Abstract
Convergent evidence from task-based functional magnetic resonance imaging (fMRI) studies suggests a posterior-to-anterior shift as an adaptive compensatory scaffolding mechanism for aging. This study aimed to investigate whether brain functional dynamics at rest follow the same scaffolding mechanism for aging using a large Chinese sample aged from 22 to 79 years (n = 277). We defined a probability of brain regions being hubs over a period of time to characterize functional hub dynamic, and defined variability of the functional connectivity to characterize dynamic functional connectivity using resting-state fMRI. Our results revealed that both functional hub dynamics and dynamic functional connectivity posited an age-related posterior-to-anterior shift. Specifically, the posterior brain region showed attenuated dynamics, while the anterior brain regions showed augmented dynamics in aging. Interestingly, our analysis further indicated that the age-related episodic memory decline was associated with the age-related decrease in the brain functional dynamics of the posterior regions. Hence, these findings provided a new dimension to view the scaffolding mechanism for aging based on the brain functional dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Scaffolding Theory of Aging and Cognition (STAC) posits that age-related brain functional alternations operate under a compensatory mechanism, that is, less engagement of posterior (i.e., occipital and temporal) brain regions but greater involvement of anterior (i.e., frontal) brain regions when performing tasks (Goh and Park 2009; Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014; Sala-Llonch et al. 2015). Indeed, it is observed that task-induced functional activation shifts from occipitotemporal to frontal brain regions in aging population, suggesting the presence of adaptive scaffolding for aging brain in the posterior-to-anterior direction (Cabeza et al. 2004a; Davis et al. 2008; Ansado et al. 2012; Alperin et al. 2015). Specifically, during the encoding or retrieval stage of episode memory tasks, older subjects as compared to young subjects demonstrate decreased activation in the occipitotemporal region but increased activation in the frontal region (Cabeza et al. 2004a; Park et al. 2013). Furthermore, an age-related increase of frontal activation is positively correlated with episodic memory performance (Davis et al. 2008), suggesting that the aging brain attempts to recruit additional anterior neural substrates to alleviate episodic memory decline in aging.
Complementary to task-based functional magnetic resonance imaging (fMRI), resting-state fMRI (rs-fMRI) has increasingly been used in aging studies to further understand complex functional organization of aging brain via synchronization of resting-state blood-oxygen-level-dependent (BOLD) signals among brain regions (Buckner et al. 2009; Ferreira et al. 2015; La Corte et al. 2016). BOLD signal during rest or passive fixation has the advantage of minimizing task-evoked BOLD fluctuations, in the sense that brain regions with activation signal during tasks would have deactivation signal during rest (Van Dijk et al. 2010). Hence, it is not surprising that rs-fMRI studies reveal age-related brain functional connectivity alterations in the opposite direction as seen in task-fMRI studies, that is, an anterior-to-posterior direction (Sala-Llonch et al. 2015). Specifically, decreasing functional connectivities among frontal regions, and increasing functional connectivities among occipitotemporal regions and between the occipitotemporal and frontal regions are all as a function of age (Onoda et al. 2012; Ferreira et al. 2015; Lee et al. 2015). Beyond the examination of functional connectivity in aging, increasing studies have focused on brain hubs for understanding aging brain functional organization, mainly because they are densely connected with other brain regions. They form a central backbone of brain functional networks for global brain communication and play a critical role in the brain’s integrative processes supporting fast and efficient communication across brain regions (Buckner et al. 2009; van den Heuvel and Sporns 2013; Dai et al. 2014; Wang et al. 2015; Lee et al. 2016). Similar to the pattern of age-related alteration in the functional connectivity, degree centrality of hubs in the frontal region also shows a reduction in aging (Drzezga et al. 2011; Tomasi and Volkow 2012; Agosta et al. 2013). Importantly, both the anterior-to-posterior shift of the resting brain and the posterior-to-anterior shift of the task-functional brain reflect consistency of the complementary mechanisms for aging (Buckner et al. 2008, 2009). To compensate for aging neurodegeneration, the frontal region is more widespread engaged in performing tasks partly because of the reduction in its centrality (Grady 2012; Lee et al. 2015). In contrast, increased functional connectivities between posterior and prefrontal brain regions are detrimental to episodic memory in older adults (Gardini et al. 2015; Zhang et al. 2016). These findings further suggest for compensatory mechanisms of the resting brain in aging.
However, the brain is not static even at rest, but undergoes rapid fluctuation between different functional states (Allen et al. 2014; Hansen et al. 2015). Brain dynamics during rest are related to processing efficiency (de Pasquale et al. 2016) and cognitive demand (Cole et al. 2013, 2016). Dynamic functional connectivity in the resting brain reflects the switching of real-time cognitive states associated with neurophysiology (Kucyi and Davis 2014; Calhoun et al. 2014; Wilson et al. 2015). Rapidly switching functional connections is beneficial for cognitive functioning (Cocchi et al. 2013; Cole et al. 2013; Armbruster-Genc et al. 2016). As such, the longer the rs-fMRI acquisition is, the more the brain should be treated as dynamic, which is a current limitation of most rs-fMRI studies in aging (Hutchison et al. 2013a, b; Calhoun et al. 2014). Accordingly, based on the STAC model, one would expect that the frontal region becomes more flexible to compensate for less flexibility of the posterior region and cognitive functioning in aging. It is, therefore, important to understand the role of brain functional dynamics and its contribution to cognitive functioning in aging.
