Abstract
Adult T cell leukemia/lymphoma (ATLL) is a life-threatening malignancy of HTLV-1 infected Th lymphocytes. In the present study host–virus interactions were investigated by assessment of HTLV-1 proviral load (PVL) and host gene expression. A cross-sectional study was carried out on 18 ATLL, 10 HAM/TSP patients and 18 HTLV-1 asymptomatic carriers (ACs). DNA and mRNA of the peripheral blood mononuclear cells were extracted for PVL and LAT, BIM, c-FOS and RAD51 gene expression measurement using qRT-PCR. The mean PVL in ATLL patients was 11,430 ± 3770 copies/104 which was statistically higher than ACs, 530 ± 119 copies/104, (p < 0.001). The expression of BIM, and c-FOS in ATLL patients were higher than HTLV-1 ACs; however, there were no statistically significant differences. The expression of RAD51 as an essential player on DNA repair showed around 160 times increase in ATLL group (166 ± 95) compared to ACs (1.04 ± 0.34) which is statistically significant (p < 0.001). Interestingly, there was a positive correlation between RAD51 expression and HTLV-PVL. The expression of LAT as a central adaptor in TCR signaling interestingly was around 36 times higher in ATLL group than ACs (ATLL; 41.33 ± 19.91 vs. ACs; 1.15 ± 0.22, p < 0.001). This finding showed that TCR signaling pathway mainly provides the growth factors for transformed cells. Furthermore, the overexpression of RAD51 which has been induced in HTLV-1 infected cells as a consequence of virus replication is not able to overcome the DNA damage toward cell transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human T-cell leukemia virus type 1 (HTLV-1) is a member of Retroviridea, Delta genera that has been extensively studied for more than 30 years [1]. Approximately 10–20 million people are infected with HTLV-1 worldwide [2]. However, only 2–5% of infected people develop adult T-cell leukemia/lymphoma (ATLL) or HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [3, 4]. The virus may also be a risk factor for infective dermatitis, uveitis, and even cardiovascular diseases [4, 5].
Endemic areas include South America, Southwestern Japan, Central Africa, the Melanesian Islands, the Caribbean basin, and the Middle East [6, 7]. In Iran, HTLV-1 is endemic at least in two provinces, including Razavi Khorasan (5,994,402 population) and Northern Khorasan (811,572 population) [8]. Mashhad, the center of Razavi Khorasan province is a pilgrimage city, and Nishabur, are the most infected areas with a HTLV-1 prevalence of 2.1 and 7%, respectively [6, 9]. However, HTLV-1-associated disorders have been reported from other provinces such as Golestan, Alborz, and East Azarbayejan [6, 10,11,12].
Adult T-cell leukemia/lymphoma is an aggressive proliferation of mature activated CD4+ T cells associated with the human T-cell lymphotropic virus type 1 (HTLV-1) [5]. Leukemia develops after a very long latency period and is preceded by clonal expansion of HTLV-1-infected activated T cells [13]. ATLL was classified into four clinical subtypes by Shimoyama, According to the lymphocyte count, serum calcium concentration, lactatedehydrogenase (LDH) level, solid organ involvement, and the severity of systemic symptoms include smoldering, acute, chronic and lymphomatose forms [46]. Flower-like lymphocytes are representative of aneuploidy or abnormal chromosomal content which develops due to aberrant mitotic divisions [14]. The viral proteins, particularly Tax, play a central role for increasing the number of HTLV-1-infected cells by promoting oligoclonal proliferation and suppressing apoptosis [15]. Therefore, Tax expression fortifies the proviral load (PVL) and frequency of infected cells that might be indicating the progress to associated diseases [2]. A prospective study from carrier to ATLL revealed that such clonal proliferation is directly associated with the onset of ATLL; therefore, these studies illustrated that HTLV-1-infected clones can transform to malignancy during the carrier state [16].
Furthermore, many studies have demonstrated that HTLV-1 Tax and HBZ proteins can induce various cellular abnormalities, genetic and epigenetic alterations and induce inappropriate host immune responses involved in the leukemogenesis in ATLL [17]. The PX region of HTLV-1 genome, encoded Tax protein, is essential for the oncogenic transformation, transactivation, dysregulation of apoptosis pathway, DNA repair distraction, and cell cycle disruption within HTLV-1-infected cells [18, 19]. However, there are no clear determinants to differentiate the subjects who develop ATLL from those who remain asymptomatic. Findings of many studies supported that interaction between host biological responses and HTLV-1 main virulence elements such as PVL, Tax, and HBZ might be implicated in development and progression of ATLL [7, 10]. Therefore, alterations and errors during proliferation are accumulated progressively by several viral proteins in the host genome during the latent period and finally lead to the onset of ATLL [19].
