Abstract
Solitary fibrous tumors (SFTs) are rare mesenchymal tumors that can occur at any location. Since the identification of specific NAB2-STAT6 fusion in SFTs, the fusion gene variants, NAB2 exon 4-STAT6 exon 2/3 and NAB2 exon 5/6/7-STAT6 exon 16/17/18, have been reported to be associated with clinicopathological features, and the latter variant is predominant in meningeal SFTs. SFTs developing in the salivary glands are rare, and more rarely, those involving ectopic salivary glands (ESGs) have been reported in the cerebellopontine angle (CPA); however, their characteristics remain not well understood. In this study, we performed a clinicopathological and molecular analysis of 3 cases of meningeal SFT with ESGs. All cases presented with an extra-axial mass in the CPA, which is a rarer location for intracranial ESGs compared to the sellar region. Histologically, except for the presence of ESGs, there was no significant difference between current cases and ordinary SFTs. The ESGs demonstrated no cellular atypia, and although the spindle tumor cells were immunopositive for STAT6, the ESGs were negative in all cases, supporting that the ESGs are non-neoplastic components. In 1 case, ESGs were found only in the primary tumor and disappeared in recurrence/dissemination. Of note, molecular analysis identified NAB2 exon 4-STAT6 exon 2 in all cases. In conclusion, our results suggest that ESGs particularly in the CPA may be associated with SFTs and that meningeal SFTs with ESGs may be associated with the minor fusion variant of NAB2-STAT6 in the intracranial lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solitary fibrous tumors (SFTs) are rare mesenchymal tumors that can occur at any location [1,2,3,4]. In the 2021 World Health Organization (WHO) classification of tumors of the central nervous system (CNS), SFTs are graded from 1 to 3 according to mitotic activity and tumoural necrosis [5]. SFTs with < 2.5 mitoses/mm2 (< 5 mitoses/10 high-power field [HPF]) and no necrosis were considered grade 1; SFTs with ≥ 2.5 mitoses/mm2 (≥ 5 mitoses/10 HPFs) and no necrosis were considered grade 2; tumors with ≥ 2.5 mitoses/mm2 (≥ 5 mitoses/10 HPFs) and necrosis were considered grade 3. Most intracranial SFTs are dura-based; thus, the skull base, parasagittal, and falcine regions are common sites, whereas the cerebellopontine angle (CPA), pineal gland, and sellar region are uncommon sites [5].
SFT and hemangiopericytoma (HPC) were initially thought to be different tumors; however, the identification of NAB2-STAT6 in both SFT and HPC suggests that they comprise the same tumor entity [3, 6, 7]. The presence of the fusion gene results in nuclear expression of STAT6 by immunohistochemistry [6]. NAB2-STAT6 fusion variants have been classified into two major groups based on their hypothesized functional effects: NAB2 exon 4-STAT6 exon 2/3 and NAB2 exon 5/6/7-STAT6 exon 16/17/18 [1, 5, 8]. Several studies have suggested that the fusion gene variants are associated with clinicopathological features [1, 2, 5, 7, 9]. NAB2 exon 4-STAT6 exon 2/3 fusion variants have been reported to be associated with pleuro-pulmonary development, lower mitotic counts, and a lower risk of adverse events [1, 2, 5, 7, 9]. On the other hand, NAB2 exon 5/6/7-STAT6 exon 16/17/18 fusion variants have been reported to be associated with development from the meninges/soft tissue/head and neck, higher cellularity, and recurrent tumors [1, 3, 5, 7]. In addition to the fusion gene variants, TP53 and TERT promoter mutations have been found to offer clinicopathological value in SFT; aberrant p53 overexpression and/or TP53 mutations have been demonstrated in dedifferentiated areas of SFT, and TERT promoter mutation has been shown to be associated with worse prognosis [4, 7, 9,10,11,12,13].
Salivary gland tissue outside the major and minor salivary glands is considered as ectopic salivary glands (ESGs), with frequent sites being the mandible, ear, and mylohyoid muscle [14]. Intracranial ESGs are rare and mainly found in the sellar region. Only 3 cases of salivary gland tissue presented in the CPA have been reported [15,16,17]. Neoplasms involving ESGs are uncommon; outside the CNS, 35 cases have been reported, and they all were salivary gland-related tumors (Table S1) [18,19,20,21,22,23,24,25,26,27,28,29,30]. As intracranial tumors involving ESGs, in addition to 2 salivary gland–related tumors, 2 SFTs in the CPA have been reported; however, SFT with ESGs has not been reported outside the CNS (Table S1) [15,16,17, 31]. Genetic analysis was not performed for the 2 reported SFT with ESGs.
