cox transformation. Subsequently, the dataset was partitioned into 1-year age stratifications and subdivided according to gender. After the stratification, outliers were removed using the method by Tukey [28]. We then examined the classification of samples by determining the percentage of samples from our dataset that would be classified as above or below the RI for each transferred RI based on 1-year age intervals. We furthermore calculated the overall percentage of local samples above and below each RI. A quality demand (QD) based on desirable performance in relation to biological variance was applied [11]. With this, we determined whether RIs were transferable (i.e. validated the transference of the RI). According to the QD, RIs were deemed suitable for application in the local population if no more than 6% of local samples were outside of the RI in total, with a maximum of 4.5% outside one RI limit [17].
Frequencies are presented as numbers and percentages. Normally distributed data were presented as mean and standard deviation (SD) and median and interquartile ranges (IQRs) for non-normally distributed data. Data were analysed with Stata software package (Stata 15.1; StataCorp, College Station, TX, USA) and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
Published paediatric RIs for transference
We found 12 studies on paediatric RIs with a combined 28 RIs. Eleven RIs were found for plt count, ten for hgb and seven for WBC count. Descriptive data of the studies are presented in Table 2.
The designs of the selected published studies on RI are shown in Table 2.
The majority of studies use a direct sampling approach, as only Soldin et al. [23] and Zierk et al. [33, 34] based their RIs on laboratory data. Health assessment of reference individuals in the studies using a direct sampling approach varied considerably, and two studies used different means of health assessment depending on the children’s age [2, 21]. Range and number of age partitions furthermore differed, as well as the use of gender subclasses. Two studies [1, 2] used the CLSI recommended method for partitioning, whilst four studies [8, 23, 33, 34] did not disclose the method used, five relied on subjective evaluation [12,13,14, 21, 27] and one used other means of partitioning [3]. Similarly, four studies adhered to the recommendations for handling outlying observations, using either Tukey’s method [1, 33, 34] or Dixon’s rule [2]. Venepuncture was used to draw samples in all studies using a direct approach, whereas this was not addressed in the studies using the indirect sampling method. Time from sampling until analysis varied across studies, ranging from ‘immediately’ [1], within 1–6 h [3, 12, 13, 21, 27] to a maximum of 3 days post sampling [8]. Four studies did not disclose time from sampling till analysis [14, 23, 33, 34]. Different analytical platforms were used across studies. RIs were mainly reported as the 2.5th–97.5th percentile [1,2,3, 21, 23, 33, 34].
The LH/OUH population
Selection and characteristics of individuals in the local population is shown in Fig. 1. First sampling from 20,597 individuals < 18 years with combined 17,704 hgb, 13,849 plt and 18,658 WBC analyses constituted the final local population.
The majority of samples were taken from girls (55%). Distributions of data were non-Gaussian. Median plt count was 280 × 109/L (IQR 240 - 327 × 109/L), median hgb level was 13.37 g/dL (IQR 12.73 g/dL–14.18 g/dL) (8.3 mmol/L (IQR 7.9–8.8 mmol/L), and median WBC count was 6.8 × 109/L (IQR 5.6–8.4 × 109/L). A total of 831 outlying observations (227 for plt, 243 for hgb and 361 for WBC) were removed using Tukey’s method [28].
RI classification of samples
The classification of the LH/OUH samples by the individual RIs was evaluated and the percentage of samples classified as normal or abnormal (i.e. above or below the upper and lower RI limit) calculated. Details can be seen in Fig. 2. Overall, 0.6% of children had hgb results that were above the upper limits of all RIs, and 0.4% had results below all lower RI limits. In relation hereto, 36% of children had hgb results within all RI limits, meaning 63% of children in the LH/OUH population had hgb results that would change classification from normal to abnormal, depending on which RI was applied. Thus, 4.088 (42%) children had results that would change classification from normal to anaemic, whilst 2.020 (21%) children would change from normal to polycythaemic. For plt, 66% of children were within the RI limits, with 33% of children changing classification depending on RI, as 938 (12%) children would change classification form normal to thrombocytopenic and 1.570 (21%) from normal to thrombocytotic. For WBC, 22% of children would change classification, as 1.154 (12%) children would change from normal to leucopoenia, and 968 (10%) would change from normal to leukocytosis, depending on which RI was applied. Details are shown in Fig. 2, which furthermore show the distribution of LH/OUH samples in relation to the published RI limits for hgb, plt and WBC, respectively.