The host immune responses are able to eliminate the virus infected or malignant cells, mainly with programmed cell death, induced by NK cells and CTLs. Therefore, to disseminate, HTLV-1 needs escaping from these host immune responses. HTLV-1 Tax has developed numerous mechanisms to subvert this control and induce cell cycle promotion in the absence of physiological signals [12]. In addition, host cell factors, particularly the ones which are involved in cell cycle progression, are implicated in ATLL development. For example, abnormal expression of c-FOS as a component of the inducible AP-1 transcriptional complex is crucial in T cell cycle promotion and in pathological conditions contributes to the cell transformation. The inducible transcriptional AP-1, composed of c-Fos and c-Jun proteins, is a regulator of major physiological processes such as cell proliferation, differentiation, and response to stress. It is also a necessary factor in a wide range of pathological situations, mainly tumorigenesis depending on the cell context [20]. Thus, Tax may affect c-FOS upstream signaling pathways and regulate expression of this gene to leukemogenesis of infected T cells. However, the direct effects of HTLV-1 infection on programmed cell death is less clear, Tax has been shown to affect cell immortalization by activating PI3K pathway and repressing Bax gene-expression [46]. Since Bax promotes apoptosis by inhibiting Bcl-2, this may imply a molecular mechanism for the resistance of HTLV-1-infected T cell lines to apoptosis inducing stimuli [21]. However, several reports have shown that HTLV-1-infected T cells can be induced to undergo apoptosis [20, 22]. Three groups of proto-oncogenic proteins (c-Myc/Max, c-FOS/c-Jun, and Bcl2/Bax) are known to exert a synergistic effect to enhance their roles in the pro- or anti-apoptotic action [23].
BIM (BCL-2-interacting mediator of cell death) induces apoptosis and is antagonized by anti-apoptotic BCL-2 family members. BIM plays a critical role in tumor cell biology, including the regulation of tumorigenesis through activities as a tumor suppressor, tumor metastasis, and tumor cell survival. Consequently, BIM has become the focus of intense interest as a potential target for cancer chemotherapy [24].
An additional disruptive effect of Tax emanates from its impact on the ability of the cell-cycle machinery to regulate DNA replication and cell division [12]. Furthermore, another possible pathway for cell cycle disruption is distraction of DNA repair. As results of the aneuploidy and polyploidy variation in infected T cell nuclear genome by HTLV-1, Tax could induce this kind of DNA variation by interrupting DNA repair mechanisms. RAD51 (repair protein of ds DNA breaks) is one of the main factors during the DNA double-strand break repair which play a renovating role in homologous recombination of DNA [25]. This molecule is responsible for monitoring strand pairing and homology stages of homologous recombination which cooperates with the other ssDNA-binding proteins similar to RAD52, PALB2, BRCA2, and RPA [26, 27].
Linker for activation of T cells (LAT) is an integral membrane protein that plays an important role in T cell activation [28]. Tyrosine phosphorylation of LAT induces the recruitment of signaling proteins that activate major signaling pathways for activating of the T cell functions [29].
Therefore, in this study HTLV-1 PVL and LAT, c-FOS, BIM and RAD51 gene expression was evaluated in ATLL patients and carrier subjects to determine interaction and correlations between host cell signaling, apoptosis, and DNA repair elements with virulence properties of HTLV-1 throughout these populations.
Materials and methods
Study design
Patients were admitted to the Department of hematology and oncology of Emam reza and Ghaem hospitals, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran, between January 2012 and December 2014. The inclusion criteria for studied subjects were newly diagnosed ATLL according to the World Health Organization criteria, no history of medications, autoimmunity or present infectious diseases. The study was approved by the Biomedical Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (No: 911304).
RNA extraction and cDNA synthesis
Whole-blood samples were obtained from 19 ATLL patients and 18 HTLV-1 asymptomatic carriers (ACs); then peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-treated blood samples by Ficoll density gradient (Cederline corporation, Canada).
Total RNA extraction from PBMCs was conducted using the Trizol reagents (Tripure Roche-Germany) to purify total RNA according to the manufacturer’s guidelines. All purified RNAs from all samples were treated with DNase before constructing complementary DNA (cDNA) to remove genomic DNA contamination. Then, cDNA was generated using random primers and reverse transcriptase according to the manufacturer’s instructions (Bioneer, South Korea).
For any probable infection, a sample was sent to the microbiology lab (Ghaem Hospital) for direct examination and culture.