The present study aimed to study functional dynamics of aging brain using a large Chinese sample aged from 22 to 79 years (n = 277). Given the aforementioned importance of hubs and functional connectivity in aging, this study aimed to examine dynamics of these brain functional network measures in relation with age, which could potentially provide a new dimension to view the STAC model. For this, we defined the probability of a brain region being a hub in a certain period of time to assess hub dynamic, and defined the variability of functional connectivity over a period of time to assess dynamic of the functional connectivity. Based on the compensatory idea of the STAC model, we expected that as age increased, frontal brain hubs would lose their central and stable roles, which would then be compensated by engaging more brain regions as hubs in the occipital region. This could be reflected by a decreased hub probability in the frontal region and an increased hub probability in the occipital lobe. This could also be reflected by more variability of the functional connectivity in the frontal regions to adapt to neuronal challenges in aging. We further tested our hypothesis by examining the relationship between episodic memory performance and the brain functional dynamics for the interpretation of brain functional dynamics in aging, since decreased episodic memory is a well-known marker for aging (Lee et al. 2013; Pudas et al. 2013; Salami et al. 2016; Zhang et al. 2016).
Methods
Subjects
This study was approved by the National University of Singapore Institutional Review Board and all participants provided written informed consent prior to participation. Three hundred and forty-three healthy Singaporean Chinese volunteers aged 21–80 years were recruited (173 males, 170 females). Volunteers with the following conditions were excluded: (1) major illnesses/surgery (heart, brain, kidney, and lung surgery); (2) neurological or psychiatric disorders; (3) learning disability or attention deficit; (4) head injury with loss of consciousness; (5) non-removable metal objects on/in the body such as cardiac pacemaker; (6) diabetes or obesity; and (7) Mini-Mental State Examination (MMSE) score less than 24 (Ng et al. 2007). In addition, this study also excluded subjects who showed large head motion during rs-fMRI scanning with framewise displacement greater than 0.5 mm (Power et al. 2012). This resulted in the inclusion of 277 subjects from 22 to 79 years (132 males, 145 females). The demographic information is reported in Table 1.
MRI acquisition and pre-processing
All subjects were scanned using a 3T Siemens Magnetom Trio Tim scanner with a 32-channel head coil at the Clinical Imaging Research Centre of the National University of Singapore. The image protocols were: (1) high-resolution isotropic T1-weighted Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE; 192 slices, 1 mm thickness, sagittal acquisition, field of view 256 × 256 mm, matrix 256 × 256, repetition time 2300 ms, echo time 1.90 ms, inversion time 900 ms, flip angle 9°); (2) isotropic axial rs-fMRI imaging protocol (single-shot echo-planar imaging; 48 slices with 3 mm slice thickness, no inter-slice gaps, matrix 64 × 64, field of view 192 × 192 mm, repetition time 2300 ms, echo time 25 ms, flip angle 90°, scanning time 8 min). During the rs-fMRI scan, the subjects were asked to close their eyes.
For the T1-weighted images, gray matter (GM), white matter (WM), and cerebral spinal fluid (CSF) were automatically segmented using FreeSurfer (Fischl et al. 2002). The T1-weighted image was then transformed to the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al. 2002) via large deformation diffeomorphic metric mapping (LDDMM) (Du et al. 2011; Tan and Qiu 2016). The parcellation information of the AAL atlas (90 cerebrum and 26 cerebellum regions) was used as regions of interest (ROIs) in the resting-state fMRI analyses below.
The rs-fMRI data were pre-processed with slice timing, motion correction, skull stripping, band-pass filtering (0.01–0.08 Hz), and grand mean scaling of the data (to whole brain modal value of 100). Framewise displacement (head motion characteristics) was computed, and subjects with rs-fMRI data of framewise displacement greater than 0.5 mm were excluded from this study as mentioned above (Power et al. 2012). Then, the six parameters from head motion, CSF, and WM signals were regressed out from the rs-fMRI signal. Temporal band-pass filtering (0.01–0.08 Hz) was applied. Finally, the rs-fMRI data were transformed to the AAL atlas based on the LDDMM transformation between the corresponding T1-weighted image and the AAL atlas (Du et al. 2011; Tan and Qiu 2016).
Functional network construction and its dynamics
To construct brain network graphs, the AAL atlas was employed to parcellate the brain into 90 cerebrum and 26 cerebellum regions (n = 116). Dynamic functional network analysis was performed for each subject using a sliding-time window approach with the window size of 15 TR (34.5 s) (Kucyi et al. 2013; Zalesky and Breakspear 2015). The time interval of the two subsequent time windows was 1 TR, resulting 192 time windows for each subject. Comparisons between window sizes suggest that cognitive states can be correctly identified with as short as 30–60 s of data (Shirer et al. 2012), with topological assessments estimated to stabilize for window lengths greater than 30 s (Jones et al. 2012). Variation in window size between 30 s and 2 min had little effect on functional brain dynamics (Allen et al. 2014). Within each window, a 116 × 116 weighted brain network graph matrix was built using Pearson correlation analysis on the time series of two brain regions. We preserved only positive correlations in each network graph (Fair et al. 2009), and only considered the network graphs with sparsity ranging from 0.1 to 0.4 for the following analysis (Achard and Bullmore 2007).
Functional hub probability (dynamics)
Hubs in individual graphs were identified using degree-based approach (Sporns et al. 2007; van den Heuvel and Sporns 2011; Zuo et al. 2012; Power et al. 2013). The degree centrality of each region in a single graph was computed as the number of edges connected to the region using brain connectivity toolbox (Rubinov and Sporns 2010). Under each time window, hubs were identified as brain regions with degree centrality greater than one standard deviation above the mean value (Buckner et al. 2009). Functional hub probability was evaluated by the percentage of brain region acting as a hub over time and it was used to indicate functional hub dynamics. Thus, brain regions with a lower hub probability suggest less stability (or more dynamic) of being a hub.
