Abstract
We present two cases of meningeal solitary fibrous tumor (SFT)/hemangiopericytoma (HPC) with immunohistochemistry of STAT6 and analysis of NAB2–STAT6 fusion genes. Case 1 was a 37-year-old male with a left middle fossa tumor; case 2 was a 68-year-old female with a cerebellar tumor. They showed late metastasis to the lung or bone 8 or 13 years, respectively, after the first surgery. Histology of both primary and metastatic tumors showed a cellular hemangiopericytomatous pattern with nuclear atypia. The primary tumors showed nuclear staining of STAT6, but both metastatic tumors showed nuclear and cytoplasmic STAT6. DNA sequencing revealed two kinds of NAB2–STAT6 fusion genes. One consisted of exon 6 of NAB2, intron 6 of NAB2, and the middle of exon 17 of STAT6 (observed in the primary and metastatic tumors of case 1); the other consisted of exon 6 of NAB2 and the beginning of exon 17 of STAT6 (observed in the metastatic tumor of case 2). The primary tumor of case 2 had both fusion genes. To the best of our knowledge, we are the first to report NAB2–STAT6 fusion gene analysis in primary and metastatic meningeal SFT/HPCs and a case showed different fusion gene status in the metastatic tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solitary fibrous tumor (SFT) and hemangiopericytoma (HPC) are rare soft tissue and meningeal tumors. In soft tissue tumor pathology, “HPC” has been omitted from the WHO blue book since 2013 [1]. Because both HPC and SFT have been associated with the same NAB2–STAT6 fusion gene, they are regarded as tumors of the same entity, but different grade. HPC was included in the 2007 WHO classification of the central nervous system [2], but recently several reports revealed that HPC and SFT in the meninges are also associated with the same NAB2–STAT6 fusion gene [3] and nuclear STAT6 immunoreactivity both in meningeal SFT and HPC [3, 4]. Recently, different variants of NAB2–STAT6 fusion genes have been reported in SFT of the soft tissue and are associated with different histology and biological behavior [5]. Here, we report two cases of meningeal SFT/HPC with extracranial metastases as well as NAB2–STAT6 fusion gene status in each primary and metastatic tumor.
Clinical summary
Case 1
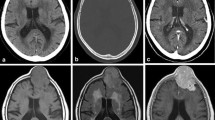
A 37-year-old male complained of left hearing impairment and left facial anesthesia. Cranial computed tomography (CT) revealed a mass at the left middle fossa. After embolization of the feeder artery, the meningeal tumor in the left middle fossa was partially resected, and supplementary radiation therapy was prescribed. The tumor recurred locally six times after the first operation, each of which was treated with radiation or surgery. Eight years after the first operation, he complained of chest pain; chest CT showed a 20-cm metastatic tumor in the lower lobe of the right lung. Right lower lobectomy was performed. Another local recurrence and a subcutaneous metastasis in the right buttock were resected. After a 9-year history, he died with generalized multiple metastases. Autopsy was performed with the informed consent of his family.
Case 2
An 81-year-old female complained of left ischial pain. Pelvic CT showed a destructive 4.5-cm tumor on the left ischial bone. Pathology of the resected tumor and medical history revealed that she had a 2-cm meningeal tumor in the left cerebellar tentorium 13 years previously. The ischial tumor recurred 10 months after resection, but regressed after radiotherapy.