Local LH/OUH samples in relation to the age-specific lower and upper RI limits set by the included studies. Black lines reflect published reference limits. Grey dots are measurements from the local dataset. Percentage of samples changing classification depending on which RI was applied on the local population is shown for each parameter. Hgb haemoglobin, plt platelet count, WBC white blood cell count
Validation of transferred RIs
In accordance with the QD, RIs were suitable for implementation in the LH/OUH population if ≤ 6% of local samples were outside the RI limits in total, with a maximum of 4.5% of samples outside either the upper or lower limit. The total percentage of samples outside an RI varied from 3.7% (hgb RI, Romeo et al. [21]) to 39.3% (hgb RI, Hinchliffe et al. [13]). The hgb RI from Romeo et al. [21] was applicable in the LH/OUH paediatric population, but the remainder were not. Table 3 shows the percentage of samples from our LH/OUH population that were above or below each published RI.
The mean percentage of local samples outside the RI limits differed according to the analytical equipment used, with 14% (SD 9%) for the Sysmex analytical platform (i.e. the same platform as used in the local LH/OUH population), 17% (SD 11%) for the Beckman Coulter and 18% (SD 14%) for the Siemens/Bayer Advia equipment. On average, the mean percentage of local samples outside the RI limits furthermore differed according to the method applied for handling outlying observations, as 33% (SD 13%) of local samples were outside the RI limits of studies using the recommended methods (i.e. Dixon’s rule or Tukey’s method), whilst 43% (SD 29%) of local samples were outside the RI limits for studies using alternative methods for handling outlying observations (i.e. Chauvenet’s Criterion, Hoffmann truncation, distribution evaluation, Winsorization, or not disclosed). Percentage of samples outside the RI for studies using the indirect method was 39% (SD 21%) and 39% (SD 27%) for studies using a direct sampling approach.
Discussion
Twenty-eight paediatric RIs for plt, hgb and WBC from populations comparable to our local population were identified. The RIs differed considerably, as nearly two thirds of the local population would change classification from normal to abnormal, depending on which RI was chosen. When applying a QD based on desirable biological variation, 1 of the 28 RIs was transferable onto the LH/OUH population.
Comparison of studies was difficult, as there was considerable variation in methodology, including preanalytical variables, where congruency between studies was limited, making it problematic to determine whether they could apply for our local population. Preanalytical considerations are critical in the establishment of RIs and interpretation of blood sample results in general, as several preanalytical factors may influence results [4, 29]. In young children, capillary sampling is commonly used in daily practice [16]. Several CBC analytes yield different values in capillary compared to venous blood in children [7, 15, 19], especially plt count, as significant underestimations have been reported in capillary blood [10, 26]. Even so, Hollowell et al. [14] was the only study addressing capillary sampling. It is essential for laboratories to be aware of the sampling method and to take it into account when conveying results to the clinicians, as interpretation of capillary samples based on venous RIs should be done with caution.
Discrepancy in means of health assessment was also prominent, with two studies even applying different approaches for health evaluation depending on the children’s age [2, 12]. In large reference populations, data mining of hospital samples supersedes health, as the majority of hospital samples will be within the normal range [33, 34]. Interestingly, we observed no difference in the RIs based on direct and indirect sampling in terms of the percentage of samples outside the RI. For transference of RIs in general, a data mining process is also more easily standardised and thus easier to evaluate. Another advantage of laboratory date is the size of reference population attainable. Especially in paediatrics, it is difficult to establish sufficiently large populations with the direct sampling method, which is highlighted by only two of the studies evaluated here [1, 3] having sufficient individuals for each partition (i.e. minimum 120 individuals) for all their RI parameters. Insufficient number of reference individuals diminishes the statistical validity of a RI, thus limiting its use.
Methods applied for partitioning and the ensuing age stratifications were exceedingly different among studies. Partitions are only justified in situations where they depict clinically relevant differences. As many analytes are influenced by the physiological changes during childhood [6, 9, 18, 20, 22, 24], incorrect partitioning can contribute to misclassification and hence misinterpretation of results. To amend this, Zierk et al. [33, 34] argued that continuous RIs reflect the physiological changes better than conventional RIs. In a paediatric population, this approach however only seems feasible with an indirect sampling method.
The number of samples classified as healthy in our dataset (i.e. within the RI) differed substantially according to which RI was applied. Of clinical relevance, 63% of the LH/OUH children had hgb values that would change classification from normal to diseased, depending on which RI was applied. For WBC and plt, percentages were 22% and 33%, respectively. As several precautions were taken in the present study to ensure a healthy local population, the variation is not likely to be due to underlying disease in the local population. Our study included all paediatric RIs available that were conducted on a population comparable to the local paediatric population in terms of ethnicity and health status. The difference in classification of samples is thus more likely due to the heterogeneity in study designs, rather than physiological differences between the populations and emphasises the difficulties in choosing an appropriate RI from the literature.