Functional connectivity
We evaluated both static and dynamic functional connectivity. Static functional connectivity between each pair of regions was computed as the functional connectivity of these two regions averaged over time (Allen et al. 2014). Its variance over time (coefficient of variation, CV = SD/mean), dynamic functional connectivity, was computed to indicate the temporal fluctuation of functional connectivity between two brain regions (Shen et al. 2015).
To provide threshold-independent assessment on the hub probability as well as static and dynamic functional connectivity, area under curve (AUC) was computed as a sum of each measure over the network sparsity ranging from 0.1 to 0.4 (He et al. 2009; Wang et al. 2011). In addition, to streamline the number of statistical analyses needed to investigate aforementioned hypothesis, we grouped the anatomical regions of our network into a coarser level of anatomical groups. The 116 brain regions were classified into 12 anatomical groups (see the grouping in Fig. 1 and column 2 of Table S1 in the Supplementary Material) that have been suggested as major structures with greater age-related effects (Andrews-Hanna et al. 2007; Vergun et al. 2013; Geerligs et al. 2014; Lee et al. 2015). The network metrics were averaged across the brain structures in each anatomical group for statistical testing below.
Anatomical parcellation. DLPFC dorsolateral prefrontal cortex, MPFC medial prefrontal cortex, ACC anterior cingulate cortex, PCC posterior cingulate cortex, PCu precuneus, SMC sensorimotor cortex, LPC lateral parietal cortex, LOC lateral occipital cortex, MOC medial occipital cortex, LTL lateral temporal lobe, MTL medial temporal lobe
Cognitive tasks
The computerized language-independent cognitive tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB, Cambridge Cognition, Cambridge, UK) (Luciana and Nelson 2002) were employed to test episodic memory function. Participants were screened on two motor and learning tasks to verify the ability to follow simple instructions. As ours and other previous studies showed prominent age-related decline in the performance of Paired Associates Learning (PAL) task (Egerházi et al. 2007; Junkkila et al. 2012; Lee et al. 2013, 2016), this study, therefore, focused on this episodic memory task to examine the relationship between cognitive performance and functional brain dynamics.
In detail, PAL is a visuospatial associative learning task (Egerházi et al. 2007). Participants are presented with six white squares arranged in a circle. Patterns are sequentially presented in each of the six squares for 3 s in a random order. Subsequently, the same patterns are represented in the center of the screen in a different order. Participants are instructed to select the square indicating the original location of the pattern. No time limit is enforced. The number of patterns presented increases if participants correctly locate the original location of every pattern on the first attempt. In the first stage, only one pattern is presented, increasing to two patterns in stage 2, three patterns in stage 3, six patterns in stage 4, and finally eight patterns in stage 5. If participants fail to recall the locations correctly, the patterns in that stage are represented for up to ten attempts per stage. Failure to recall the correct order after ten attempts results in the termination of the task. To evaluate participant’s episodic memory ability, the first trial memory score was utilized. The first trial memory score was calculated as the number of objects correctly associated with their locations in the first attempt for each trial, summed across the stages completed. The full score for the task is 26 points. A higher score indicates better episodic memory ability.
Statistical analysis
Multiple regression models were employed to examine age effects on the hub probability of 116 AAL regions. In the full regression model, linear and quadratic terms of age were entered as main factors and the hub probability as the dependent variable. If the quadratic term was not significant, a reduced regression model was employed with only the linear term of age as main factor. Gender and education level were considered as covariates. False discovery rate (FDR) was carried out for correcting the multiple comparisons of the 116 brain regions. Second, the same regression model was performed to the hub probability, and static and dynamic functional connectivity at the level of the 12 anatomical groups (see grouping in Fig. 1 and column 2 of Table S1 in the Supplementary Material). FDR was carried out for correction for multiple comparisons at the level of the 12 anatomical groups.
To determine the relationship between the brain network measures and episodic memory performance, the age effect was examined on episodic memory using linear regression analysis between age and the first trail memory score of PAL, while controlling for gender and educational level. Subsequently, the same analysis was employed to assess the association between brain network measures and the first trail memory score of PAL. To reveal the neural substrates shifting from posterior to anterior regions in cognitive aging, the hub probability was averaged over the posterior regions (i.e., LTL, MTL, LOC, and MOC) or the anterior regions (i.e., DLPFC, MPFC, ACC/Insula, PCC/PCu, SMC, and LPC). Here, the grouping of the anterior and posterior regions was based on evidence of the proposed posterior-to-anterior shift in aging model (Davis et al. 2008) and on the similarity of age-related brain functional adaptation in these regions (Sala-Llonch et al. 2015). Likewise, the averaged values in the anterior and posterior regions were also computed for the static and dynamic functional connectivity.
Results
Age effects on functional hub probability
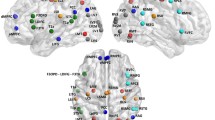
Figure 2a shows an example of the functional hub probability for individual subjects under the sparsity of the functional network of 0.2. Figure S1 in the Supplementary Material illustrates similar examples when the sparsity of the functional network is at 0.1, 0.15, 0.25, 0.3, 0.35, and 0.4. Figure 2b illustrates examples of the functional hub probability on the three-dimension brain atlas.