Oligonucleotide designing and real-time PCR
Real-time PCR assay (TaqMan method) was carried out to measure host BIM, RAD51, c-FOS, and LAT expression and PVL of HTLV-1 in PBMCs using specific primers and a fluorogenic probe by Real-Time PCR in a Q 6000 Rotor gene machine (Qiagene, Germany) using the AccuPower® Plus DualStar™Master Mix (South Korea). Table 1 shows the nucleotide sequence of primers and probes. All samples were performed and related to the expression of an appropriate reference gene, β2-microglobulin (β2m) expression. The gene expression of BIM, RAD51, c-FOS, LAT, and β2m was relatively calculated from two 6-point standard curves. The normalized value of the expression for each gene was calculated as the ratio of relative copies number of the mRNA of interest/relative mRNA copies number of reference gene, indicated as the expression index.
Proviral load measurement
To assess the HTLV-1 PVL, PBMCs were isolated from EDTA-treated blood samples and cellular DNA was extracted for proviral load quantification, as explained earlier. Real-time PCR was performed using a commercial absolute quantification kit (Novin Gene, Iran) to measure the PVL of HTLV-1 using specific primers and a fluorogenic probe by a Rotorgen Q 6000 machine (Qiagen, Germany). The HTLV-1 copies number was reported as an actual amount of cellular DNA by means of quantification of the albumin gene as the reference gene (Albumin). HTLV-1 and albumin DNA concentrations were calculated from two 5-point standard curves. The normalized value of the HTLV-1 PVL was calculated as the ratio of HTLV-1 DNA copies number/albumin DNA copies number/2 × 104 and expressed as the number of HTLV-1 proviruses per 104 PBMCs [30].
Statistical analysis
The experimental results are statistically presented as the mean ± SEM. Data analysis was performed by SPSS software ver.13.0 (SPSS, Chicago, IL, USA). As Kolmogorow–Smirnow normality test showed none of the variables were admitted the normal distributions; therefore, nonparametric statistical tests including Man–Whitney and spearman correlation with Tukey post-test were used to compare the data between the groups and the correlation of the parameters, respectively. p values <0.05 were considered as a statistically significant.
Results
Descriptive analysis of patients
The study investigated host–microbe interactions by assessment of alteration in gene expression of the proteins in 19 patients (13 females and 6 males) with ATLL and 18 ACs (11 females and 7 males, Table 2). The mean age in ATLL group was 53 ± 8 and in ACs was 39 ± 9 years old (Table 2). There were not any significant differences in age and gender between groups.
As shown in Fig. 1, patients were classified based on birth place in this endemic area, Razavi Khorasan province, although most of the patients were Mashhad residents. There were not any differences in the frequency of the ATLL among these cities adjusted based on the population.
Out of 19 ATLLs, 17 patients had lymphomatosis, 5 patients had skin lesion, and 6 patients had opportunistic infections, mainly candidiasis. Moreover, in these immune-compromised subjects, two patients had aspergillosis, one patient Pneumocystis jiroveci, and two patients had P. aeruginosa in their skin lesions. Moreover, two patients had lymphadenopathy and skin lesion and one patient had immunodeficiency and skin lesion, simultaneously. None of the patients showed three clinical symptoms together (Fig. 2).
The clinical symptoms in ATLL subjects. None of the patients showed three clinical symptoms together. In immunodeficient patients, all of the six subjects had candidiasis, two patients had aspergillosis, and one patient had Pneumocystis jiroveci. Moreover, two patients had P. aeruginosa in their skin lesions. There was not any strongyloidiasis, tuberculosis or HIV infection
Gene expression
The mRNA expression level of BIM, a cell pro-apoptotic factor in ATLL patients (3.86 ± 1.11), was around three times higher than in HTLV-1 ACs (1.36 ± 0.39); however, there was no statistically significant difference between the two groups (Fig. 3a).
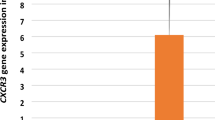
a The BIM expression in ATLL and healthy carriers. There was no statistically significant difference between two groups. b RAD51 expression in ATLL and healthy carriers. Significant difference was observed between two groups (p < 0.001). c The c-FOS expression in ATLL and healthy carriers. Despite the increased relative expression of c-FOS and BIM (a) in ATLL patients compared to ACs, there were no significant differences between ATLLs and ACs. d LAT expression in ATLL and healthy carriers; significant difference was observed between two groups (p < 0.001)
The expression of RAD51 as an essential player on DNA repair showed around 160 times increase in ATLL group (166 ± 95) compared to ACs (1.04 ± 0.34) which is statistically significant (p < 0.001; CI 95%; Fig. 3b).
