Abstract
Human T-cell lymphotropic virus type 1 (HTLV-1) can induce a neuroinflammatory condition that leads to myelopathy. Pentraxin 3 (PTX3) is an acute-phase protein that its plasma concentration increases during inflammation. We aimed to determine whether PTX3 serum level is elevated in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients and HTLV-1 asymptomatic carriers (ACs) and evaluate its association with proviral load and clinical features. The serum level of PTX3 was measured using an enzyme-linked immunosorbent assay in 30 HAM patients, 30 HTLV-1 ACs, and 30 healthy controls. Also, the HTLV-1 proviral load was determined via real-time PCR technique. The findings showed that PTX3 serum level was significantly higher in HAM patients than in both asymptomatic carriers and healthy controls (p values < 0.0001). No correlation between PTX3 and the proviral load was observed in HAM patients and asymptomatic carriers (r = − 0.238, p = 0.205 and r = − 0.078, p = 0.681, respectively). The findings showed that there was no significant correlation between PTX3 and motor disability grading (MDG) (r = − 0.155, p = 0.41) nor urinary disturbance score (UDS) (r = − 0.238, p = 0.20). Higher levels of PTX3 are associated with HTLV-1-associated myelopathy compared to asymptomatic carriers. This finding may support the idea that PTX3 has the potential as a diagnostic biomarker.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) is a human retrovirus that affects 5–10 million people worldwide [1]. Razavi Khorasan province, in the northeast of Iran has been considered an endemic region [2]. HTLV-1 causes two major diseases, including malignancy of mature CD4+ T-cells named adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [3]. Although most individuals infected by HTLV-1 remain asymptomatic carriers (ACs) lifelong, almost 2–3% of the infected individuals develop HAM/TSP [4]. HAM/TSP is an inflammatory disorder affecting the central nervous system (CNS), in which demyelination of the thoracic cord has been observed, resulting in progressive loss of motor function [5]. HAM/TSP manifests with lower limbs weakness, lumbar pain, neuropathic pain, paresthesia, erectile dysfunction, and constipation [6, 7]. The precise mechanism by which HAM/TSP damages the CNS is still not fully elucidated [6]. However, some studies reported that the development of neuroinflammation in HAM/TSP patients is associated with a higher proviral load (PVL), increased infiltration of CD8+ T-cells and more secretion of proinflammatory cytokines such as interleukin (IL)-6, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α along with a decrease in Th2 cytokines such as IL-4 and IL-10 [8, 9]. It has been demonstrated that inflammation biomarkers provide more substantial evidence of HAM/TSP progression than neuronal injury markers, including Tau proteins and neurofilament [10].
Pentraxin 3 (PTX3), a member of the long pentraxin subfamily [11], is an acute-phase protein after trauma, injury, and infections [12]. PTX3 highly binds to various bacteria, fungi, and viruses [13], and activates the complement component C1q to enhance the destruction and clearance of these pathogens [14]. Upregulation of PTX3 expression has been observed in the CNS following inflammatory cytokine release, ischemia, and seizure-induced neurodegeneration [15,16,17]. Also, PTX3 serum level has been suggested as a new marker in Parkinson’s disease [18]. Furthermore, growing evidence points to the involvement of PTX3 in CNS infections [19, 20]. However, whether PTX3 levels are elevated in HAM/TSP patients has remained uncertain. We measured plasma levels of PTX3 protein in HAM/TSP patients, HTLV-1 asymptomatic carriers, and healthy control individuals to evaluate the correlation between PTX3 serum level and HTLV-1 proviral load as well as major clinical features of HTLV-1-associated myelopathy patients and whether PTX3 could be used as a useful biochemical marker to determine the infection progression and disease severity.
Method
Study design
The study protocol was approved by the ethics committee of Mashhad University of Medical Sciences with approval code IR.MUMS.MEDICAL.REC.1399.44.7. All the participants were informed about the study procedure and provided written consent before they participated in the study.
Population study
This study included, 90 participants divided into three groups: 30 HTLV-1 associated myelopathy (HAM) patients, 30 HTLV-1 asymptomatic carriers (ACs), and 30 healthy controls (HCs) who were admitted to the HTLV-1 clinic of Ghaem Hospital affiliated with Mashhad University of Medical Sciences (MUMS), Mashhad, Iran in 2021. It should be noted that the subjects in this study have been recruited from the first national HTLV-1 registry in Iran (Code: 941637).