The troubles associated with accepting a transferred RI is supported by other parts of the present study. Ideally, when implementing a published RI to a local population, the QD should approximate the definition of an RI, thus having a maximum of 2.5% of samples outside either RI [17]. In reality, this is however not feasible. The CLSI guideline suggests accepting a transferred RI if ≤ 10% of local samples are outside the RI. This validation approach is based on direct sampling of 20 healthy representative individuals, which is often unsuitable in paediatrics, especially in young children. The alternative, subjective validation is not well described. Given how vastly different the RI classified samples from healthy children are, it is important to bear in mind that seemingly suitable RIs with comparable reference populations is not enough for this subjective evaluation. We therefore used a QD based on biological variation which also accounts for bias [11]. As demonstrated in this article, it albeit leads to very few RIs deemed acceptable for transference.
The present study clearly shows the heterogeneity in published reference intervals and emphasises the challenges associated with transference of RIs and highlights the necessity for standardisation of the methodology applied for the establishment of paediatric reference intervals in order to minimise potential misinterpretation and misclassification of paediatric samples.
In conclusion, published paediatric RIs constitute a very heterogeneous group in terms of results and methodology. As a result, classification of samples by different RIs exhibit marked divergence. Assessment of RI transferability is problematic with seemingly few transferable RIs. Laboratories seeking to transfer RIs onto their local paediatric population therefore face a wide range of pitfalls in the process, which ultimately influence the interpretation of paediatric samples, thus possibly affecting the diagnosis and treatment of children.
Abbreviations
- RI:
-
Reference interval
- plt:
-
Platelet count
- hgb:
-
Haemoglobin level
- WBC:
-
White blood cell
- GP:
-
General practitioner
- CLSI:
-
Clinical and Laboratory Standards Institute
- QD:
-
Quality demand
- IFCC:
-
Federation of Clinical Chemistry and Laboratory Medicine
- CBC:
-
Complete blood count
- LH:
-
Lillebaelt Hospital
- OUH:
-
Odense University Hospital
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- ER:
-
Emergency room
References
Adeli K, Raizman JE, Chen Y, Higgins V, Nieuwesteeg M, Abdelhaleem M, Wong SL, Blais D (2015) Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian health measures survey. Clin Chem 61(8):1075–1086. https://doi.org/10.1373/clinchem.2015.240531
Aldrimer M, Ridefelt P, Rodoo P, Niklasson F, Gustafsson J, Hellberg D (2013) Population-based pediatric reference intervals for hematology, iron and transferrin. Scand J Clin Lab Invest 73(3):253–261. https://doi.org/10.3109/00365513.2013.769625
Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, Ulivi S, Di Castelnuovo A, Gogele M, Nutile T, Francavilla M, Sala C, Pirastu N, Cerletti C, Iacoviello L, Gasparini P, Toniolo D, Ciullo M, Pramstaller P, Pirastu M, de Gaetano G, Balduini CL (2013) Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects' data. PLoS One 8(1):e54289. https://doi.org/10.1371/journal.pone.0054289
Carraro P, Vettore G, Padoan A, Piva E, Plebani M (2015) Complete blood count at the ED: preanalytic variables for hemoglobin and leukocytes. Am J Emerg Med 33(9):1152–1157. https://doi.org/10.1016/j.ajem.2015.05.011
CLSI (2010) Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline - third edition. CLSI document EP28-A3C, vol Third edition. Clinical and Laboratory Standards Institute, Wayne
Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58(5):854–868. https://doi.org/10.1373/clinchem.2011.177741
Daae LN, Hallerud M, Halvorsen S (1991) A comparison between haematological parameters in ‘capillary’ and venous blood samples from hospitalized children aged 3 months to 14 years. Scand J Clin Lab Invest 51(7):651–654
Dortschy R, Rosario AS, Scheidt-Nave C, Thierfelder W, Thamm M, Gutsche J, Markert A (2009) Bevölkerungsbezogene Verteilungswerte ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS), vol 2017, Berlin
Favaloro EJ, Lippi G (2017) Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different. Ann Transl Med 5(10):212. https://doi.org/10.21037/atm.2017.04.18
Feusner JH, Behrens JA, Detter JC, Cullen TC (1979) Platelet counts in capillary blood. Am J Clin Pathol 72(3):410–414
Fraser CG, Hyltoft Petersen P, Libeer JC, Ricos C (1997) Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem 34(Pt 1):8–12. https://doi.org/10.1177/000456329703400103