Pathological findings
Case 1
Primary, recurrent, and metastatic tumors had a similar macroscopic and microscopic appearance. Gross observation showed the tumors were whitish-gray to brown and solid. The primary tumor of the middle fossa consisted of polygonal or short spindle cells with a cellular hemangiopericytomatous pattern, nuclear atypia, and geographic necrosis (Fig. 1a, b). Embolized materials were occasionally seen in the vessels. Mitotic count was four per high-power field (/HPF). Recurrent and metastatic tumors also showed a hemangiopericytomatous pattern, but cellularity was higher and necroses were more abundant (Fig. 1d, e). Mitotic count of the right buttock tumor was 17/HPF. At autopsy, a recurrent tumor was found in the left middle fossa. The tumor directly invaded the temporal, frontal, and posterior lobes of the brain and the posterior region of the ear. Multiple metastatic nodules were found in the liver, lungs, chest wall, pancreas, kidneys, esophagus, and heart. Immunohistochemistry showed the primary tumor was positive for Bcl-2 (clone 124, Dako, Carpinteria, CA), LEU7 (clone HNK-1, Becton–Dickinson, San Jose, CA), and keratin (clone AE1/AE3, Leica Biosystems, Newcastle, UK), and the metastatic tumor of the right buttock was focally positive for CD34 (clone NU-4A1, Nichirei, Tokyo, Japan), EMA (clone E29, Nichirei), Bcl-2, and keratin. Both tumors were negative for S-100 protein (clone 2A10, IBL, Takasaki, Japan), GFAP (polyclonal, Nichirei), CD99 (clone 12E7, Dako), and progesterone receptor (PgR) (clone PgR636, Dako). The primary tumor showed mostly nuclear staining of STAT6 (polyclonal, Santa Cruz, CA), and the metastatic tumor showed nuclear and more cytoplasmic staining of STAT6 (Fig. 1c, f, l, i).
Histology (a, b of a primary meningeal tumor, and d, e of a metastatic tumor of thigh of case 1, g, h of a primary meningeal tumor and j, k of a metastatic tumor of bone of case 2) and immunohistochemistry for STAT6 (c of the primary tumor and f of the metastatic tumor of case 1, i of the primary tumor and l of the metastatic tumor of case 2). The tumors of case 1 show polygonal or short spindle cells with a cellular hemangiopericytomatous pattern with nuclear atypia (a–f). Necrosis was more abundant in the metastatic tumor (d, e). In case 2, the primary and metastatic tumors show a similar histology with case 1, but their nuclear atypia is somewhat milder (g–l). Immunohistochemistry of STAT6 showed nuclear positivity in all tumors and predominant cytoplasmic positivity in metastatic tumors (c, f, i, l). (original magnifications of a, d, g and j are ×20, and those of b, c, e, f, h, i, k, and l are ×400)
Case 2
In gross appearance, the ischial tumor was 4.8 cm in size, tan-white in color, and solid. The ischial tumor was composed of small round cells or short spindle cells with diffuse or hemangiopericytomatous pattern and high cellularity. Tumor cells were monotonous and showed a high nuclear–cytoplasmic ratio with mild to moderate nuclear atypia (Fig. 1g, h, j, k). Mitotic count was 6/HPF. Immunohistochemistry showed that the ischial tumor was positive for vimentin (clone V9, Dako) and CD99, but negative for keratin (clone AE1/AE3, Dako), EMA (clone E29, Dako), αSMA (clone 1A4, Dako), S-100 protein (polyclonal, Dako), CD34 (clone QBEnd 10, Dako), CD31 (clone JC70A, Dako), Bcl-2, LCA (clone 2B11+PD7/26, Dako), and CD138 (clone MI15, Dako). Ki-67 (clone SP6, Lab Vision Corporation, Fremont, CA, USA) was indeterminate because of artificial effects, probably due to decalcification. Histopathological differential diagnosis included a borderline or low-grade fibrous histiocytic tumor, a giant cell tumor of the tendon sheath, and a metastatic tumor. Based on the patient’s history of cerebellar meningeal tumor, metastasis from the cerebellar meningeal hemangiopericytoma was diagnosed. As with case 1, the primary tumor of case 2 showed mostly nuclear STAT6 staining and the metastatic ischial tumor showed nuclear and more cytoplasmic STAT6 staining (Fig. 1c, g, i, l).