The inclusion criteria for HAM patients included HTLV-1 positive serology confirmed by polymerase chain reaction (PCR) or Western blot, spastic paraparesis symptoms, and the presence of anti-HTLV-1 antibody in cerebrospinal fluid (CSF). The inclusion criteria for HTLV-1 AC cases were as follows: HTLV-1 positive serology confirmed by PCR or Western blot without any myelopathy-related symptoms and signs. On the other hand, a history of cardiovascular diseases, surgery during the last three months, acute cerebral infarction, rheumatoid arthritis, and Parkinson's disease, as well as the use of immunomodulatory drugs, were defined as exclusion criteria for all three groups.
Data measurements
The individuals were asked to complete a checklist on the demographic data (age, sex, duration of HTLV-1 disease, drug history as well as a history of blood transfusion, breast-feeding in infancy, surgery, dialysis, dental procedures, tattooing, cupping, and unsafe intercourse). All patients underwent neurological evaluation by a neurologist, including muscle strength examination using the Medical Research Council (MRC) scale [21], deep tendon reflexes examination, spasticity evaluation based on the modified Ashworth scale [22], and motor disability based on motor disability grading (MDG) ranging from normal walking and running (score 0) to complete bedridden (score 13), and urinary complaint evaluation using Urinary Disturbance Score (UDS) ranging from being normal (score 0) to severe disturbance based on a feeling of residual urine, incontinence and frequency (score 3) [23].
Blood collection
Plasma samples were obtained from all participants between 9:00 AM and noon and put in heparin tubes. The samples were centrifuged at 2000g for 15 min to eliminate cells and other insoluble materials and then were aliquoted in polypropylene tubes.
PTX3 measurement by plasma by enzyme-linked immunosorbent assay (ELISA)
PTX3 plasma levels were measured using a commercially available sandwich ELISA kit (ZellBio, GmbH, Germany) according to the manufacturer’s instructions in all three studied groups. According to the manufacturer, the kit sensitivity was 0.096 ng/mL, and the standard curve range was 0.312–20 ng/mL.
HTLV-1 proviral load
HTLV-1 Proviral load was quantified in HAM patients and ACs, using a Real-time PCR commercial Kit (Novin Gene, Iran) on extracted DNA from peripheral blood mononuclear cells (PBMCs), using Rotor-Gene Q 6000 machine (Qiagen, Germany) that was reported in details elsewhere [24].
Statistical analyses
The Statistical Package for Social Sciences version 16 was used for data analysis. Descriptive analysis was performed using mean ± standard deviation and median (first quartile- third quartile). Inferential analysis regarding differences in the PTX3 concentration between different study groups was performed using one-way ANOVA and post hoc test (LSD). Proviral load values were analysis between HAM and AC groups was performed with Mann–Whitney U test. The correlation between quantitative variables was reported based on the spearman correlation coefficient. Chi-squared test was used to compare the frequency of qualitative variables in three study groups. All tests were two-tailed, and p value < 0.05 was considered statistically significant.
Results
This study was carried out on 90 individuals. The study population was divided into three groups, and each group consisted of 30 participants. The demographic characteristics are shown in Table 1.
Serum level of PTX3
Serum levels of PTX3 mean ± SD, in HAM patients, ACs and HCs were 12.40 ± 4.85 ng/ml, 9.19 ± 2.46 ng/ml, and 8.95 ± 0.95 ng/ml, respectively (Fig. 1). PTX3 serum level was the highest in the HAM patients group and the lowest in the HC group (p < 0.0001). It was found that PTX3 serum level in the HAM patients was significantly higher than in AC and HC groups (p values < 0.0001), but no statistically significant difference was observed between AC and HC groups (p = 0.76).
Serum levels of PTX3 in HAM patients, asymptomatic carriers (ACs), and healthy controls (HCs). Serum PTX3 was higher in the HAM patients compared to AC and HC groups (p-values < 0.0001). No significant difference was observed between AC and HC groups (p = 0.76). Error bars show standard deviation. *** denotes p values < 0.0001 and N.S denotes non significant
Correlation between PTX3 plasma level and proviral load
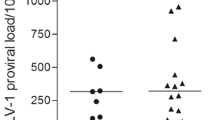
Quantitative real-time PCR was performed on the DNA extract from PBMCs, as previously described. The mean ± SD (median, 1st quartile–3rd quartile) of proviral load (PVL) was 273.2 ± 302.3 (199.9, 102.5–287.4) copies/104 PBMCs in HAM patients and 57.18 ± 111.14 (20.7, 12.3–68.8) copies/104 PBMCs in ACs, respectively. The difference in PVL between HAM patients and ACs was statistically significant (p < 0.001). There was no statistically significant correlation between PTX3 plasma level and PVL in HAM patients (r = − 0.238, p = 0.205) and ACs (r = − 0.078, p = 0.681) ( Fig. 2).