In this study, we investigated a series of 3 cases of meningeal SFT with ESGs, including 1 previously reported case, for clinicopathological and molecular features.
Materials and methods
Tumor samples
Three cases were collected for this study. Case 1 was previously reported [17]. Case 2 was from the consultation files of 1 of the authors (J.H). Case 3 was from the pathology archives of the Japan Brain Tumor Reference Center. Sections for histologic and genetic analyses were prepared from formalin-fixed paraffin-embedded (FFPE) tissue specimens. This study was conducted in accordance with the ethical committees of Gunma University (HS2016-075).
Conventional histological analysis
Three micrometer thick tissue sections were cut and stained with hematoxylin and eosin. Immunohistochemical staining was performed on FFPE tissue sections. Primary antibodies directed against the following antigens were applied: CD34 (NU-4A1; 1:100; Nichirei, Tokyo, Japan), STAT6 (S-20; 1:300; Santa Cruz, Dallas, TX, USA), p53 (DO-7; 1:50; Leica Microsystems, Wetzlar, Germany), and Ki-67 (MIB-1; 1:100; Dako, Glostrup, Denmark). For coloration, a commercially available biotin-streptavidin immunoperoxidase kit (Histofine, Nichirei, Tokyo, Japan) and diaminobenzidine were employed.
Detection of NAB2-STAT6 fusion transcripts
Reverse transcription polymerase chain reaction (RT-PCR) was performed, as previously described [32]. Briefly, total RNA was extracted from FFPE samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen) to prepare the first-strand complementary DNA. NAB2-STAT6 fusion assays were performed using primers described previously [32]. Each PCR product (5 μL) was loaded onto 2% agarose gel with ethidium bromide and visualized under UV illumination. The PCR products were also evaluated by direct sequence analysis using the Big Dye Terminator method (version 1.1; Applied Biosystems, Foster City, CA, USA) to confirm the breakpoints of the fusion transcripts.
DNA extraction and direct DNA sequencing for TP53 and TERT promoter mutations
Genomic DNA was extracted from the FFPE tissue sections, as previously described [33], and was amplified and sequenced using previously described primer sets [34, 35]. PCR products were analyzed by agarose gel electrophoresis, and were then sequenced on a 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) with the Big Dye Terminator v.1.1 Cycle Sequencing Kit (Applied Biosystems) following standard procedures.
Statistical analysis
Extensive review of the English-language literature was conducted to identify previously reported cases of meningeal SFTs without ESG components with known NAB2-STAT6 fusion variants. Fisher’s exact test was performed to analyze the correlation between the fusion variants and presence of ESGs.
Results
Clinical findings
Relevant clinical data are summarized in Table 1. All 3 cases were adult females (27 to 41 years old) and presented with an extra-axial mass in the CPA. Tumors were cystic in cases 1 and 2, which showed high-signal intensity on T2-weighted magnetic resonance images (Fig. 1a) with capsule and septa showing contrast enhancement (Fig. 1b). In case 3, the recurrent tumor was solid, which showed iso- to high-signal intensity on T2-weighted images (Fig. 1c) and homogeneous enhancement on gadolinium-enhanced T1-weighted images (Fig. 1d).
Magnetic resonance images of case 2 at initial onset (a, b) and case 3 at first recurrence (c, d). a, b A lobulated mass in the right cerebellopontine angle compresses right cerebellum and middle cerebellar peduncle. The mass shows very high signal intensity on T2-weighted images (a), suggesting a multilocular cystic mass, and the capsule and septa show contrast enhancement on gadolinium-enhanced T1-weighted images (b). c, d A lobulated mass in the left cerebellopontine angle compresses middle cerebellar peduncle. The mass shows mildly high signal intensity on T2-weighted images (c) and shows homogeneous enhancement on gadolinium-enhanced T1-weighted images (d)
All cases underwent gross total tumor resection. In case 2, the tumor recurred locally 8 (first recurrence, R1), 18 (R2), and 19 (R3) years after the initial surgery, and excisions were performed. In case 3, although the tumor had been initially controlled by the first surgery and a gamma knife treatment at 7 years, its recurrence was more aggressive, and the patient received additional excisions at 13 (R1), 14 (R2), and 16 (R3) years after initial surgery. Multiple dissemination throughout posterior fossa and cervical spinal cord had developed at 18 years, and 2 more surgeries (R4 and R5) and 4 stereotactic radiosurgeries were performed in the next 4 years. The patient died 22 years after the initial surgery at her age of 57 years.
Histopathological findings
Representative histological images are shown in Fig. 2, and the histological features are summarized in Table 2. Specimens from R3 and R5 of case 3 were not available.