NAB2–STAT6 fusion gene analysis
NAB2–STAT6 fusion genes were analyzed in formalin-fixed, paraffin-embedded tissues (FFPE) of each primary and metastatic tumor, except for a frozen tissue specimen of the metastatic tumor in case 2. Reverse-transcriptase polymerase chain reaction (RT-PCR) and cDNA sequencing of the NAB2–STAT6 fusion gene were performed. Total RNA was isolated from FFPE or frozen tumor samples. For FFPE samples, tissue were deparaffinized and treated with proteinase K in 1 % SDS HMW buffer at 55 °C. RNA was extracted using ISOGEN (Nippon Gene, Tokyo, Japan). cDNA was synthesized from 500 ng RNA by AMV (TAKARA BIO, Otsu, Japan). Primers were designed based on the cDNA sequences of NAB2 (NM_005967.3) and STAT6 (NM_001178078.1) and are listed in Table 1. PCR was performed at 94 °C for 2 min, followed by 40 cycles of denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 10 min. After electrophoresis in 1.5 % agarose gels, the subjective bands were excised and DNA was extracted with a gel extraction kit (QIAGEN K.K., Tokyo, Japan). Direct sequencing was performed on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Life Technologies, Tokyo, Japan).
RT-PCR yielded 163-bp products in the primary and metastatic tumors of case 1, 163-bp and 202-bp bands in the primary tumor of case 2, and a 202-bp band in the metastatic tumor of case 2 (Fig. 2a). Fusion products were confirmed by direct sequencing. The genomic breakpoint of the 202-bp band was located at the end of exon 6 of NAB2 and the beginning of exon 17 of STAT6. In contrast, the breakpoint of the 163-bp band was located at the end of exon 6 of NAB2, intron 6 of NAB2, and the middle of exon 17 of STAT6 (Fig. 2b). The size of NAB2 intron 6 in the 163-bp band was 15 bp and that of exon 17 of STAT6 was 36 bp; thus, exon 17 was 54 bp shorter than in the 202-bp band. Both fusion genes were in-frame fusions (data not shown).
NAB2–STAT6 fusion gene analysis. a An electrophoresis of RT-PCR for NAB2–STAT6 fusion gene shows bands of PCR products by only a primer set for exon 6 of NAB2 and exon 17 of STAT6. 163-bp PCR products were detected in both primary and metastatic tumors of case 1. There were 163- and 202-bp bands in primary tumor and only a 202-bp band for a metastatic tumor of case 2. P stands for primary and M stands for metastasis. b DNA sequences of the 163- and 202-bp bands obtained by electrophoresis. The 163-bp band consisted of exon 6 of NAB2, intron 6 of NAB2, and shorter exon 17 of STAT6. The 202-bp band was formed by exon 6 of NAB2 and longer exon 17 of STAT6
Discussion
We report two cases of malignant SFT/HPC including STAT6 immunohistochemistry and NAB2–STAT6 fusion gene analysis in primary and metastatic tumors. The primary tumors showed nuclear staining of STAT6 and the metastatic tumors exhibited nuclear and cytoplasmic staining in both cases. We analyzed the NAB2–STAT6 fusion genes in the primary and metastatic meningeal SFT/HPCs and showed that one SFT/HPC had two kinds of NAB2–STAT6 fusion genes (case 2). In this case, the primary tumor expressed two NAB2–STAT6 fusion genes and the metastatic tumor expressed only one.
Meningeal SFT/HPC is frequently associated with local recurrence and late metastasis to extracranial sites. Extracranial metastatic rates at 5, 10, and 15 years are about 10, 31, and 77 %, respectively [6], with a mean period of 8 years after initial therapy [7]. They metastasize to virtually any organ including bone, liver, lung, abdominal cavity, lymph node, skeletal muscle, kidney, pancreas, skin, subcutaneous tissue, breast, adrenal gland, gallbladder, diaphragm, retroperitoneum, and heart [7]. Bony metastases are most frequently reported, especially in the pelvis, spine, and scapula [6]. In case 1, the pulmonary metastasis was found 8 years after resection of the primary tumor; in case 2, ischial bone metastasis was documented 13 years after the first brain tumor resection. If a hemangiopericytomatous pattern is seen in tumors of the bones, liver, or lungs, confirmation of the history of a brain tumor is essential to avoid misdiagnosis.