Giacomini A, Legovini P, Gessoni G, Antico F, Valverde S, Salvadego MM, Manoni F (2001) Platelet count and parameters determined by the Bayer ADVIA 120 in reference subjects and patients. Clin Lab Haematol 23(3):181–186
Hinchliffe RF, Bellamy GJ, Bell F, Finn A, Vora AJ, Lennard L (2013) Reference intervals for red cell variables and platelet counts in infants at 2, 5 and 13 months of age: a cohort study. J Clin Pathol 66(11):962–966. https://doi.org/10.1136/jclinpath-2013-201742
Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, Centers for Disease C, Prevention NCfHS (2005) Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 11(247):1–156
Kayiran SM, Ozbek N, Turan M, Gurakan B (2003) Significant differences between capillary and venous complete blood counts in the neonatal period. Clin Lab Haematol 25(1):9–16
Krleza JL, Dorotic A, Grzunov A, Maradin M, Croatian Society of Medical B, Laboratory M (2015) Capillary blood sampling: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med (Zagreb) 25(3):335–358. https://doi.org/10.11613/BM.2015.034
Lykkeboe S, Nielsen CG, Christensen PA (2018) Indirect method for validating transference of reference intervals. Clin Chem Lab Med 56(3):463–470. https://doi.org/10.1515/cclm-2017-0574
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S (2006) Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 95(2):362–372. https://doi.org/10.1160/TH05-01-0047
Ozbek N, Gurakan B, Kayiran SM (2000) Complete blood cell counts in capillary and venous blood of healthy term newborns. Acta Haematol 103(4):226–228 doi:41056
Ridefelt P, Hellberg D, Aldrimer M, Gustafsson J (2014) Estimating reliable paediatric reference intervals in clinical chemistry and haematology. Acta Paediatr 103(1):10–15. https://doi.org/10.1111/apa.12438
Romeo J, Warnberg J, Gomez-Martinez S, Diaz LE, Moreno LA, Castillo MJ, Redondo C, Baraza JC, Sola R, Zamora S, Marcos A, group A (2009) Haematological reference values in Spanish adolescents: the AVENA study. Eur J Haematol 83(6):586–594. https://doi.org/10.1111/j.1600-0609.2009.01326.x
Sankaran VG, Orkin SH (2013) The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 3(1):a011643. https://doi.org/10.1101/cshperspect.a011643
Steven J, Soldin CB, Wong EC (2011) Pediatric reference ranges, 7th edn. AACC Press, Washington, DC
Stirnadel-Farrant HA, Galwey N, Bains C, Yancey C, Hunt CM (2015) Children’s liver chemistries vary with age and gender and require customized pediatric reference ranges. Regul Toxicol Pharmacol 73(1):349–355. https://doi.org/10.1016/j.yrtph.2015.07.013
Tahmasebi H, Higgins V, Fung AWS, Truong D, White-Al Habeeb NMA, Adeli K (2017) Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC 28(1):43–63
Tai DY, Chan KW, Chee YC, Mak KH (1995) Comparison of platelet counts in simultaneous venous and capillary blood samples using an automated platelet analyser. Singap Med J 36(3):263–266
Taylor MR, Holland CV, Spencer R, Jackson JF, O'Connor GI, O'Donnell JR (1997) Haematological reference ranges for schoolchildren. Clin Lab Haematol 19(1):1–15
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading
Unger G, Benozzi SF, Campion A, Pennacchiotti GL (2018) Preanalytical phase: effects of water ingestion during fasting on routine hematological parameters in a small cohort of young women. Clin Chim Acta 483:126–129. https://doi.org/10.1016/j.cca.2018.04.019
van Assendelft OW (1987) The international system of units (SI) in historical perspective. Am J Public Health 77(11):1400–1403
World HO (2016) Under-five mortality rate (per 1000 live births). http://apps.who.int/gho/data/view.xgswcah.10-viz. Accessed Accessed June 2018 June 2018
World HO (2018) Density of physicians (total number per 1000 population, latest available year). WHO. http://www.who.int/gho/health_workforce/physicians_density/en/. Accessed Accessed June 2018 2018
Zierk J, Arzideh F, Haeckel R, Rascher W, Rauh M, Metzler M (2013) Indirect determination of pediatric blood count reference intervals. Clin Chem Lab Med 51(4):863–872. https://doi.org/10.1515/cclm-2012-0684
Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, Rauh M (2015) Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem 61(7):964–973. https://doi.org/10.1373/clinchem.2015.239731
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the present study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alnor, A.B., Vinholt, P.J. Paediatric reference intervals are heterogeneous and differ considerably in the classification of healthy paediatric blood samples. Eur J Pediatr 178, 963–971 (2019). https://doi.org/10.1007/s00431-019-03377-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03377-w