Clinical features of HAM patients
The correlation between PTX3 plasma level and clinical features of the HAM/TSP patients, including motor disability and urinary disturbance, were assessed. Motor disability and urinary disturbance were evaluated by motor disability grading (MDG) and Urinary Disturbance Score (UDS), respectively. The findings showed that there was no significant correlation between PTX3 serum level and MDG (r = − 0.155, p = 0.41) nor UDS (r = − 0.238, p = 0.20) (Figs. 3, 4).
Discussion
We assessed the serum level of PTX3 in HTLV-1-associated myelopathy (HAM) patients, HTLV-1 asymptomatic carriers (AC), and healthy controls (HC), as well as its association with proviral load and clinical symptoms. There was a statistically significant gender preference in HAM/TSP group, which also was reported by Shoeibi et al. in the HTLV-1 data registry in northeast of Iran [26]. It showed that the plasma level of PTX3 in HAM patients was significantly higher than ACs and HCs, whereas no significant difference was observed between AC and HC groups. Pentraxin 3 (PTX3) is recently discovered as an acute-phase protein belonging to the same family as C-reactive protein (CRP) [25]. PTX3 is stored in neutrophils and rapidly released upon pathogen challenge or tissue injury [27]. PTX3 has a vital role in the early stage of inflammation by recognizing microorganisms, enhancing the identification of the pathogen by phagocytes, and activating the classical and lectin complement pathway [28, 29].
PTX3 expression can be induced in neurons, microglia, and, or astrocytes following cytokine release or neuroinflammation. PTX3 upregulation has been revealed in other animal studies under different experimental neuroinflammatory models, such as limbic seizure [17], autoimmune encephalomyelitis [13], neurotrauma [30], globoid cell leukodystrophy [13], and ischemia [15]. Additionally, high serum level of PTX3 has been shown in patients with neurodegenerative conditions, including multiple sclerosis [31], Parkinson’s disease [18], and ischemic stroke [32]. An experimental study showed that PTX3 has a role in facilitating myelin phagocytosis during neuroinflammation, whereas it does not affect controlling the autoimmune neuroinflammation development [13]. However, only a few studies evaluated the correlation between PTX3 and CNS infections.
According to the literature, high serum PTX3 level was correlated with the prognosis and severity of infectious diseases [33]. Patients with dengue virus infection, pulmonary aspergillosis, tuberculosis, shigellosis, meningococcal disease, and leptospirosis have elevated PTX3 serum levels, which associated with disease severity and could serve as predictors of adverse outcomes [34,35,36,37,38,39,40].
In addition to the application of serum PTX3 level as a biomarker for detecting infections, it has also been used to monitor the response to therapy. In a study consisting of 220 lately diagnosed patients with mycobacterium tuberculosis, PTX3 serum level was remarkedly reduced in people who responded to treatment compared to patients with therapy failure [35]. Moreover, patients with community-acquired pneumonia [41] or pyelonephritis [42] showed a decreased level of PTX3 after antibiotic treatment. To the best of our knowledge, this study is the first one to evaluate the role of PTX3 levels in blood in the setting of CNS involvement with HTLV-1.
Proviral load is an essential factor in determining the outcomes of chronic viral infections, such as hepatitis B virus, hepatitis C virus, and HIV-1 and 2 [43]. Further, a recent study has emphasized the importance of PVL in the outcome of HTLV-1 infection [44]. Consistent with the literature [43], the current study showed that PVL was markedly higher in HAM/TSP patients than that of HTLV-1 HCs, indicating that active HTLV-I viral replication might have significant impact on the disease development.