Meningeal SFT/HPCs are typically positive for CD34 (100 % of SFT and 33 % of HPC), Bcl-2 (85 % of HPC), and CD99 (95 % of HPC); focally positive for EMA (0 % of SFT and 4–37 % of HPC), cytokeratin (0 % of SFT and 20 % of HPC), claudin-1 (13 % of HPC), and desmin (13 % of SFT and 20 % of HPC); and negative for S100 protein [8, 9]. The combination of these antibodies yields high sensitivity, but the specificity is relatively poor because many soft tissue tumors are positive for CD34, Bcl-2, or CD99. Since the recent discovery of the NAB2–STAT6 fusion gene in SFT/HPC [10, 11], several reports have suggested that nuclear expression of STAT6 is highly specific and quite useful for the diagnosis of SFT/HPC [3–5, 12, 13].
STAT6 is a member of the STAT family of transcription factors; induced by interleukin-4 and phosphorylated by JAK, STAT6 forms homo- or heterodimers that translocate to the nucleus where they act as transcription activators [14]. The NAB2 gene encodes NGFI-A-binding protein 2 (NAB2), also known as early growth response-1 (EGR-1)-binding protein 2 or melanoma-associated delayed early response protein (MADER). Both the human STAT6 and NAB2 genes have been localized to chromosome 12q13, a region that is rearranged in several solid tumors, such as lipomas, uterine leiomyomas, and liposarcomas [15]. The normal NAB2 protein functions in the nucleus to repress transcription induced by EGR-1 in a negative feedback loop; in contrast, the NAB2–STAT6 fusion gene turns into a potent transcriptional activator of EGR-1, thus activating EGR-1 target genes and driving neoplastic progression [10, 16]. Robinson reported that EGR1 target genes, such as FGFR1, NAB1, IGF2, FGF2, PDGFD, and receptor tyrosine kinases such as FGFR1 and NTRK1, are most highly enriched in SFT versus non-SFT sarcomas [10]. It is possible that strong expression of the NAB2–STAT6 fusion gene increases proliferation and expression of EGR-1 target genes, thus leading to tumorigenesis and tumor progression of SFT [10].
The two variant fusion genes reported here have been reported previously [5, 10, 17]. Barthelmeß et al. [5] reported that the most common fusion variant (exon 4 of NAB2 and exon 2/3 of STAT6) corresponds to classic pleuropulmonary SFTs with mostly benign behavior and occurs in older patients. In contrast, the second-most common fusion variant (exon 6 of NAB2 and exon 16/17 of STAT6) is found in much younger patients and represents typical HPC from deep soft tissue with a more aggressive phenotype [5]. The present cases showed that fusion variants correspond to the more aggressive phenotype, though the patient of case 2 was older. Case 2 had two different types of fusion genes in the primary tumor, but only one type in the metastatic tumor. To the best of our knowledge, there have been no reports about the difference of fusion gene variants between primary and metastatic SFT/HPCs, even in extra-cranial non-meningeal SFT/HPCs. Mohajeri et al. [17] reported 3 cases of extracranial SFTs having more than two types of fusion genes by next-generation sequencing; however, there was no comment on metastatic tumors in the report. The two fusion genes in this report have not been associated with any clinicopathological differences. These findings may simply reflect an instability of the splicing process of unknown significance during transcription, or relate to subclonal evolution and metastatic potential of the tumor [11]. Because there have been no studies of the relationship between different fusion variants and clinicopathological features of meningeal SFT/HPC [5], accumulation of sufficient information in larger studies is necessary to define the significance of differences in these fusion variants.
Metastatic tumors of both cases showed nuclear and cytoplasmic STAT6 staining, although nuclear stain was predominant in both primary tumors. The reason for this is not clear. STAT6 protein translocates from the cytoplasm to the nucleus by phosphorylation [18], and most of the phosphorylation sites, such as 641, 645, 707, and 756, are present in variant NAB2–STAT6 fusion genes. The phosphorylation state of STAT6 might differ between the primary and metastatic tumors, or degradation of NAB2–STAT6 gene products in the cytoplasm might occur in metastatic tumors.
Nuclear expression of STAT6 has also been detected in some cases of dedifferentiated liposarcoma, undifferentiated pleomorphic sarcoma, and nodular fasciitis [4, 13]. NAB2–STAT6 fusion genes have also been detected in some cases of dedifferentiated liposarcoma [13, 19]. Morphological differentiation of SFT/HPC from undifferentiated pleomorphic sarcoma and nodular fasciitis is generally straightforward and the latter diseases have shown weaker nuclear STAT6 expression in only a proportion of tumor cells [4, 13]. Since SFT/HPC rarely has a lipomatous feature [20], differentiating it from dedifferentiated liposarcoma may be problematic. However, dedifferentiated liposarcoma can be distinguished by immunopositivity for MDM2 and CDK4, or MDM2 amplification by FISH, which are negative in SFT/HPC [13].