Functional hub probability of individual subjects. a Functional hub dynamics of individual subjects at the sparsity level of the brain functional network of 0.2. x-axis represents age of individual subjects, while y-axis represents individual anatomical structures. Color encodes the hub probability. The higher probability indicates that the node more frequently plays a role of a functional hub over time, which is a stable hub. b Functional hub dynamics of selected subjects mapped on the three-dimension brain atlas. The nodes are located according to their centroid stereotaxic coordinates. The color of each node represents its hub probability
For the hub probability of 116 individual brain regions, no quadratic age effects were identified. However, linear regression analysis revealed a significant age-related decrease of the hub probability in the anterior brain regions, including bilateral superior, middle and inferior frontal gyrus, precentral gyrus, supplementary motor area, anterior and middle cingulate gyrus, as well as right insula, inferior parietal lobule, supramarginal gyrus, etc. (see details in Table S2 of the Supplementary Material and Fig. 3; p < 0.05, FDR corrected). Regions located in the posterior brain regions, on the other hand, showed a significant age-related increase of hub probability, including bilateral superior, middle, and inferior occipital gyrus, cuneus, calcarine sulcus, middle temporal gyrus, as well as left inferior temporal gyrus, fusiform gyrus, parahippocampus, middle temporal pole, etc. (see details in Table S2 of the Supplementary Material and Fig. 3; p < 0.05, FDR corrected). These results suggest that the frontal regions are more flexible for being hubs and are dynamically changing over time in aging, which is in contrast of the posterior brain regions.
Age effects on functional hub probability. Nodes are located according to their centroid stereotaxic coordinated. The color of each node represents its standardized β value from the linear regression analysis, and the range of β value is from −0.46 to 0.36. The nodes revealed that significant age effects are shown in bigger size (p < 0.05, FDR corrected)
The statistical analysis was further simplified by grouping into 12 brain regions (see the brain structure grouping in column 2 of Table S1 in the Supplementary Material) for examining the STAC model. Our results revealed linear age effects but not quadratic age effects (p < 0.05, FDR corrected). Linear effects showed a decreased hub probability (an increased functional hub dynamic) in the anterior brain region and an increased hub probability (a decreased functional hub dynamic) in the posterior brain regions as age increases. Specifically, significant negative effects of age were observed on functional hub probability of the anterior brain regions, including DLPFC, ACC/Insula, PCC/PCu, LPC, and SMC, as well as subcortical regions (see Table 2; p < 0.05, FDR corrected). In contrast, significant positive effects of age on the functional hub probability were revealed in the posterior brain regions, including LTL, MTL, LOC, and MOC (see Table 2; p < 0.05, FDR corrected). Again, these findings showed more flexibility of the anterior regions but less flexibility of the posterior regions in aging, supporting the idea of the posterior-to-anterior shift in aging.
Age effects on static functional connectivity
To examine age effects on static functional connectivity, regression analysis revealed 34 out of 66 functional connections (i.e., according to the 12 anatomical groups) that exhibited significant linear age effects but no significant quadratic age effects. Among these 34 functional connections, 28 of them showed age-related decreases in static functional connectivity, while 6 of them showed age-related increases in static functional connectivity (see Fig. 4a; p < 0.05, FDR corrected). Specifically, static functional connectivities with age-related decreases were predominantly within the anterior brain regions (within DLPFC, PCC/PCu, SMC, and between DLPFC–ACC/Insula, DLPFC–PCC/PCu, DLPFC–LPC, ACC/Insula–PCC/PCu, ACC/Insula–SMC, ACC/Insula–LPC, PCC/PCu–SMC), between anterior and posterior regions (between DLPFC–LTL, DLPFC–MTL, PCC/PCu–LTL, SMC–LTL, SMC–MTL, SMC–MOC, LPC–LTL, and LPC–MTL), as well as between anterior and subcortical/cerebellar regions, which is in line with existing literature (Onoda et al. 2012; Ferreira et al. 2015; Lee et al. 2015). Moreover, static functional connectivities with age-related decreases were also identified in connections within MOC, within subcortical, and between subcortical and cerebellar regions. On the other hand, age-related increase of static functional connectivity was located within the posterior regions (between LTL–MTL and MTL–MOC), between MPFC and posterior regions (LOC and MOC), as well as between posterior and cerebellar regions. These findings are consistent with literature, suggesting that static resting brain shows the anterior-to-posterior shift in aging (Onoda et al. 2012; Ferreira et al. 2015; Lee et al. 2015).
Age effects on functional connectivity. Each line indicates significant age effect on static (a) and dynamic (b) functional connectivity. Each circle represents 12 brain regions and significant age-related increasing (red lines) and decreasing (green lines) connections between regions. The width of the line indicates p values. The thicker the line is, the smaller p value is. *p < 0.05, FDR corrected and **p < 0.01, FDR corrected
Age effects on dynamic functional connectivity
In examining age effects on dynamic functional connectivity, quadratic age effects was revealed in 5 out of 66 connections (Figure S2 in the Supplementary Material). These were the dynamic functional connectivities between MTL and DLPFC (β = −0.239; p = 0.030, FDR corrected), between MTL and SMC (β = −0.215; p = 0.050, FDR corrected), between subcortical and SMC (β = −0.245; p = 0.031, FDR corrected), between cerebellum and PCC/PCu (β = −0.219; p = 0.045, FDR corrected), as well as between cerebellum and SMC (β = −0.284; p < 0.001, FDR corrected). Figure S2 in the Supplementary Material illustrates the scatter plots of these dynamic functional connectivities in relation with age. Moreover, regression analysis also found linearly increased age effects on 9 of 66 dynamic functional connectivities within the anterior regions (within DLPFC, SMC, and between DLPFC–ACC/Insula, DLPFC–LPC, ACC/Insula–PCC/PCu, ACC/Insula–SMC), as well as between SMC and LTL, within MOC, and between ACC/Insula and subcortical regions. In contrast, linearly decreased age effects were observed on 27 of 66 dynamic functional connectivities (see Fig. 4b; p < 0.05, FDR corrected) within the posterior regions (between LTL–LOC, MTL–LOC, and MTL–MOC), between posterior and anterior regions (between LTL–MPFC, MTL–PCC/PCu, MTL–SMC, MTL–LPC, LOC–DLPFC, LOC–MPFC, LOC–ACC/Insula, LOC–PCC/PCu, LOC–SMC, LOC–LPC, MOC–DLPFC, MOC–MPFC, and MOC–LPC), between posterior and subcortical/cerebellar regions, between MPFC and SMC, and between anterior (PCC/PCu and SMC) and cerebellar regions. These findings further support the idea of the posterior-to-anterior shift of dynamic aging brain.