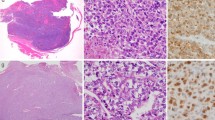
Microscopic appearance. a, b, c The lesion of case 1 demonstrates a mixture of tumor tissue and ectopic salivary gland (ESG) components. The tumor is composed of spindle to ovoid cells with eosinophilic cytoplasm separated by hyalinized collagenous stroma. d. R2 of case 2 shows a proliferation of small round or short spindle cells with high cellularity. ESGs are mixed in the tumor. e, f R3 of case 2 is composed of atypical mesenchymal cells with pleomorphic nuclei (e). ESGs remained in the tumor (f). g P of case 3 is composed of short spindle to oval shaped cells with high cellularity. ESGs are observed in the tumor. h, i R4 of case 3 demonstrates brisk mitoses (h) and necrosis (i). P, primary; R2-R4, second-fourth recurrence. Original magnification: a, i × 40; b, d, e, f, g × 200; c, h × 400
The lesion of case 1 demonstrated a mixture of tumor tissue and ESG components (Fig. 2a, b). The tumor was composed of spindle to ovoid cells with eosinophilic cytoplasm separated by hyalinized collagenous stroma and had variable cellularity (Fig. 2c). Necrosis and mitosis were absent, and this tumor was considered to be SFT, WHO grade 1. In addition, components of glandular structures were present in the tumor (Fig. 2a, b). The components consisted of serous and mucous acini and dilated ducts surrounded by myoepithelial cells. No cellular atypia was observed in these glandular ducts. These characteristic structures were consistent with ESGs.
The primary tumor (P) and R1–2 of case 2 showed a mixture of tumor tissue and ESG components (Fig. 2d). These tumors were focally composed of small round or short spindle cells with high cellularity with no necrosis or mitosis, and were considered to be SFT, WHO grade 1. ESGs were mixed in these tumors. Meanwhile, R3 of case 2 was composed of atypical mesenchymal cells with pleomorphic nuclei (Fig. 2e). Necrosis was absent. The mitotic rate was up to 6 per 10 high-power fields (HPFs), corresponding to SFT, WHO grade 2, and atypical mitoses were observed. These histological features were judged as dedifferentiation. ESGs remained in the tumor (Fig. 2f).
P, R1–2, and R4 of case 3 were composed of short spindle to oval shaped cells with high cellularity (Fig. 2g, h). In P and R1–2, mitotic counts ranged from 4 to 9 per 10 HPFs and necrosis was absent. These tumors were considered to be SFT, WHO grade 1 or 2. Meanwhile, brisk mitoses (21 per 10 HPFs) and necrosis were observed in R4 (Fig. 2h, i). This tumor was classified as SFT, WHO grade 3. ESGs were observed only in P (Fig. 2g).
The immunohistochemical findings are summarized in Table 2. A diffuse and strong expression of CD34 was demonstrated in case 1 and P and R1–2 of case 3. In R1 of case 2, a focal and strong expression of CD34 was demonstrated. Nuclear STAT6 positivity was observed in the spindle tumor cells but not in the ESGs in all specimens analyzed (Fig. 3a–c). The pleomorphic tumor cells in R3 of case 2 were also positive for STAT6. A diffuse and moderate expression of p53 was de monstrated only in R2 of case 2 (Fig. 3e), whereas the expression of p53 in the other specimens was negative or weakly/focally positive (Fig. 3d, f). In cases 2 and 3, the MIB-1 labeling index tended to increase along with tumor recurrence (Fig. 3g–i).
Immunohistochemistry. a, b, c STAT6 staining. Nuclear positivity is observed in the spindle tumor cells but not in the ectopic salivary glands in all 3 cases (a, case 1; b, R1 of case 2; c, P of case 3). d, e, f p53 staining. p53 is diffusely and moderately positive in R3 of case 2 (e). The expression of p53 was negative in case 1 (d) and focally/weakly positive in R4 of case 3 (f). g, h, i Ki-67 staining (g, case 1; h, R3 of case 2; i, R1 of case 3). P, primary; R1, R3, and R4, first, third, and fourth recurrence, respectively. Original magnification: d, e, f, g, h, i × 200; a, b, c × 400
Genetic analysis
The results of genetic analysis are summarized in Table 2. RT-PCR for detecting NAB2-STAT6 was performed for all 3 cases (for cases 2 and 3, R1 and R2 were analyzed, respectively), and they all harbored NAB2 exon 4-STAT6 exon 2 (Fig. 4a–c).