In summary, two cases of meningeal SFT/HPC showed late distant metastases, and both primary tumors showed mostly nuclear staining of STAT6, but both metastatic tumors showed nuclear and more cytoplasmic staining. To the best of our knowledge, we are the first to report the presence of two NAB2–STAT6 fusion genes in a single case of meningeal SFT/HPC. The significance of these findings is not clear at this time. Further studies on the association of NAB2–STAT6 fusion gene status with clinicopathological features of meningeal SFT/HPC are needed.
References
Fletcher CDM, Bridge JA, Lee JC (2013) Extrapleural solitary fibrous tumour. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds) WHO classification of tumours of soft tissue and bone, 4th edn. IARC Press, Lyon, pp 80–82
Paulus W, Scheithauer BW, Perry A (2007) Mesenchymal, non-meningoethelial tumours. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the central nervous system, 4th edn. IARC Press, Lyon, pp 173–177
Schweizer L, Koelsche C, Sahm F et al (2013) Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2–STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125:651–658
Yoshida A, Tsuta K, Ohno M et al (2014) STAT6 Immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 38:552–559
Barthelmeß S, Geddert H, Boltze C et al (2014) Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2–STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol 184:1209–1218
Damodaran O, Robbins P, Knuckey N et al (2014) Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci 21:1310–1314
Fountas KN, Kapsalaki E, Kassam M et al (2006) Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev 29:145–153
Perry A, Scheithauer BW, Nascimento AG (1997) The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol 21:1354–1360
Rajaram V, Brat DJ, Perry A (2004) Anaplastic meningioma versus meningeal hemangiopericytoma: immunohistochemical and genetic markers. Hum Pathol 35:1413–1418
Robinson DR, Wu Y-M, Kalyana-Sundaram S et al (2013) Identification of recurrent NAB2–STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 45:180–185
Chmielecki J, Crago AM, Rosenberg M et al (2013) Whole-exome sequencing identifies a recurrent NAB2–STAT6 fusion in solitary fibrous tumors. Nat Genet 45:131–132
Doyle LA, Vivero M, Fletcher CD et al (2014) Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 27:390–395
Koelsche C, Schweizer L, Renner M et al (2014) Nuclear relocation of STAT6 reliably predicts NAB2/STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology 65:613–622
Reich NC (2013) STATs get their move on. JAKSTAT 2:e27080
Svaren J, Sevetson BR, Apel ED et al (1996) NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol 16:3545–3553
Bhattacharyya S, Fang F, Tourtellotte W, Varga J (2013) Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J Pathol 229:286–297
Mohajeri A, Tayebwa J, Collin A et al (2013) Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosom Cancer 52:873–886
Shirakawa T, Kawazoe Y, Tsujikawa T et al (2011) Deactivation of STAT6 through serine 707 phosphorylation by JNK. J Biol Chem 286:4003–4010
Doyle LA, Tao D, Mariño-Enríquez A (2014) STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol 27:1231–1237
Guillou L, Gebhard S, Coindre JM (2000) Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohistochemical, and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol 31:1108–1115
Acknowledgments
The authors specially thank Mr. Manabu Yamashita for technical assistance of fusion gene analysis. This study was supported in part by the Health and Labor Sciences Research Expenses for Commission, Applied Research for Innovative Treatment of Cancer from the Ministry of Health, Labor and Welfare H26-084 (T. N.) and by JSPS KAKENHI Grant Number 26460660 (H. M.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakada, S., Minato, H., Takegami, T. et al. NAB2–STAT6 fusion gene analysis in two cases of meningeal solitary fibrous tumor/hemangiopericytoma with late distant metastases. Brain Tumor Pathol 32, 268–274 (2015). https://doi.org/10.1007/s10014-015-0220-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-015-0220-x