Relationship between functional brain dynamics and episodic memory performance
The first trial PAL memory score showed a negative relationship with age (β = −0.254, p < 0.002), suggesting an age-related decline of episodic memory performance.
For the functional hub dynamics, the first trial PAL memory score was negatively associated with the functional hub probability of the posterior regions (β = −0.195, p = 0.018), suggesting that the decreased functional hub dynamics of posterior regions was associated with reduced episodic memory performance in aging. However, no significant result was revealed between the functional hub probability of the anterior regions and the first trial PAL memory score (β = 0.084, p = 0.312).
For the functional connectivity, no significant results were found between static functional connectivity and the first trial PAL memory score (among anterior regions: β = 0.017, p = 0.838; among posterior regions: β = −0.074, p = 0.371; between anterior and posterior regions: β = −0.093, p = 0.263). However, positive correlation was revealed between dynamic functional connectivity in the posterior region and PAL memory score (β = 0.186, p = 0.025), suggesting that age-related decrease in dynamic functional connectivity within the posterior region was consistent with age-related decrease in episodic memory performance. No significant result was found between the first trial PAL memory score and dynamic functional connectivity in the anterior regions (β = 0.009, p = 0.913), as well as between anterior and posterior regions (β = 0.055, p = 0.506).
Discussion
This study examined age effects on brain functional dynamics using a large Chinese sample aged from 22 to 79 years. Consistent with most of existing literature (Onoda et al. 2012; Ferreira et al. 2015; Sala-Llonch et al. 2015; Lee et al. 2015), the static functional connectivity manifested an anterior-to-posterior shift in aging; that is, the posterior brain regions showed an age-related increase in the functional connectivity while the anterior brain regions demonstrated an age-related decrease in the functional connectivity in aging. This study provided new evidence on brain functional dynamics in aging that is consistent and complementary to the static resting brain in aging. In detail, our results revealed that both functional hub probability and dynamic functional connectivity posited an age-related posterior-to-anterior shift. Specifically, the posterior brain regions showed attenuated dynamics, while the anterior brain regions showed augmented dynamics in aging. Interestingly, our analysis further indicated that the age-related episodic memory decline was associated with the age-related decrease in brain functional dynamics of the posterior regions. Hence, these findings provided a new dimension to view the STAC model based on the brain functional dynamics, further suggesting that adaptive posterior-to-anterior shift of the brain functional dynamics occurs in response to aging.
Our study revealed a posterior-to-anterior shift of the brain functional dynamics in aging. This was supported by both the dynamics of hubs and functional connectivity. The frontal region in general has a less chance to be hubs in aging brain, implicating an age-related attenuated central role of the frontal region in the brain functional network architecture. This is congruent with the idea of the STAC model on more frontal region engaged to perform tasks as one ages (Cabeza et al. 2004b; Davis et al. 2008; Ansado et al. 2012). This is also in line with the frontal regions attempt to compensate for the loss of functional specificity in the occipital region during tasks (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014). Our study additionally provided a new dimension of the compensatory mechanism of the frontal region in aging. The frontal region increased its flexibility to switch on and off its functional connections as reflected by an increase in the variability of its functional connectivity in aging. In contrast, the influence of aging on the functional dynamics of the posterior region was opposite to that in the anterior region. Interestingly, the attenuation of brain functional dynamics in the posterior regions was associated with poor episodic memory function in aging. Recent studies suggest that rapidly switching functional connectivities is beneficial for cognitive functioning (Cocchi et al. 2013; Cole et al. 2013; Armbruster-Genc et al. 2016). An increase in brain dynamicity promotes the flexibility of the brain in configuring its functional architecture to adapt to cognitive need (Cole et al. 2013). On the other hand, being less dynamic reflects that the brain’s functional architecture is less adaptive or un-refreshed, which subsequently leads to poor cognitive ability in aging (Turner and Spreng 2015). Our results implicated that the posterior region of aging brain could operate under a less adaptive functional architecture, which accounted for poor episodic memory in aging. Moreover, there is evidence indicating that the brain functional dynamics are dependent on the underlying structural connections (Shen et al. 2015). Using the same sample, we previously found a posterior-to-anterior shift of the structural connectivity in aging, which was assessed using high angular resolution diffusion imaging (HARDI) (Lee et al. 2015). Taken together, these findings underscore the importance of the posterior-to-anterior shift in adaptiveness of brain functional architecture in aging brain, which provided an additional view on the STAC model for aging brain (Park and Reuter-Lorenz 2009; Reuter-Lorenz and Park 2014).