Although c-FOS expression in the ATLL group was >tenfold higher than in the carrier group (35 ± 20 vs. 1.08 ± 0.46, respectively), there was no significant difference (Fig. 3c).
The mRNA expression of LAT as a central adaptor in TCR signaling was interestingly increased around 36 times in ATLL group compared to carrier group (p < 0.001, ATLL; 41.33 ± 19.91 vs. ACs; 1.15 ± 0.22; CI 95%; Fig. 3d).
Proviral load
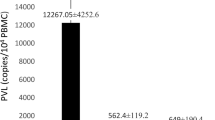
The mean PVL of HTLV-1 in ATLL patients was 11,430 ± 3770 copies/104 which was statistically different from HTLV-1 PVL in healthy carriers, 530 ± 119 copies/104, (p < 0.001; CI 95%; Fig. 4).
Discussion
Although most of ACs remain infected lifetime without developing any major clinical symptoms, a small part of infected individuals develop an aggressive lymphoproliferative disorder called adult T cell leukemia/lymphoma. Given the fact that only 2–5% of infected individuals develop ATLL, it seems that HTLV-1 infection alone is not sufficient for the transformation of infected cells. However, over 30 years after the discovery of HTLV-1, it is still not fully understood how HTLV-1 transforms mature TCD4+ cells. Therefore, viral–host interactions are assumed the main players of disease’s development and progression; thus host genetic, epigenetic abnormalities and host immunological status should be considered in attempting to understand mechanism of the oncogenesis of ATLL.
Like other studies, in the present study the ATLL patients showed a variety of clinical manifestations due to involvement of many organs by malignant cells, including lymphadenopathy, hypercalcemia, skin lesionsin and/or opportunistic infections which often contribute to the extremely high mortality of the disease [31]. In the present study lymphadenopathy had a strong negative correlation with presence of skin lesions in ATLL patients. These findings show coincidence probability of cutaneous manifestation and lymphomatosis. Moreover, around 21% of ATLL patients had opportunistic infection manifestations like pneumonia, vaginitis or thrush. High incidence of respiratory infectious episodes with C. albicans, Aspergillus, and Pneumocystis jiroveci in these patients might be due to immunosuppressive activity of the virus and malignancy condition [31, 32]. However, in contrast with superinfections in other endemic areas strongyloidiasis, tuberculosis or HIV infection was not detected. Therefore, in HTLV-1 endemic areas pulmonary complications should be considered during pregnancy. According to accumulated data, escaping from immune responses, increased HTLV-1 PVL, and clonal expansion of HTLV-1 infected cells are the main players of leukemogenesis in HTLV-1 carriers [16].
However, Cook et al. demonstrated that the PVL correlates with the total number of infected clones, not with the degree of oligoclonal proliferation. ATLL is frequently accompanied by a population of unusually abundant HTLV-1-infected T-cell clones [33]. The present study showed significant differences in HTLV-1 PVL between ATLL patients and ACs. In the same study group Akbarin et al. [30] demonstrated that HTLV-1 PVL was higher in ATLL patients in comparison with HAM/TSP and ACs and suggested that HTLV-1 PVL is a prognostic factor for development of HTLV-1 associated diseases and can be used as a monitoring marker for the efficiency of the therapeutic regime [30].
There are scant data on the mechanisms of pathogenesis of ATLL. Boxus M et al. showed that HTLV-1 has developed advanced mechanisms to guarantee persistence. HTLV-1 regulatory elements such as Tax and HBZ permit favored proliferation of the infected cells. HBZ, p12, and p30 either decrease viral expression or constrain immune recognition. Enduring Tax-induced proliferation and abnormal expansion of infected cells produce DNA lesions characteristic of ATLL. Advanced stabilization of these abnormalities provides an increased proliferative ability of the infected cells and finally leads to ATLL [34].
Furthermore, the HTLV-1-Tax protein is another crucial element for viral replication and for initiating malignant transformation leading to the progression of ATLL. Marriott et al. demonstrated that HTLV1- Tax protein, is necessary and sufficient for cell proliferation and consequently cell transformation, thus it can be considered as a viral oncoprotein. Tax interacts with numerous cellular proteins to reprogram cellular processes including, but not limited to, transcription, cell cycle regulation, DNA repair, and apoptosis [35]. Thus, in this study some intended processes were investigated (signaling, apoptosis pathways, and DNA repair).