To interpret the correlation between the serum levels of PTX3 and HTLV-1 proviral load in HAM/TSP and AC groups, we applied a non-parametric statistical technique, since the serum PTX3 levels and viral load values did not follow the normal distribution. However the correlation between serum levels of PTX3 and proviral load was not statistically significant in those groups. Regarding previous studies, PTX3 decreased the viral load of influenza [45] and Human cytomegalovirus [27] but increased the viral load of chikungunya and Ross River virus [46]. Although further studies might elucidate whether these types of results could be affected by experimental conditions or whether the findings remain true for various pathogen infections, the fact is that PTX3 has a complex role in immunoregulation.
This study has not demonstrated the association between PTX3 plasma level and HTLV-1 proviral load or the clinical features of HAM patients, such as motor disability nor urinary disturbance assessed by MDG and UDS scales. However, in one study that evaluated the PTX3 plasma levels in multiple sclerosis (MS) and Neuromyelitis Optica (NMO) patients, plasma levels of PTX3 were significantly increased in the relapse phase of both groups, and it also had a correlation with EDSS scores of the patients. In addition, the PTX3 plasma levels were decreased and did not correlate with EDSS scores in the remission phase [32]. The inconsistency between our study and the mentioned study in MS and NMO patients might be due to different inflammatory mechanisms involved in the pathophysiology of the diseases and also different disease courses of HTLV-1 associated myelopathy as a chronic evolving myelopathy and MS/NMO myelopathy as an acute or subacute evolving myelopathy.
Future longitudinal studies of HTLV-1 associate myelopathy patients and asymptomatic carriers seem to be needed to evaluate the exact role of PTX3 in the pathophysiology of HTLV-1 infection and disease progression and to define its diagnostic and prognostic value in HTLV-1infected individuals. This study demonstrated that plasma PTX3 level was higher in HAM patients than in both HTLV1 asymptomatic carriers and healthy controls. This finding may support the idea that PTX3 has the potential as a diagnostic biomarker in HAM patients. Further investigation to clarify the exact role of PTX3 in HTLV-1 infection may provide insights, enabling the recognition of possible drug targets for treatment.
Data availability statement
The data generated or analyzed during this study are available from corresponding author on reasonable request.
References
Gessain A, Cassar O (2012) Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 15(3):388. https://doi.org/10.3389/fmicb.2012.00388
Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, Sohgandi L, Azarpazhooh MR, Rezaee SA, Farid R, Bazarbachi A (2011) High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol 52(3):172–176. https://doi.org/10.1016/j.jcv.2011.07.004
Nozuma S, Kubota R, Jacobson S (2020) Human T-lymphotropic virus type 1 (HTLV-1) and cellular immune response in HTLV-1-associated myelopathy/tropical spastic paraparesis. J Neurovirol 26(5):652–663. https://doi.org/10.1007/s13365-020-00881-w
Mohammadi A, Fazeli B, Poursina Z, Tehranian F, Vakili V, Boostani R, Rafatpanah H (2019) HTLV-1-infected asymptomatic carriers compared to HAM/TSP patients over-express the apoptosis- and cytotoxicity-related molecules. Med Microbiol Immunol 208(6):835–844. https://doi.org/10.1007/s00430-019-00625-6
Gudo ES, Silva-Barbosa SD, Linhares-Lacerda L, Ribeiro-Alves M, Real SC, Bou-Habib DC, Savino W (2015) HAM/TSP-derived HTLV-1-infected T cell lines promote morphological and functional changes in human astrocytes cell lines: possible role in the enhanced T cells recruitment into central nervous system. Virol J 12(12):165. https://doi.org/10.1186/s12985-015-0398-x