Direct DNA sequencing was performed for all samples available from all 3 cases except for P of case 2. Among all samples analyzed, TP53 mutation (c.535C > T, p.H179Y) was detected only in the sample that demonstrated dedifferentiation, R3 of case 2 (Fig. 4d). No TERT promoter mutation was found in any samples.
Statistical analysis
Review of the English literature yielded 78 cases of meningeal SFTs with NAB2 exon 4-STAT6 exon 2/3 and 185 cases of those with NAB2 exon 5/6/7-STAT6 exon 16/17/18 [6,7,8,9, 13, 32, 34, 37,38,39,40,41,42,43]. We explored the association of NAB2-STAT6 fusion variants versus the presence of ESGs, and the presence of ESGs was significantly associated with NAB2 exon 4-STAT6 exon 2/3 (p = 0.028).
Discussion
In the present study, we described clinicopathological and molecular features of 3 meningeal SFT with ESGs in the CPA. All 3 cases harbored NAB2 exon 4-STAT6 exon 2, which is a minor fusion variant in meningeal SFTs.
Immunohistochemically, although the spindle tumor cells were positive for STAT6, the ESG cells were negative in all 3 cases (Fig. 3a–c), supporting that the ESG cells were non-neoplastic components. That is also in line with the findings that the ESG cells demonstrated no cellular atypia and disappeared in the specimens of recurrence/dissemination of case 3. The histologies of our cases were similar to those of reported SFTs involving non-neoplastic epithelial components other than ESGs, such as eccrine glands, orthotopic minor salivary glands, kidney, Mullerian epithelium in the omentum, and prostate [44,45,46]. As discussed in the previous report of case 1, due to the lack of cellular atypia in the ESG cells, carcinosarcomas as a whole or carcinomas metastasizing in SFTs were not considered [17].
Although the number of cases investigated was small, the current study showed that the presence of ESGs was associated with NAB2 exon 4-STAT6 exon 2 in meningeal SFTs. This may reflect a difference in pathogenesis between ordinary meningeal SFTs and those with ESGs. In addition, SFTs developing in the orthotopic salivary glands are rare and 4 cases have been tested for NAB2-STAT6 fusion variants: 2 cases with NAB2 exon 6-STAT6 exon 16 and 2 cases with NAB2 exon 3-STAT6 exon 19, with the latter being a rare variant [1, 43, 47,48,49]. Therefore, SFTs in the orthotopic salivary glands and those with ESGs may show different distributions of NAB2-STAT6 fusion variants.
In case 3, ESG components were found only in the primary tumor (P) and disappeared in the recurrent and disseminated tumors (R1, R2, and R4) (Table 2, Fig.2 g–i). Considering this phenomenon, some of the meningeal SFTs with ESGs may have been diagnosed as ordinary SFTs without ESGs, as the salivary gland components disappeared like case 3 in the current study, probably because of the overrunning of ESGs by the growth of SFTs. Sampling errors, unawareness, and underestimation may also mislead the diagnosis.
We understand that the presence of ESGs contributes to the tumorigenesis of salivary gland–related tumors, such as Warthin’s tumor, pleomorphic adenoma, and mucoepidermal carcinoma; however, it is unknown whether the same is true for SFTs or whether they are only involved in the growth of tumors. If the former is true, we would expect to find SFT with ESGs in the sellar region or extracranial sites, which are more common for ESGs than CPA as mentioned before; however, there have so far not been any reports of this. Asayama et al.’s hypothesis about the developmental mechanism of SFTs with ESGs was that some salivary gland primordial cells are misplaced into dura mater during embryonic migration and then neoplastic transformation into SFTs is brought about by the result of differentiation [17]. Along with this theory, we speculated that ESGs particularly (or only) in the CPA, as well as orthotopic salivary glands, may possess precursor cells that give rise to SFT.
On the other hand, ESGs found in 3 cases of SFTs in the current study may be the pre-existing tissue that was only embedded in the growth of tumors. If so, the absence of reports of SFT with ESGs in the sellar region, except for those in extracranial sites, does not contradict the higher incidence of conventional SFTs in the CPA than in the sellar region [50, 51]. As for meningiomas, it is now widely accepted that differences in genetic mutations (NF2, KLF4/TRAF7, AKT1/TRAF7, SMO) are associated with tumor location [52, 53]. Between NAB2 exon 4-STAT6 exon 2/3 and NAB2 exon 5/6/7-STAT6 exon 16/17/18, functional differences associated with the presence or absence of CHD4-interacting domain in NAB2 which regulates activity of ERG1, an important regulator of fibrosis, were suggested [1]. Similar to meningiomas, it may be of interest to see whether the fusion gene variants of NAB2-STAT6 are associated with the location of meningeal SFTs, and whether those in the CPA predominantly have NAB2 exon 4-STAT6 exon 2/3 fusion. However, most of the literature of meningeal SFTs that analyzed NAB2-STAT6 variants did not describe the detailed site of lesion [6,7,8,9, 32, 37,38,39]; thus, further studies are needed to investigate this.