Our study revealed that static functional connectivity of the resting brain shows an anterior-to-posterior shift in aging, which is also supported by substantial existing literature (Onoda et al. 2012; Geerligs et al. 2014; Ferreira et al. 2015; Lee et al. 2015). This finding is in line with the posterior-to-anterior shift of the brain functional dynamics in aging. Less frontal functional connectivity of the stationary brain in aging partially reflects that frontal brain regions reduce their connection strength with other brain regions and hence play a less central role in the brain functional architecture, which is consistent to a reduction in the frontal hub probability in aging. Likewise, the occipital lobe increases its connectivity with other brain regions, which corresponds to an increase in the occipital hub probability in aging. Importantly, our study provided additional information on the variability of the functional connectivity that implicated the temporal dynamics of the brain. Interestingly, a recent study demonstrated that an increased static functional connectivity was accompanied by a decreased variability of the functional connectivity (Demirtaş et al. 2016), which reflects the correspondence between the static and dynamic brains. Hence, both static and dynamic aspects of the resting brain support the idea of the STAC model. Furthermore, Jockwitz and colleagues (2017) recently provided a structural correlate for the functionally established STAC theory, focusing on the default mode network (DMN), which is complementary to our study from the structural network point of view. This finding suggested that the aging brain might follow the posterior-to-anterior shift pattern even within one specific brain network (Buckner et al. 2008; Jones et al. 2011; Mormino et al. 2011). Hence, this warrants future investigation of age effects on the temporal variability in specific functional networks to detail the STAC model.
Our study also showed that a few of dynamic functional connectivities followed the inverted U-shape relationship with age. These were the dynamic functional connectivities of the anterior regions (e.g., DLPFC, SMC, etc.) with the posterior and subcortical regions. First, these findings are beyond evidence derived from task-based fMRI on the posterior-to-anterior shift in aging, since our study reported the functional connectivity between the anterior and posterior regions rather than functional activity in an anterior or posterior region in aging. Second, inverted U-shape effects of age were also shown on the functional and structural connectivity in the frontal cortex (Wang et al. 2012; Cao et al. 2014; Zhao et al. 2015). These findings may provide additional dimension of the STAC model, that is, brain functional dynamics in the frontal cortex may not always be reorganized in the monotonous direction of age-related increase/decrease.
This study is best considered as exploratory using a Chinese sample and intends to provide a new measure that quantifies the brain functional dynamics. While preliminary, our findings suggest convincing evidence on a posterior-to-anterior shift of the brain functional dynamics in aging, which is consistent with the STAC model. In constructing the dynamic brain functional networks, this study employed parcellations from the AAL atlas that was further grouped into 12 large regions (Tzourio-Mazoyer et al. 2002) for the convenience of the examination of our hypothesis. This may not be the best atlas for studying the STAC model based on the brain functional dynamics in aging. The choice of brain parcellations for rs-fMRI studies remains a challenging question (Wang et al. 2009). Nevertheless, this study mainly focused on the anterior and posterior regions as a whole in the sense that our findings may not be sensitive to the choice of the brain atlas. Moreover, the rs-fMRI data in this study were acquired with long TR (2.3 s), which may not be desirable for analyzing brain functional dynamics. However, Liao and colleagues (2017) employed rs-fMRI data from the Human Connectome Projects with TR of 0.72 s and showed that brain modular patterns were highly variable within each subject. This finding is in line to ones observed based on our data with long TR, e.g., high variabilities in terms of hub probability and dynamic functional connectivity, providing the support on the feasibility of using rs-fMRI data with long TR to study brain functional dynamics. Moreover, we noticed that the orbital cortex (superior frontal gyrus, orbital part in Table S2) showed positive age effects, which was contradictory to the findings of the other frontal regions. This was perhaps because of fMRI signal loss in the orbital frontal region due to its surrounding air and bone. Finally, this study used a cross-sectional design. Future research with longitudinal data is needed to investigate age-related trajectory of brain functional dynamics and its relation to cognitive performance for further understanding the STAC model.
In sum, this study provided new evidence on the dynamics of the brain functional architecture in relation with age by introducing the hub probability and variability of the functional connectivity, which was associated with age-related episodic memory decline. A posterior-to-anterior shift of the brain functional dynamics in aging was in line with that of the static brain functional architecture at rest and individual brain activations in tasks, which thus provided a new dimension to view the STAC model by emphasizing the compensatory mechanisms of the brain functional dynamics in aging.