Despite the increased relative expression of c-FOS and BIM in ATLL patients compared to ACs, there were not any significant differences between ATLLs and ACs. It has been shown that c-FOS as an important transcription factor in MAPK signaling pathway and BIM as an element of intrinsic apoptosis pathway are not major players in ATLL cell transformation. Nagata et al. suggested that cellular genes such as c-FOS, which regulate normal T-cell growth, are also activated directly or indirectly by p40tax and p40tax-induced modulation of gene expression plays a crucial role in T-cell transformation by HTLV-1 [36]. However, there was no association found between c-FOS expression and ATLL onset in the study; consequently other signaling pathway molecules such as pI3K/AKT and NF-kB pathways were investigated deeper. Jeong et al. suggested that AKT plays a role in the activation of pro-survival pathways in HTLV-1-transformed cells, possibly through NF-kB activation and inhibition of p53 transcription activity [37]. Saggioro et al. emphasized the importance of NF-κB pathways and CREB as a survival factor in various cell systems to HTLV-1 infection and progression of ATLL [38]. Moreover, Piazza et al. believed that BIM is epigenetically silenced in cell lines and anaplastic large cell lymphoma cells and should be involved in apoptosis and cancer development [39]. In the present study, BIM did not show any association with ATLL; this finding revealed that another apoptosis pathways and molecules may be involved. Soderquist et al. found Gossypol increased Noxa (mitochondrial apoptosis pathway molecule) and decreased Bcl2 and Bclxl expression in CLL patients; on the other hand, Miller et al. suggested that paclitaxel-induced apoptosis is BAK-dependent, but BAX-independent in human breast cancer cells [40, 41]. There are no reliable studies to investigate these apoptotic molecules in ATLL individuals. In another study we are trying to evaluate the role of these molecules in ATLL development (unpublished data).
LAT expression, a central adaptor in TCR signaling, was significantly increased in ATLL subjects compared to HTLV-1 ACs. Therefore, it seems that LAT acts as activator of growth and transformation element in ATLL cell progression toward the cancer. Januchowski et al. demonstrated that trichostatin A (TSA) resulted in ZAP-70, LAT, and SLP-76 transcript and protein down-regulation in Jurkat leukemia T cells and may be considered as an immunosuppressive effector [42]. Another study indicated that reactive oxygen species (ROS) induced T-cell receptor-induced lipid raft formation and T cell activation toward proliferation. Oxygen radicals activate LAT, phospho-LAT, and PLC-γ in T-cell hybridomas, T leukemia cells, and normal T cells. In other words, oxidative stress via LAT activation is a very important mechanism in activation, proliferation, and clonal expansion of T cells [43]. Therefore, preventing LAT-mediated T cell activation, by cell stress such as ROS, viruses and oncogenes, should be an important implication of therapeutic strategy, particularly a combined therapy regime with ROS scavengers for T cell malignancies [43].
DNA damage has been reported as a main cause of transformation in many studies; however, a preventive transformation strategy to overcome the DNA damage and consequently inducing cell cycle called RAD51 protects the cells from DNA damage. Klein et al. showed that there is a propensity for RAD51 to be overexpressed in tumor cells that results in increased resistance to DNA injury and medicines used in chemotherapies. This event leads to increased genomic instability and may contribute to carcinogenesis [44].
Regarding QueryRAD51, although in the present study its expression was strongly increased in ATLL patients compared to HTLV-1 ACs, it could not constrain DNA damage because of elevated activity of HTLV-1 in infected cells. It seems that ATLL cells attempt to overexpress RAD51 to overcome the DNA damage in transformed cell but fails. Thus, RAD51 overexpression in ATLL cells could serve as an effective biomarker for diagnosis and a possible target for the treatment.
Zhu et al. demonstrated that abnormally elevated RAD51 function and hyperactive homologous recombination (HR) rates have been found in a panel of cancers, including breast cancer and chronic myeloid leukemia (CML). They believed that directly targeting RAD51 and attenuating the deregulated RAD51 activity has, therefore, been proposed as an alternative and supplementary strategy for cancer treatment [45].
However, there are limitations for such studies. Although the present study was conducted on primary cells of ATLL patients, (1) the actual expression of proteins was not investigated. (2) The roles of other modulatory signals associated with RAD51, LAT or TCR signaling pathways together with HTLV-1 factor expression using next-generation sequencing based on mRNA and microRNA analysis must be validated. (3) The actual mechanisms in which these proteins contribute to cell transformation in ATLL are largely not understood [46].
Interestingly, there was a positive correlation between RAD51 expression and HTLV-1 PVL in our study group. This finding showed that overexpression of RAD51 has been induced in HTLV-1 infected cells as a consequence of virus replication activities; however, RAD51 overexpression is not able to overcome the DNA damages toward cell transformation. Furthermore, according to our results, it can be suggested that TCR signaling pathway mainly provides the growth factors for transformed cells.