In conclusion, we report 3 meningeal SFT with ESGs harboring NAB2 exon 4-STAT6 exon 2 in the CPA; however, the etiological association between SFTs and the ESGs, the reason why ESGs only in the CPA have been found to be associated with the development of SFTs, and the association between the variant of NAB2 exon 4-STAT6 exon 2 fusion and SFT with ESGs remain to be elucidated. Further clinicopathological and genetic analyses on more cases are needed to clarify these issues.
Data availability
Derived data supporting the findings of this study are available from the corresponding author on request.
The present study was approved by the Gunma University Ethical Committee.
References
Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, Haller F (2014) Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol 184:1209–1218. https://doi.org/10.1016/j.ajpath.2013.12.016
Tai HC, Chuang IC, Chen TC, Li CF, Huang SC, Kao YC, Lin PC, Tsai JW, Lan J, Yu SC, Yen SL, Jung SM, Liao KC, Fang FM, Huang HY (2015) NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol 28:1324–1335. https://doi.org/10.1038/modpathol.2015.90
Nakada S, Minato H, Nojima T (2016) Clinicopathological differences between variants of the NAB2-STAT6 fusion gene in solitary fibrous tumors of the meninges and extra-central nervous system. Brain Tumor Pathol 33:169–174. https://doi.org/10.1007/s10014-016-0264-6
Machado I, Morales GN, Cruz J, Lavernia J, Giner F, Navarro S, Ferrandez A, Llombart-Bosch A (2020) Solitary fibrous tumor: a case series identifying pathological adverse factors-implications for risk stratification and classification. Virchows Arch 476:597–607. https://doi.org/10.1007/s00428-019-02660-3
Giannini C, Bouvier C, Demicco EG, Figarella-Branger D, Fritchie KJ, Macagno N, Perry A (2021) Solitary fibrous tumor. In: WHO classification of tumours editorial board (ed) WHO classification of the central nervous system tumours, 5th edn. International Agency for Research on Cancer, Lyon, pp 301–305
Fritchie KJ, Jin L, Rubin BP, Burger PC, Jenkins SM, Barthelmeß S, Moskalev EA, Haller F, Oliveira AM, Giannini C (2016) NAB2-STAT6 gene fusion in meningeal hemangiopericytoma and solitary fibrous tumor. J Neuropathol Exp Neurol 75:263–271. https://doi.org/10.1093/jnen/nlv026
Park HK, Yu DB, Sung M, Oh E, Kim M, Song JY, Lee MS, Jung K, Noh KW, An S, Song K, Nam DH, Kim YJ, Choi YL (2019) Molecular changes in solitary fibrous tumor progression. J Mol Med 97:1413–1425. https://doi.org/10.1007/s00109-019-01815-8
Vogels R, Macagno N, Griewank K, Groenen P, Verdijk M, Fonville J, Kusters B; French CNS SFT/HPC Consortium; Dutch CNS SFT/HPC Consortium, Figarella-Branger D, Wesseling P, Bouvier C, Flucke U (2019) Prognostic significance of NAB2-STAT6 fusion variants and TERT promotor mutations in solitary fibrous tumors/hemangiopericytomas of the CNS: not (yet) clear. Acta Neuropathol 137:679–682. https://doi.org/10.1007/s00401-019-01968-3
Akaike K, Kurisaki-Arakawa A, Hara K, Suehara Y, Takagi T, Mitani K, Kaneko K, Yao T, Saito T (2015) Distinct clinicopathological features of NAB2-STAT6 fusion gene variants in solitary fibrous tumor with emphasis on the acquisition of highly malignant potential. Hum Pathol 46:347–356. https://doi.org/10.1016/j.humpath.2014.11.018
Schirosi L, Lantuejoul S, Cavazza A, Murer B, Brichon PY, Migaldi M, Sartori G, Sgambato A, Rossi G (2008) Pleuro-pulmonary solitary fibrous tumors: a clinicopathologic, immunohistochemical, and molecular study of 88 cases confirming the prognostic value of de Perrot staging system and p53 expression, and evaluating the role of c-kit, BRAF, PDGFRs (alpha/beta), c-met, and EGFR. Am J Surg Pathol 32:1627–1642. https://doi.org/10.1097/PAS.0b013e31817a8a89