Freitas NL, Gomes YCP, Souza FDS, Torres RC, Echevarria-Lima J, Leite ACCB, Lima MASD, Araújo AQC, Silva MTT, Espíndola OM (2022) Lessons from the cerebrospinal fluid analysis of HTLV-1-infected individuals: biomarkers of inflammation for HAM/TSP development. Viruses 14(10):2146. https://doi.org/10.3390/v14102146
Lopes Martins AL, Rios Grassi MF, de Aquino FA, Lacerda Araujo JP, Paixao TS, Galvão-Castro B, Boa-Sorte N (2018) Human T-lymphotropic virus-1-associated myelopathy/tropical spastic paraparesis is associated with sexual dysfunction in infected women of reproductive age. Sex Med 6(4):324–331. https://doi.org/10.1016/j.esxm.2018.07.002. (Epub 2018 Sep 1)
Hatatian N, Bosstani R, Mohammadi A, Mehraban S, Mahdifar M, Zemorshidi F, Mozhgani SH, Haji Ghadimi A, Foroughipour M, Rafatpanah H (2021) Evaluation of interleukin-32 and cyclooxygenase-2 expression in HAM/TSP patients and HTLV-1 asymptomatic carriers. Iran J Basic Med Sci 24(7):992–996. https://doi.org/10.22038/ijbms.2021.50821.11569
Guerra M, Luna T, Souza A, Amorim C, Carvalho NB, Carvalho L, Tanajura D, Cardoso LS, Carvalho EM, Santos S (2018) Local and systemic production of proinflammatory chemokines in the pathogenesis of HAM/TSP. Cell Immunol 334:70–77. https://doi.org/10.1016/j.cellimm.2018.09.009
Souza FDS, Freitas NL, Gomes YCP, Torres RC, Echevarria-Lima J, da Silva-Filho IL, Leite ACCB, de Lima MASD, da Silva MTT, Araújo AQC, Espíndola OM. Following the clues: usefulness of biomarkers of neuroinflammation and neurodegeneration in the investigation of HTLV-1-associated myelopathy progression. Front Immunol. 2021;12:737941. https://doi.org/10.3389/fimmu.2021.737941
Yi L, Tang J, Shi C, Zhang T, Li J, Guo F, Zhang W (2020) Pentraxin 3, TNF-α, and LDL-C are associated with carotid artery stenosis in patients with ischemic stroke. Front Neurol 10(10):1365. https://doi.org/10.3389/fneur.2019.01365
Qi S, Zhao F, Li Z, Liang F, Yu S (2020) Silencing of PTX3 alleviates LPS-induced inflammatory pain by regulating TLR4/NF-κB signaling pathway in mice. Biosci Rep 40(2):BSR20194208. https://doi.org/10.1042/BSR20194208
Ummenthum K, Peferoen LA, Finardi A, Baker D, Pryce G, Mantovani A, Bsibsi M, Bottazzi B, Peferoen-Baert R, van der Valk P, Garlanda C, Kipp M, Furlan R, van Noort JM, Amor S (2016) Pentraxin-3 is upregulated in the central nervous system during MS and EAE, but does not modulate experimental neurological disease. Eur J Immunol 46(3):701–711. https://doi.org/10.1002/eji.201545950
Moalli F, Paroni M, Véliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, Garlanda C (2011) The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol 186(9):5425–5434. https://doi.org/10.4049/jimmunol.1002035
Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E (2015) Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflamm 24(12):15. https://doi.org/10.1186/s12974-014-0227-y
Polentarutti N, Bottazzi B, Di Santo E, Blasi E, Agnello D, Ghezzi P, Introna M, Bartfai T, Richards G, Mantovani A (2000) Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol 106(1–2):87–94. https://doi.org/10.1016/s0165-5728(00)00214-9
Ravizza T, Moneta D, Bottazzi B, Peri G, Garlanda C, Hirsch E, Richards GJ, Mantovani A, Vezzani A (2001) Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience 105(1):43–53. https://doi.org/10.1016/s0306-4522(01)00177-4
Lee HW, Choi J, Suk K (2011) Increases of pentraxin 3 plasma levels in patients with Parkinson’s disease. Mov Disord 26(13):2364–2370. https://doi.org/10.1002/mds.23871
Bourgeois MA, Denslow ND, Seino KS, Barber DS, Long MT (2011) Gene expression analysis in the thalamus and cerebrum of horses experimentally infected with West Nile virus. PLoS ONE 6(10):e24371. https://doi.org/10.1371/journal.pone.0024371
Zatta M, Di Bella S, Bottazzi B, Rossi F, D’Agaro P, Segat L, Fabbiani M, Mantovani A, Luzzati R (2020) Determination of pentraxin 3 levels in cerebrospinal fluid during central nervous system infections. Eur J Clin Microbiol Infect Dis 39(4):665–670. https://doi.org/10.1007/s10096-019-03767-w
Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka-Moser V (2008) Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 40(8):665–671. https://doi.org/10.2340/16501977-0235
Charalambous CP (2014) Interrater reliability of a modified Ashworth scale of muscle spasticity. Classic papers in orthopaedics. Springer, Berlin, pp 415–417
Izumo S, Usuku K, Osame M, Arimura K, Igata A (1988) Effect of alpha-interferon treatment on HTLV-I associated myelopathy (HAM). Neurology 39(Suppl 1):242
Ramezani S, Shirdel A, Rafatpanah H, Akbarin MM, Tarokhian H, Rahimi H, Bari A, Jahantigh HR, Rezaee SA (2017) Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med Microbiol Immunol 206(4):327–335. https://doi.org/10.1007/s00430-017-0506-1
Staubli SM, Schäfer J, Rosenthal R, Zeindler J, Oertli D, Nebiker CA (2019) The role of CRP and Pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci Rep 9(1):18340. https://doi.org/10.1038/s41598-019-54910-8
Shoeibi A, Rafatpanah H, Azarpazhooh A, Mokhber N, Hedayati-Moghaddam MR, Amiri A, Hashemi P, Foroghipour M, Hoseini RF, Bazarbachi A, Azarpazhooh MR (2013) Clinical features of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in northeast Iran. Acta Neurol Belg 113(4):427–433. https://doi.org/10.1007/s13760-013-0194-6
Bozza S, Bistoni F, Gaziano R, Pitzurra L, Zelante T, Bonifazi P, Perruccio K, Bellocchio S, Neri M, Iorio AM, Salvatori G, De Santis R, Calvitti M, Doni A, Garlanda C, Mantovani A, Romani L (2006) Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood 108(10):3387–3396. https://doi.org/10.1182/blood-2006-03-009266
Porte R, Davoudian S, Asgari F, Parente R, Mantovani A, Garlanda C, Bottazzi B (2019) The long pentraxin PTX3 as a humoral innate immunity functional player and biomarker of infections and sepsis. Front Immunol 12(10):794. https://doi.org/10.3389/fimmu.2019.00794
Vengen IT, Enger TB, Videm V, Garred P (2017) Pentraxin 3, ficolin-2 and lectin pathway associated serine protease MASP-3 as early predictors of myocardial infarction—the HUNT2 study. Sci Rep 20(7):43045. https://doi.org/10.1038/srep43045
Oggioni M, Mercurio D, Minuta D, Fumagalli S, Popiolek-Barczyk K, Sironi M, Ciechanowska A, Ippati S, De Blasio D, Perego C, Mika J, Garlanda C, De Simoni MG (2021) Long pentraxin PTX3 is upregulated systemically and centrally after experimental neurotrauma, but its depletion leaves unaltered sensorimotor deficits or histopathology. Sci Rep 11(1):9616. https://doi.org/10.1038/s41598-021-89032-7
Wang H, Wang K, Wang C, Zhong X, Qiu W, Hu X (2013) Increased plasma levels of pentraxin 3 in patients with multiple sclerosis and neuromyelitis optica. Mult Scler 19(7):926–931. https://doi.org/10.1177/1352458512457845
Ryu WS, Kim CK, Kim BJ, Kim C, Lee SH, Yoon BW (2012) Pentraxin 3: a novel and independent prognostic marker in ischemic stroke. Atherosclerosis 220(2):581–586. https://doi.org/10.1016/j.atherosclerosis.2011.11.036
Mauri T, Bellani G, Patroniti N, Coppadoro A, Peri G, Cuccovillo I, Cugno M, Iapichino G, Gattinoni L, Pesenti A, Mantovani A (2010) Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med 36(4):621–629. https://doi.org/10.1007/s00134-010-1752-5
Ciancarella V, Lembo-Fazio L, Paciello I, Bruno AK, Jaillon S, Berardi S, Barbagallo M, Meron-Sudai S, Cohen D, Molinaro A, Rossi G, Garlanda C, Bernardini ML (2018) Role of a fluid-phase PRR in fighting an intracellular pathogen: PTX3 in Shigella infection. PLoS Pathog 14(12):e1007469. https://doi.org/10.1371/journal.ppat.1007469
Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D’Elios MM, Mantovani A, Del Prete G (2005) IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect 7(1):1–8. https://doi.org/10.1016/j.micinf.2004.09.004
Wagenaar JF, Goris MG, Gasem MH, Isbandrio B, Moalli F, Mantovani A, Boer KR, Hartskeerl RA, Garlanda C, van Gorp EC (2009) Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. J Infect 58(6):425–432. https://doi.org/10.1016/j.jinf.2009.04.004
Sprong T, Peri G, Neeleman C, Mantovani A, Signorini S, van der Meer JW, van Deuren M (2009) Pentraxin 3 and C-reactive protein in severe meningococcal disease. Shock 31(1):28–32. https://doi.org/10.1097/SHK.0b013e31817fd543