Conclusions
TCR signaling pathway mainly provides the growth factors for transformed cells. Furthermore, the overexpression of RAD51 which has been induced in HTLV-1 infected cells as a consequence of virus replication is not able to overcome the DNA damage toward cell transformation.
Abbreviations
- ACs:
-
Asymptomatic carriers
- AKT:
-
Protein kinase B
- ATLL:
-
Adult T-cell leukemia/lymphoma
- β2m:
-
β2 microglobulin
- cDNA:
-
Complementary DNA
- CML:
-
Chronic myeloid leukemia
- CREB:
-
c-AMP response element binding protein
- HAM/TSP:
-
HTLV-1-associated myelopathy/tropical spastic paraparesis
- HBZ:
-
HTLV-1 basic zipper factor of oncogenesis
- HR:
-
Homologous recombination
- HTLV-1:
-
Human T-cell leukemia virus type 1
- LAT:
-
Linker for activation of T cells
- LDH:
-
Lactate dehydrogenase
- MUMS:
-
Mashhad University of Medical Sciences
- PBMCs:
-
Peripheral blood mononuclear cells
- PVL:
-
Proviral load
- ROS:
-
Reactive oxygen species
- TCD4+ cells:
-
CD4 T-lymphocytes
- TCR:
-
T-cell receptor
References
Kannian P, Green PL (2010) Human T lymphotropic virus type 1 (HTLV-1): molecular biology and oncogenesis. Viruses 2(9):2037–2077. doi:10.3390/v2092037
Gessain A, Cassar O (2012) Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 3:388. doi:10.3389/fmicb.2012.00388
Shoeibi A, Etemadi M, Moghaddam Ahmadi A, Amini M, Boostani R (2013) “HTLV-1 infection” twenty-year research in neurology department of mashhad university of medical sciences. Iran J Basic Med Sci 16(3):202–207
Farid Hosseni R, Jabbari F, Shabestari M, Rezaee SA, Gharivani Y, Valizadeh N, Sobhani M, Moghiman T, Mozayani F (2013) Human T lymphotropic virus type 1 (HTLV-1) is a risk factor for coronary artery disease. Iran J Basic Med Sci 16(3):217–220
Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, Matsuo R, Takiuchi Y, Uchino H, Hanaoka M (1982) Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer 29(6):631–635
Rafatpanah H, Hedayati-Moghaddam MR, Fathimoghadam F, Bidkhori HR, Shamsian SK, Ahmadi S, Sohgandi L, Azarpazhooh MR, Rezaee SA, Farid R, Bazarbachi A (2011) High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. J Clin Virol 52(3):172–176. doi:10.1016/j.jcv.2011.07.004
Azarpazhooh MR, Hasanpour K, Ghanbari M, Rezaee SA, Mashkani B, Hedayati-Moghaddam MR, Valizadeh N, Farid Hosseini R, Foroghipoor M, Soltanifar A, Sahebari M, Azadmanesh K, Hassanshahi G, Rafatpanah H (2012) Human T-lymphotropic virus type 1 prevalence in northeastern Iran, Sabzevar: an epidemiologic-based study and phylogenetic analysis. AIDS Res Hum Retroviruses 28(9):1095–1101. doi:10.1089/aid.2011.0248
Kiadaliri AA, Najafi B, Haghparast-Bidgoli H (2011) Geographic distribution of need and access to health care in rural population: an ecological study in Iran. Int J Equity Health 10(1):39
Hedayati-Moghaddam MR, Fathimoghadam F, Eftekharzadeh Mashhadi I, Soghandi L, Bidkhori HR (2011) Epidemiology of HTLV-1 in Neyshabour, northeast of Iran. Iran red crescent Med J 13(6):424–427
Boostani R, Mellat Ardakani A, Ashrafi H (2011) Khorasan Disease: prevalence of HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP) in west Azarbaijan from 2004 to 2007. Iran red crescent med J 13(6):428–430
Kalavi K, Moradi A, Tabarraei A (2013) Population-based seroprevalence of HTLV-1 infection in Golestan province, south east of Caspian sea, Iran. Iran J Basic Med Sci 16(3):225–228
Ahmadi Ghezeldasht S, Shirdel A, Assarehzadegan MA, Hassannia T, Rahimi H, Miri R, Rezaee SA (2013) Human T lymphotropic virus type 1 (HTLV-1) oncogenesis: molecular aspects of virus and host interactions in pathogenesis of adult T cell leukemia/lymphoma (ATL). Iran J Basic Med Sci 16(3):179–195
Wattel E, Vartanian JP, Pannetier C, Wain-Hobson S (1995) Clonal expansion of human T-cell leukemia virus type 1-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol 69(5):2863–2868