Demicco EG, Wani K, Ingram D, Wagner M, Maki RG, Rizzo A, Meeker A, Lazar AJ, Wang WL (2018) TERT promoter mutations in solitary fibrous tumour. Histopathology 73:843–851. https://doi.org/10.1111/his.13703
Bahrami A, Lee S, Schaefer IM, Boland JM, Patton KT, Pounds S, Fletcher CD (2016) TERT promoter mutations and prognosis in solitary fibrous tumor. Mod Pathol 29:1511–1522. https://doi.org/10.1038/modpathol.2016.126
Maekawa A, Kohashi K, Yamada Y, Nakamizo A, Yoshimoto K, Mizoguchi M, Iwaki T, Oda Y (2015) A case of intracranial solitary fibrous tumor/hemangiopericytoma with dedifferentiated component. Neuropathology 35:260–265. https://doi.org/10.1111/neup.12181
Martinez-Madrigal F (2019) Major salivary glands. In: Mills SE (ed) Histology for pathologists, 5th edn. Lippincott Williams & Wilkins, Philadelphia, pp 440–466
Curry B, Taylor CW, Fisher AW (1982) Salivary gland heterotopia: a unique cerebellopontine angle tumor. Arch Pathol Lab Med 106:35–38
Rodriguez F, Scheithauer BW, Ockner DM, Giannini C (2004) Solitary fibrous tumor of the cerebellopontine angle with salivary gland heterotopia: a unique presentation. Am J Surg Pathol 28:139–142. https://doi.org/10.1097/00000478-200401000-00017
Asayama B, Seo Y, Ozaki Y, Tanikawa S, Hirose T, Tanaka S, Nakamura H (2019) A 41 year-old woman with a mass in the posterior cranial fossa. Brain Pathol 29:699–700. https://doi.org/10.1111/bpa.12771
Perzin KH, Livolsi VA (1980) Acinic cell carcinoma arising in ectopic salivary gland tissue. Cancer 45:967–972. https://doi.org/10.1002/1097-0142(19800301)45:5%3c967::AID-CNCR2820450522%3e3.0.CO;2-A
Marucci DD, Lawson K, Harper J, Sebire NJ, Dunaway DJ (2009) Sialoblastoma arising in ectopic salivary gland tissue. J Plast Reconstr Aesthet Surg 62:241–246. https://doi.org/10.1016/j.bjps.2007.09.047
Wilson DF, MacEntee MI (1974) Papillary cystadenoma of ectopic minor salivary gland origin. Oral Surg Oral Med Oral Pathol 37:915–918. https://doi.org/10.1016/0030-4220(74)90444-7
Smith A, Winkler B, Perzin KH, Wazen J, Blitzeet A (1985) Mucoepidermoid carcinoma arising in an intraparotid lymph node. Cancer 55:400–403. https://doi.org/10.1002/1097-0142(19850115)55:2%3c400::aid-cncr2820550218%3e3.0.co;2-4
Ismı O, Vayısoğlu Y, Arpaci RB, Eti C, Pütürgeli T, Gorur K, Ozcan C (2015) Carcinoma ex pleomorphic adenoma originating from ectopic salivary gland in the neck region: case report. Gland Surg 4:567–571. https://doi.org/10.3978/j.issn.2227-684X.2015.01.03
Luksić I, Suton P, Manojlović S, Macan D, Dediol E (2008) Pleomorphic adenoma in ectopic salivary tissue of the neck. OTO Open 2:13–15
Al-Sukhun J, Lindqvist C, Hietanen J, Leivo I, Penttilä H (2006) Central adenoid cystic carcinoma of the mandible: case report and literature review of 16 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:304–308. https://doi.org/10.1016/j.tripleo.2005.06.029
Faras F, Abo-Alhassan F, Bastaki J, Al-Sihan MK (2015) Primary mucoepidermoid carcinoma arising from ectopic salivary tissue within an intraparotid lymph node. Case Rep Otolaryngol 2015:1–3. https://doi.org/10.1155/2015/879137
Daniel E, McGuirt WF Sr (2005) Neck masses secondary to heterotopic salivary gland tissue: a 25-year experience. Am J Otolaryngol 26:96–100. https://doi.org/10.1016/j.amjoto.2004.08.009
Tay HL, Howitt RJ (1995) Heterotopic pleomorphic adenoma in the neck. J Laryngol Otol 109:445–448. https://doi.org/10.1017/s0022215100130403
Badia L, Weir JN, Robinson AC (1996) Heterotopic pleomorphic adenoma of the external nose. J Laryngol Otol 110:376–378. https://doi.org/10.1017/s0022215100133675
Hata T, Iga H, Imai S, Hirokawa M (1997) Heterotopic salivary gland adenocarcinoma in the cervical region. Int J Oral Maxillofac Surg 26:290–292. https://doi.org/10.1016/s0901-5027(97)80872-7