References
Achard S, Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17. doi:10.1371/journal.pcbi.0030017
Agosta F, Sala S, Valsasina P et al (2013) Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 81:134–143. doi:10.1212/WNL.0b013e31829a33f8
Allen EA, Damaraju E, Plis SM et al (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. doi:10.1093/cercor/bhs352
Alperin BR, Tusch ES, Mott KK et al (2015) Investigating age-related changes in anterior and posterior neural activity throughout the information processing stream. Brain Cogn 99:118–127. doi:10.1016/j.bandc.2015.08.001
Andrews-Hanna JR, Snyder AZ, Vincent JL et al (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56:924–935. doi:10.1016/j.neuron.2007.10.038
Ansado J, Monchi O, Ennabil N et al (2012) Load-dependent posterior–anterior shift in aging in complex visual selective attention situations. Brain Res 1454:14–22. doi:10.1016/j.brainres.2012.02.061
Armbruster-Genc DJN, Ueltzhoffer K, Fiebach CJ (2016) Brain signal variability differentially affects cognitive flexibility and cognitive stability. J Neurosci 36:3978–3987. doi:10.1523/JNEUROSCI.2517-14.2016
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. doi:10.1196/annals.1440.011
Buckner RL, Sepulcre J, Talukdar T et al (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 29:1860–1873. doi:10.1523/JNEUROSCI.5062-08.2009
Cabeza R, Daselaar SM, Dolcos F et al (2004a) Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14:364–375. doi:10.1093/cercor/bhg133
Cabeza R, Nyberg L, Park D (2004b) Cognitive neuroscience of aging. Oxford University Press, Oxford
Calhoun VD, Miller R, Pearlson G, Adalı T (2014) The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84:262–274. doi:10.1016/j.neuron.2014.10.015
Cao M, Wang JH, Dai ZJ et al (2014) Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci 7:76–93. doi:10.1016/j.dcn.2013.11.004
Cocchi L, Zalesky A, Fornito A, Mattingley JB (2013) Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci 17:493–501. doi:10.1016/j.tics.2013.08.006
Cole MW, Reynolds JR, Power JD et al (2013) Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. doi:10.1038/nn.3470
Cole MW, Ito T, Bassett DS, Schultz DH (2016) Activity flow over resting-state networks shapes cognitive task activations. Nat Neurosci 19:1718–1726. doi:10.1101/055194
Dai Z, Yan C, Li K et al (2014) Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb Cortex. doi:10.1093/cercor/bhu246
Davis SW, Dennis NA, Daselaar SM et al (2008) Que PASA? The posterior–anterior shift in aging. Cereb Cortex 18:1201–1209. doi:10.1093/cercor/bhm155
de Pasquale F, Della Penna S, Sporns O et al (2016) A dynamic core network and global efficiency in the resting human brain. Cereb Cortex 26:4015–4033. doi:10.1093/cercor/bhv185
Demirtaş M, Tornador C, Falcón C et al (2016) Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum Brain Mapp 37:2918–2930. doi:10.1002/hbm.23215
Drzezga A, Becker JA, Van Dijk KR et al (2011) Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134:1635–1646. doi:10.1093/brain/awr066
Du J, Younes L, Qiu A (2011) Whole brain diffeomorphic metric mapping via integration of sulcal and gyral curves, cortical surfaces, and images. Neuroimage 56:162–173. doi:10.1016/j.neuroimage.2011.01.067
Egerházi A, Berecz R, Bartók E, Degrell I (2007) Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 31:746–751. doi:10.1016/j.pnpbp.2007.01.011
Fair DA, Cohen AL, Power JD et al (2009) Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5:14–23. doi:10.1371/journal.pcbi.1000381
Ferreira LK, Regina ACB, Kovacevic N et al (2015) Aging effects on whole-brain functional connectivity in adults free of cognitive and psychiatric disorders. Cereb Cortex. doi:10.1093/cercor/bhv190
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. doi:10.1016/S0896-6273(02)00569-X
Gardini S, Venneri A, Sambataro F et al (2015) Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. J Alzheimer’s Dis 45:457–470. doi:10.3233/JAD-142547
Geerligs L, Renken RJ, Saliasi E et al (2014) A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. doi:10.1093/cercor/bhu012
Goh JO, Park DC (2009) Neuroplasticity and cognitive aging: the scaffolding theory of aging and cognition. Restor Neurol Neurosci 27:391–403. doi:10.3233/RNN-2009-0493
Grady C (2012) The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505. doi:10.1038/nrn3256
Hansen ECA, Battaglia D, Spiegler A et al (2015) Functional connectivity dynamics: modeling the switching behavior of the resting state. Neuroimage 105:525–535. doi:10.1016/j.neuroimage.2014.11.001
He Y, Dagher A, Chen Z et al (2009) Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain 132:3366–3379. doi:10.1093/brain/awp089
Hutchison RM, Womelsdorf T, Allen E et al (2013a) Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–378. doi:10.1016/j.neuroimage.2013.05.079
Hutchison RM, Womelsdorf T, Gati JS et al (2013b) Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34:2154–2177. doi:10.1002/hbm.22058
Jockwitz C, Caspers S, Lux S et al (2017) Age- and function-related regional changes in cortical folding of the default mode network in older adults. Brain Struct Funct 222:83–99. doi:10.1007/s00429-016-1202-4
Jones DT, MacHulda MM, Vemuri P et al (2011) Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77:1524–1531. doi:10.1212/WNL.0b013e318233b33d
Jones DT, Vemuri P, Murphy MC et al (2012) Non-stationarity in the “resting brain’s” modular architecture. PLoS One. doi:10.1371/journal.pone.0039731
Junkkila J, Oja S, Laine M, Karrasch M (2012) Applicability of the CANTAB-PAL computerized memory test in identifying amnestic mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord 34:83–89. doi:10.1159/000342116
Kucyi A, Davis KD (2014) Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage 100:471–480. doi:10.1016/j.neuroimage.2014.06.044
Kucyi A, Salomons TV, Davis KD (2013) Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci 110:18692–18697. doi:10.1073/pnas.1312902110
La Corte V, Sperduti M, Malherbe C et al (2016) Cognitive decline and reorganization of functional connectivity in healthy aging: the pivotal role of the salience network in the prediction of age and cognitive performances. Front Aging Neurosci 8:1–12. doi:10.3389/fnagi.2016.00204
Lee A, Archer J, Wong C et al (2013) Age-related decline in associative learning in healthy Chinese adults. PLoS One 8:e80648