Trevino A, Aguilera A, Caballero E, Benito R, Parra P, Eiros JM, Hernandez A, Calderon E, Rodriguez M, Torres A, Garcia J, Ramos JM, Roc L, Marcaida G, Rodriguez C, Trigo M, Gomez C, de Lejarazu RO, de Mendoza C, Soriano V (2012) Trends in the prevalence and distribution of HTLV-1 and HTLV-2 infections in Spain. Virol J 9:71. doi:10.1186/1743-422x-9-71
Etoh K, Tamiya S, Yamaguchi K, Okayama A, Tsubouchi H, Ideta T, Mueller N, Takatsuki K, Matsuoka M (1997) Persistent clonal proliferation of human T-lymphotropic virus type-1 infected cells in vivo. Cancer Res 57(21):4862–4867
Okayama A, Stuver S, Matsuoka M, Ishizaki J, Tanaka G, Kubuki Y, Mueller N, Hsieh CC, Tachibana N, Tsubouchi H (2004) Role of HTLV-1 proviral DNA load and clonality in the development of adult T-cell leukemia/lymphoma in asymptomatic carriers. Int J Cancer 110(4):621–625. doi:10.1002/ijc.20144
Mesnard JM, Barbeau B, Devaux C (2006) HBZ, a new important player in the mystery of adult T-cell leukemia. Blood 108(13):3979–3982. doi:10.1182/blood-2006-03-007732
Tabakin-Fix Y, Azran I, Schavinky-Khrapunsky Y, Levy O, Aboud M (2006) Functional inactivation of p53 by human T-cell leukemia virus type 1 Tax protein: mechanisms and clinical implications. Carcinogenesis 27(4):673–681. doi:10.1093/carcin/bgi274
Cesarman E, Chadburn A, Inghirami G, Gaidano G, Knowles DM (1992) Structural and functional analysis of oncogenes and tumor suppressor genes in adult T-cell leukemia/lymphoma shows frequent p53 mutations. Blood 80(12):3205–3216
Brauweiler A, Garrus JE, Reed JC, Nyborg JK (1997) Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology 231(1):135–140
Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M (1994) The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol 68(5):3374–3379
Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi MV, Cara A, Nicot C, Giam C, Franchini G (1998) Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol 72(11):8852–8860
Kalra N, Kumar V (2004) c-FOS is a mediator of the c-myc-induced apoptotic signaling in serum-deprived hepatoma cells via the p38 mitogen-activated protein kinase pathway. J Biol Chem 279(24):25313–25319. doi:10.1074/jbc.M400932200
Harada H, Grant S (2012) Targeting the regulatory machinery of BIM for cancer therapy. Crit Rev Eukaryot Gene Expr 22(2):117–129
Ward JD, Muzzini DM, Petalcorin MI, Martinez-Perez E, Martin JS, Plevani P, Cassata G, Marini F, Boulton SJ (2010) Overlapping mechanisms promote postsynaptic RAD-51 filament disassembly during meiotic double-strand break repair. Mol Cell 37(2):259–272. doi:10.1016/j.molcel.2009.12.026
Martin JS, Winkelmann N, Petalcorin MI, McIlwraith MJ, Boulton SJ (2005) RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25(8):3127–3139. doi:10.1128/mcb.25.8.3127-3139.2005
Rinaldo C, Bazzicalupo P, Ederle S, Hilliard M, La Volpe A (2002) Roles for Caenorhabditis elegans RAD-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160(2):471–479
Fukumoto R, Dundr M, Nicot C, Adams A, Valeri VW, Samelson LE, Franchini G (2007) Inhibition of T-cell receptor signal transduction and viral expression by the linker for activation of T cells-interacting p12(I) protein of human T-cell leukemia/lymphoma virus type 1. J Virol 81(17):9088–9099. doi:10.1128/JVI.02703-06
Salek M, McGowan S, Trudgian DC, Dushek O, de Wet B, Efstathiou G, Acuto O (2013) Quantitative phosphoproteome analysis unveils LAT as a modulator of CD3zeta and ZAP-70 tyrosine phosphorylation. PLoS ONE 8(10):e77423. doi:10.1371/journal.pone.0077423
Akbarin MM, Rahimi H, Hassannia T, Shoja Razavi G, Sabet F, Shirdel A (2013) Comparison of HTLV-I proviral load in adult T cell leukemia/lymphoma (ATL), HTLV-1 associated myelopathy (HAM-TSP) and healthy carriers. Iran J Basic Med Sci 16(3):208–212