Ludmer B, Joachims HZ, Ben-Arie J, Eliachar I (1981) Adenocarcinoma in heterotopic salivary tissue. Arch Otolaryngol 107:547–548. https://doi.org/10.1001/archotol.1981.00790450023007
Erdogan S, Rodriguez FJ, Scheithauer BW, Abell-Aleff PC, Rabin M (2007) Malignant myoepithelioma of cranial dura. Am J Surg Pathol 31:807–811. https://doi.org/10.1097/PAS.0b013e31802c98ae
Yamada Y, Kohashi K, Kinoshita I, Yamamoto H, Iwasaki T, Yoshimoto M, Ishihara S, Toda Y, Itou Y, Koga Y, Hashisako M, Nozaki Y, Kiyozawa D, Kitahara D, Inoue T, Mukai M, Honda Y, Toyokawa G, Tsuchihashi K, Matsushita Y, Fushimi F, Taguchi K, Tamiya S, Oshiro Y, Furue M, Nakashima Y, Suzuki S, Iwaki T, Oda Y (2019) Clinicopathological review of solitary fibrous tumors: dedifferentiation is a major cause of patient death. Virchows Arch 475:467–477. https://doi.org/10.1007/s00428-019-02622-9
Nobusawa S, Lachuer J, Wierinckx A, Kim YH, Huang J, Legras C, Kleihues P, Ohgaki H (2010) Intratumoral patterns of genomic imbalance in glioblastomas. Brain Pathol 20:936–944. https://doi.org/10.1111/j.1750-3639.2010.00395.x
Watanabe K, Tachibana O, Sata K, Yonekawa Y, Kleihues P, Ohgaki H (1996) Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol 6:217–224. https://doi.org/10.1111/j.1750-3639.1996.tb00848.x
Gessi M, van de Nes J, Griewank K, Barresi V, Buckland ME, Kirfel J, Caltabiano R, Hammes J, Lauriola L, Pietsch T, Waha A (2014) Absence of TERT promoter mutations in primary melanocytic tumours of the central nervous system. Neuropathol Appl Neurobiol 40:794–797. https://doi.org/10.1111/nan.12138
Nakada S, Minato H, Takegami T, Kurose N, Ikeda H, Kobayashi M, Sasagawa Y, Akai T, Kato T, Yamamoto N, Nojima T (2015) NAB2-STAT6 fusion gene analysis in two cases of meningeal solitary fibrous tumor/hemangiopericytoma with late distant metastases. Brain Tumor Pathol 32:268–274. https://doi.org/10.1007/s10014-015-0220-x
Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, Iyer MK, Roychowdhury S, Siddiqui J, Pienta KJ, Kunju LP, Talpaz M, Mosquera JM, Singer S, Schuetze SM, Antonescu CR, Chinnaiyan AM (2013) Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 45:180–185. https://doi.org/10.1038/ng.2509
Yuzawa S, Nishihara H, Wang L, Tsuda M, Kimura T, Tanino M, Tanaka S (2016) Analysis of NAB2-STAT6 gene fusion in 17 cases of meningeal solitary fibrous tumor/hemangiopericytoma: review of the literature. Am J Surg Pathol 40:1031–1040. https://doi.org/10.1097/PAS.0000000000000625
Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D, Velázquez Vega JE, Perry A, Wu A, Raleigh DR, Santagata S, Louis DN, Brastianos PK, Kaplan A, Alexander BM, Rossi S, Ferrarese F, Haller F, Giannini C (2019) The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol 137:307–319. https://doi.org/10.1007/s00401-018-1952-6
Ishizawa K, Tsukamoto Y, Ikeda S, Suzuki T, Homma T, Mishima K, Nishikawa R, Sasaki A (2016) ‘Papillary’ solitary fibrous tumor/hemangiopericytoma with nuclear STAT6 expression and NAB2-STAT6 fusion. Brain Tumor Pathol 33:151–156. https://doi.org/10.1007/s10014-015-0247-z
Matsuda K, Tsugu H, Hirata Y, Yoshioka T, Nishiyama K, Nabeshima K, Inoue T, Ikeda K (2018) A case of solitary fibrous tumor of NAB2 exon 6-STAT6 exon 17. Jpn J Neurosurg 27:228–234. https://doi.org/10.7887/jcns.27.228
Yao ZG, Wu HB, Hao YH, Wang XF, Ma GZ, Li J, Li JF, Lin CH, Zhong XM, Wang Z, Wang DZ (2019) Papillary solitary fibrous tumor/hemangiopericytoma: an uncommon morphological form with NAB2-STAT6 gene fusion. J Neuropathol Exp Neurol 78:685–693. https://doi.org/10.1093/jnen/nlz053