Mairuhu AT, Peri G, Setiati TE, Hack CE, Koraka P, Soemantri A, Osterhaus AD, Brandjes DP, van der Meer JW, Mantovani A, van Gorp EC (2005) Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol 76(4):547–552. https://doi.org/10.1002/jmv.20397
Kim HS, Won S, Lee EK, Chun YH, Yoon JS, Kim HH, Kim JT (2016) Pentraxin 3 as a clinical marker in children with lower respiratory tract infection. Pediatr Pulmonol 51(1):42–48. https://doi.org/10.1002/ppul.23199
Biagi E, Col M, Migliavacca M, Dell’Oro M, Silvestri D, Montanelli A, Peri G, Mantovani A, Biondi A, Rossi MR (2008) PTX3 as a potential novel tool for the diagnosis and monitoring of pulmonary fungal infections in immuno-compromised pediatric patients. J Pediatr Hematol Oncol 30(12):881–885. https://doi.org/10.1097/MPH.0b013e318180bc1d
Kao SJ, Yang HW, Tsao SM, Cheng CW, Bien MY, Yu MC, Bai KJ, Yang SF, Chien MH (2013) Plasma long pentraxin 3 (PTX3) concentration is a novel marker of disease activity in patients with community-acquired pneumonia. Clin Chem Lab Med 51(4):907–913. https://doi.org/10.1515/cclm-2012-0459
Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F, Barbati E, Nebuloni M, Cvetko Krajinovic L, Markotic A, Valentino S, Doni A, Tartari S, Graziani G, Montanelli A, Delneste Y, Svanborg C, Garlanda C, Mantovani A (2014) The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40(4):621–632. https://doi.org/10.1016/j.immuni.2014.02.015
Vakili R, Sabet F, Aahmadi S, Boostani R, Rafatpanah H, Shamsian A, Rezaee SA (2013) Human T-lymphotropic Virus Type I (HTLV-I) proviral load and clinical features in Iranian HAM/TSP patients: comparison of HTLV-I proviral load in HAM/TSP patients. Iran J Basic Med Sci 16(3):268–272
Jeffery KJ, Usuku K, Hall SE, Matsumoto W, Taylor GP, Procter J, Bunce M, Ogg GS, Welsh KI, Weber JN, Lloyd AL, Nowak MA, Nagai M, Kodama D, Izumo S, Osame M, Bangham CR (1999) HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci USA 96(7):3848–3853. https://doi.org/10.1073/pnas.96.7.3848
Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, Crouch EC, Brooks AG, Brown LE, Bottazzi B, Romani L, Mantovani A (2008) Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol 180(5):3391–3398. https://doi.org/10.4049/jimmunol.180.5.3391
Foo SS, Chen W, Taylor A, Sheng KC, Yu X, Teng TS, Reading PC, Blanchard H, Garlanda C, Mantovani A, Ng LF, Herrero LJ, Mahalingam S (2015) Role of pentraxin 3 in shaping arthritogenic alphaviral disease: from enhanced viral replication to immunomodulation. PLoS Pathog. 11(2):e1004649. https://doi.org/10.1371/journal.ppat.1004649. (Erratum in: PLoS Pathog. 2015 Apr;11(4):e1004797)
Acknowledgements
This study was performed as the thesis for neurology specialty degree of the first author. The authors should appreciate the support of Vice-chancellor for research at Mashhad University of Medical Sciences (990557).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
MM, ZV, HR and FZ performed research; MKR Analyzed data; ZV and FZ conceived and designed research; RB supervised and coordinated the study; MM wrote the paper draft; FZ contributed to manuscript editing. The manuscript was approved by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
The study protocol was approved by the ethics committee of Mashhad University of Medical Sciences with approval code IR.MUMS.MEDICAL.REC.1399.44.7. All the participants were informed about the study procedure and provided written consent before they participated in the study.
Additional information
Edited by Matthias J. Reddehase.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manzarinejad, M., Vahidi, Z., Boostani, R. et al. Pentraxin 3, a serum biomarker in human T-cell lymphotropic virus type-1-associated myelopathy patients and asymptomatic carriers. Med Microbiol Immunol 212, 271–278 (2023). https://doi.org/10.1007/s00430-023-00770-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-023-00770-z