Lee A, Ratnarajah N, Tuan TA et al (2015) Adaptation of brain functional and structural networks in aging. PLoS One 10:1–16. doi:10.1371/journal.pone.0123462
Lee A, Tan M, Qiu A (2016) Distinct aging effects on functional networks in good and poor cognitive performers. Front Aging Neurosci 8:1–12. doi:10.3389/fnagi.2016.00215
Liao X, Cao M, Xia M, He Y (2017) Individual differences and time-varying features of modular brain architecture. Neuroimage 152:94–107. doi:10.1016/j.neuroimage.2017.02.066
Luciana M, Nelson CA (2002) Assessment of neuropsychological function through use of the Cambridge neuropsychological testing automated battery: performance in 4- to 12-year-old children. Dev Neuropsychol 22:595–624. doi:10.1207/S15326942DN2203_3
Mormino EC, Smiljic A, Hayenga AO et al (2011) Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex 21:2399–2407. doi:10.1093/cercor/bhr025
Ng T, Niti M, Chiam P, Kua E (2007) Ethnic and educational differences in cognitive test performance on mini-mental state examination in Asians. Am J Geriatr Psychiatry 15:130–139
Onoda K, Ishihara M, Yamaguchi S (2012) Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci 24:2186–2198. doi:10.1162/jocn_a_00269
Park DC, Reuter-Lorenz P (2009) The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60:173–196. doi:10.1146/annurev.psych.59.103006.093656
Park H, Kennedy KM, Rodrigue KM et al (2013) An fMRI study of episodic encoding across the lifespan: changes in subsequent memory effects are evident by middle-age. Neuropsychologia 51:448–456. doi:10.1016/j.neuropsychologia.2012.11.025
Power JD, Barnes KA, Snyder AZ et al (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. doi:10.1016/j.neuroimage.2011.10.018
Power JD, Barnes KA, Snyder AZ et al (2013) Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76:439–441. doi:10.1016/j.neuroimage.2012.03.017
Pudas S, Persson J, Josefsson M et al (2013) Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci 33:8668–8677. doi:10.1523/JNEUROSCI.2900-12.2013
Reuter-Lorenz PA, Park DC (2014) How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol Rev 24:355–370. doi:10.1007/s11065-014-9270-9
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. doi:10.1016/j.neuroimage.2009.10.003
Sala-Llonch R, Bartrés-Faz D, Junqué C (2015) Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol 6:1–11. doi:10.3389/fpsyg.2015.00663
Salami A, Wåhlin A, Kaboodvand N et al (2016) Longitudinal evidence for dissociation of anterior and posterior MTL resting-state connectivity in aging: links to perfusion and memory. Cereb Cortex. doi:10.1093/cercor/bhw233
Shen K, Hutchison RM, Bezgin G et al (2015) Network structure shapes spontaneous functional connectivity dynamics. J Neurosci 35:5579–5588. doi:10.1523/JNEUROSCI.4903-14.2015
Shirer WR, Ryali S, Rykhlevskaia E et al (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22:158–165. doi:10.1093/cercor/bhr099
Sporns O, Honey CJ, Kötter R (2007) Identification and classification of hubs in brain networks. PLoS One 2:e1049. doi:10.1371/journal.pone.0001049
Tan M, Qiu A (2016) Large deformation multiresolution diffeomorphic metric mapping for multiresolution cortical surfaces: a coarse-to-fine approach. IEEE Trans Image Process 25:4061–4074
Tomasi D, Volkow ND (2012) Aging and functional brain networks. Mol Psychiatry 17:549–558. doi:10.1038/mp.2011.81
Turner GR, Spreng RN (2015) Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: the default-executive coupling hypothesis of aging. J Cogn Neurosci 27:2462–2476. doi:10.1162/jocn_a_00409
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. doi:10.1006/nimg.2001.0978
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. doi:10.1523/JNEUROSCI.3539-11.2011
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17:683–696. doi:10.1016/j.tics.2013.09.012
Van Dijk KR, Hedden T, Venkataraman A et al (2010) Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321. doi:10.1152/jn.00783.2009
Vergun S, Deshpande AS, Meier TB et al (2013) Characterizing functional connectivity differences in aging adults using machine learning on resting state fMRI data. Front Comput Neurosci 7:38. doi:10.3389/fncom.2013.00038
Wang J, Wang L, Zang Y et al (2009) Parcellation-dependent small-world brain functional networks: a resting-state fmri study. Hum Brain Mapp 30:1511–1523. doi:10.1002/hbm.20623
Wang J, Zuo X-N, Gohel S et al (2011) Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS One 6:e21976. doi:10.1371/journal.pone.0021976
Wang L, Su L, Shen H, Hu D (2012) Decoding lifespan changes of the human brain using resting-state functional connectivity MRI. PLoS One. doi:10.1371/journal.pone.0044530
Wang Z, Dai Z, Gong G et al (2015) Understanding structural–functional relationships in the human brain: a large-scale network perspective. Neuroscientist 21:290–305. doi:10.1177/1073858414537560
Wilson RS, Mayhew SD, Rollings DT et al (2015) Influence of epoch length on measurement of dynamic functional connectivity in wakefulness and behavioural validation in sleep. Neuroimage 112:169–179. doi:10.1016/j.neuroimage.2015.02.061
Zalesky A, Breakspear M (2015) Towards a statistical test for functional connectivity dynamics. Neuroimage 114:466–470. doi:10.1016/j.neuroimage.2015.03.047
Zhang Y, Simon-Vermot L, Araque Caballero MT et al (2016) Enhanced resting-state functional connectivity between core memory-task activation peaks is associated with memory impairment in MCI. Neurobiol Aging 45:43–49. doi:10.1016/j.neurobiolaging.2016.04.018
Zhao T, Cao M, Niu H et al (2015) Age-related changes in the topological organization of the white matter structural connectome across the human lifespan. Hum Brain Mapp. doi:10.1002/hbm.22877
Zuo XN, Ehmke R, Mennes M et al (2012) Network centrality in the human functional connectome. Cereb Cortex 22:1862–1875. doi:10.1093/cercor/bhr269
Acknowledgements
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore—NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by NMRC (NMRC/CBRG/0039/2013), and Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2012-T2-2-130).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Lee, A. & Qiu, A. A posterior-to-anterior shift of brain functional dynamics in aging. Brain Struct Funct 222, 3665–3676 (2017). https://doi.org/10.1007/s00429-017-1425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1425-z