Bieg M, Moskalev EA, Will R, Hebele S, Schwarzbach M, Schmeck S, Hohenberger P, Jakob J, Kasper B, Gaiser T, Ströbel P, Wardelmann E, Kontny U, Braunschweig T, Sirbu H, Grützmann R, Meidenbauer N, Ishaque N, Eils R, Wiemann S, Hartmann A, Agaimy A, Fritchie K, Giannini C, Haller F (2021) Gene expression in solitary fibrous tumors (SFTs) correlates with anatomic localization and NAB2-STAT6 gene fusion variants. Am J Pathol 191:602–617. https://doi.org/10.1016/j.ajpath.2020.12.015
Tran TAN (2020) Primary cutaneous solitary fibrous tumor with entrapped eccrine components. J Cutan Pathol 47:845–849. https://doi.org/10.1111/cup.13717
Tapia JL, Goodloe S 3rd, Margarone JE 3rd, Markiewicz MR, Aguirre A (2011) Solitary fibrous tumor with entrapment of minor salivary gland tissue: an unusual presentation that requires exclusion of pleomorphic adenoma. Head Neck Pathol 5:314–320. https://doi.org/10.1007/s12105-011-0254-2
Zhao M, He H, Cao D, Fan D, Xu M, Zhang X, Ru G (2022) Solitary fibrous tumor with extensive epithelial inclusions. Am J Clin Pathol 158:35–46. https://doi.org/10.1093/ajcp/aqab211
Rais M, Kessab A, Sayad Z, El Mourabit S, Zrarqi R, Benazzou S, Boulaadas M, Cherradi N (2017) Solitary fibrous tumor occurring in the parotid gland: a case report. BMC Clin Pathol 17:22. https://doi.org/10.1186/s12907-017-0062-z
Lee CK, Liu KL, Huang SK (2019) A dedifferentiated solitary fibrous tumor of the parotid gland: a case report with cytopathologic findings and review of the literature. Diagn Pathol 14:20. https://doi.org/10.1186/s13000-019-0792-6
Gonzalez MF, Husain BH (2021) Solitary fibrous tumour of the submandibular gland: Novel insights from clinical practice on a close mimicker of pleomorphic adenoma and a diagnostic challenge for the cytopathologist. Cytopathology 32:261–265. https://doi.org/10.1111/cyt.12932
Ghanchi H, Patchana T, Christian E, Li C, Calayag M (2020) Pediatric sellar solitary fibrous tumor/ hemangiopericytoma: a rare case report and review of the literature. Surg Neurol Int 11:238. https://doi.org/10.25259/SNI_234_2020
Oishi M, Fujisawa H, Tsuchiya K, Nakajima Y (2020) The importance of STAT6 in a schwannoma-like grade III solitary fibrous tumor/hemangiopericytoma located in the cerebellopontine angle and Meckel’s cave. World Neurosurg 141:500–506. https://doi.org/10.1016/j.wneu.2020.05.262
Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080. https://doi.org/10.1126/science.1233009
Yuzawa S, Nishihara H, Tanaka S (2016) Genetic landscape of meningioma. Brain Tumor Pathol 33:237–247. https://doi.org/10.1007/s10014-016-0271
Acknowledgements
We thank Ms. Machiko Yokota and Mr. Tatsuya Yamazaki (Gunma University) for their technical assistance.
Author information
Authors and Affiliations
Contributions
S. Nobusawa designed the study; T.S., Y.Y., N.M., J.H., and S. Nobusawa performed the pathological analysis; T.S., Y.Y., Y.O., and S. Nobusawa performed the laboratory research; cases and clinical data were provided by B.A., Y.S., S.T., T.K., N. Komoribayashi, and N. Kubo; T.S., S. Nakata, N.O., and S. Nobusawa analyzed and interpreted the data; T.S., S. Nakata, and S. Nobusawa wrote the manuscript; J.H. and H.Y. participated in construction of the manuscript and revised it critically; and all authors accepted the final version of the manuscript.
Corresponding author
Ethics declarations
The present study was approved by the Gunma University Ethical Committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirakura, T., Yamada, Y., Nakata, S. et al. Analysis of clinicopathological features and NAB2-STAT6 fusion variants of meningeal solitary fibrous tumor with ectopic salivary gland components in the cerebellopontine angle. Virchows Arch 481, 913–923 (2022). https://doi.org/10.1007/s00428-022-03403-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03403-7