Shuh M, Beilke M (2005) The human T-cell leukemia virus type 1 (HTLV-1): new insights into the clinical aspects and molecular pathogenesis of adult T-cell leukemia/lymphoma (ATLL) and tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM). Microsc Res Tech 68(3–4):176–196. doi:10.1002/jemt.20231
White JD, Zaknoen SL, Kasten-Sportes C, Top LE, Navarro-Roman L, Nelson DL, Waldmann TA (1995) Infectious complications and immunodeficiency in patients with human T-cell lymphotropic virus I-associated adult T-cell leukemia/lymphoma. Cancer 75(7):1598–1607
Cook LB, Melamed A, Niederer H, Valganon M, Laydon D, Foroni L, Taylor GP, Matsuoka M, Bangham CR (2014) The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood 123(25):3925–3931. doi:10.1182/blood-2014-02-553602
Boxus M, Willems L (2009) Mechanisms of HTLV-1 persistence and transformation. Br J Cancer 101(9):1497–1501. doi:10.1038/sj.bjc.6605345
Marriott SJ, Semmes OJ (2005) Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 24(39):5986–5995. doi:10.1038/sj.onc.1208976
Nagata K, Ohtani K, Nakamura M, Sugamura K (1989) Activation of endogenous c-FOS proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line. Jurkat. J Virol 63(8):3220–3226
Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN (2005) Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 24(44):6719–6728. doi:10.1038/sj.onc.1208825
Saggioro D (2011) Anti-apoptotic effect of Tax: an NF-kappaB path or a CREB way? Viruses 3(7):1001–1014. doi:10.3390/v3071001
Piazza R, Magistroni V, Mogavero A, Andreoni F, Ambrogio C, Chiarle R, Mologni L, Bachmann PS, Lock RB, Collini P, Pelosi G, Gambacorti-Passerini C (2013) Epigenetic silencing of the proapoptotic gene BIM in anaplastic large cell lymphoma through an MeCP2/SIN3a deacetylating complex. Neoplasia 15(5):511–522
Soderquist RS, Danilov AV, Eastman A (2014) Gossypol increases expression of the pro-apoptotic BH3-only protein NOXA through a novel mechanism involving phospholipase A2, cytoplasmic calcium, and endoplasmic reticulum stress. J Biol Chem 289(23):16190–16199. doi:10.1074/jbc.M114.562900
Miller AV, Hicks MA, Nakajima W, Richardson AC, Windle JJ, Harada H (2013) Paclitaxel-induced apoptosis is BAK-dependent, but BAX and BIM-independent in breast tumor. PLoS ONE 8(4):e60685. doi:10.1371/journal.pone.0060685
Januchowski R, Jagodzinski PP (2007) Trichostatin A down-regulates ZAP-70, LAT and SLP-76 content in Jurkat T cells. Int Immunopharmacol 7(2):198–204. doi:10.1016/j.intimp.2006.09.010
Lu SP, Lin Feng MH, Huang HL, Huang YC, Tsou WI, Lai MZ (2007) Reactive oxygen species promote raft formation in T lymphocytes. Free radic biol med 42(7):936–944. doi:10.1016/j.freeradbiomed.2006.11.027
Klein HL (2008) The consequences of RAD51 overexpression for normal and tumor cells. DNA repair (Amst) 7(5):686–693. doi:10.1016/j.dnarep.2007.12.008
Zhu J, Zhou L, Wu G, Konig H, Lin X, Li G, Qiu XL, Chen CF, Hu CM, Goldblatt E, Bhatia R, Chamberlin AR, Chen PL, Lee WH (2013) A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO Mol Med 5(3):353–365. doi:10.1002/emmm.201201760
Banerjee K, Resat H (2016) Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer J Int Du Cancer 138(11):2570–2578
Acknowledgements
This study was supported by the Vice-chancellor for Research, Mashhad University of Medical Sciences (Grant number 911304) and was the subject of a student thesis for Ms Samaneh Ramezani. Great thanks to our colleagues in Inflammation and Inflammatory Research division, Ms N. Valizadeh, Dr. B. Fazeli, Ms S. Ahmadi Ghezeldasht, Dr. A. Mosavat and Ms A. Zeyaee Mehr.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest regarding this manuscript.
Rights and permissions
About this article
Cite this article
Ramezani, S., Shirdel, A., Rafatpanah, H. et al. Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med Microbiol Immunol 206, 327–335 (2017). https://doi.org/10.1007/s00430-017-0506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-017-0506-1