Abstract
The aim of the study was to investigate prevalence of high-risk human papillomavirus (HR-HPV) infection in sinonasal carcinomas by immunohistochemistry, in situ hybridization, and polymerase chain reaction, detecting p16INK4a protein (p16) expression and presence of both HPV DNA and HPV E6/E7 messenger RNA (mRNA). The study comprised 47 males and 26 females, aged 23–83 years (median 62 years), mostly (67 %) with a squamous cell carcinoma (SCC). Of the tumors, 53 % arose in the nasal cavity, 42 % in the maxillary sinus, and 5 % in the ethmoid complex. The follow-up period ranged 1–241 months (median 19 months). HPV16, HPV18, or HPV35 were detected in 18/73 (25 %) tumors, 17 SCCs, and 1 small cell neuroendocrine carcinoma. There was a strong correlation between results of HPV detection methods and p16 expression (p < 0.005). HPV-positive SCCs occurred more frequently in smokers (p = 0.04) and were more frequently p16-positive (p < 0.0001) and nonkeratinizing (p = 0.02), the latter occurring more commonly in nasal cavity (p = 0.025). Median survival for HPV-positive SCC patients was 30 months, while for HPV-negative SCC patients was 14 months (p = 0.23). In summary, we confirm that HR-HPV is actively involved in the etiopathogenesis of a significant subset of sinonasal SCCs. p16 may be used as a reliable surrogate marker for determination of HPV status also in sinonasal SCCs. Although we observed a trend toward better overall survival in HPV-positive SCCs, the prognostic impact of HPV status in sinonasal carcinomas needs to be elucidated by further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinomas of the nasal cavity and paranasal sinuses are rare. In 2011, 51 new cases in men and 28 in women were diagnosed in the Czech Republic, giving incidence rates of 1.0/100,000 and 0.5/100,000 for males and females, respectively [1]. These figures probably reflect the general status in Europe and worldwide [2, 3]. Sinonasal carcinomas encompass a heterogenous group of tumors arising from sinonasal mucosa and seromucinous glands, with squamous cell carcinoma (SCC) being the most common [4]. The etiopathogenesis of this group of neoplasms is still poorly understood, although a variety of agents have been implicated in the past as risk factors. These include occupational exposure to wood dust and various chemical substances, such as nickel (during refining), but only for one rarely occurring sinonasal cancer subtype, the intestinal-type adenocarcinoma [4]. Interestingly, cigarette smoking, still a major risk factor for head and neck SCC, is only weakly associated with sinonasal cancer [5].
Persistent high-risk human papillomavirus (HR-HPV) infection is now a well-recognized risk factor for a significant proportion of carcinomas of the anogenital area, including cervical, vaginal, vulvar, penile, and anal cancers, and for a subset of head and neck carcinomas [6–10]. Regarding the latter, oropharyngeal SCC, affecting mostly the palatine tonsils and root of the tongue, shows the strongest, although highly variable association with HR-HPV infection [11–13]. In sinonasal carcinoma, HPV detection rates vary significantly from 0 to 100 % [2, 14–20]. In a meta-analysis provided by Syrjänen and Syrjänen, 133/492 (27 %) cases were HPV-positive [2]. Five recent larger studies reported on tumor morphology, p16 expression and patient outcome [16, 18, 21–23]. Altogether, transcriptionally active HPV is found in 47/285 (16 %) of cases [24]. Only a single study directly investigated the presence of HPV16 messenger RNA (mRNA) E7 transcripts by real-time polymerase chain reaction (RT-PCR) [22].
We investigated the prevalence of HR-HPV infection in a large cohort of sinonasal epithelial malignant tumors including SCCs using a panel of methods, including immunohistochemistry, in situ hybridization (ISH) and PCR. We also compared clinicopathological characteristics of HPV-positive and HPV-negative carcinomas and assessed the prognostic impact of HPV status on patient outcome.
Material and methods
Clinicopathological data
A review of the surgical pathology files at The Fingerland Department of Pathology (University Hospital, Hradec Kralove, Czech Republic), Department of Pathology (General University Hospital, Prague, Czech Republic), and Department of Pathology (University Hospital, Olomouc, Czech Republic) identified all malignant epithelial tumors of the sinonasal tract diagnosed between August 1995 and August 2014. Only tumors primarily originating from the nasal cavity, maxillary sinuses, and ethmoid complex were included [25]. No tumors were found in the frontal or sphenoid sinuses. The 73 cases meeting the inclusion criteria were reclassified by experienced head and neck pathologists (J.L., A.R.) according to the current World Health Organization (WHO) classification [4] and using appropriate ancillary methods in the case of need [26, 27]. Paraffin blocks for further analysis were available in all cases. Ethical approval was obtained from the Ethics Committee, University Hospital, Hradec Kralove (Reference No. 201403 S16P).
For every patient, gender, age at the time of diagnosis, smoking history (nonsmoker vs. ex-smoker vs. concurrent smoker), occupation (risky vs. nonrisky), tumor localization including nasal cavity, maxillary sinus, and ethmoid complex, laterality and pathological TNM were recorded. During the follow-up period (until October 2014) local recurrence, regional recurrence, distant recurrence, death, and tumor-related death staging were recorded [25]. When radical surgery was not performed, clinical TNM staging was used instead. Treatment modalities were radical surgery, radiotherapy, and chemotherapy in various combinations.
The tumor types included squamous cell carcinoma (SCC, conventional, verrucous, papillary, basaloid, spindle cell, acantholytic, adenosquamous), lymphoepithelial carcinoma (LEC), sinonasal undifferentiated carcinoma (SNUC), adenocarcinoma (intestinal-type, nonintestinal-type, salivary gland-type), and neuroendocrine tumor (typical carcinoid, atypical carcinoid, small cell neuroendocrine carcinoma (SCNEC)) [4]. Conventional SCC was further subclassified into nonkeratinizing (NK-SCC), nonkeratinizing with maturation (hybrid) (NKM-SCC) and keratinizing (K-SCC) subtypes, applying the criteria recently proposed for oropharyngeal SCC [28, 29]. Only conventional K-SCC and intestinal-type adenocarcinoma were graded as well, moderately or poorly differentiated [30]. Vascular invasion, perineural spread, status of resection margins (in case of radical surgery), and microscopic findings in the surrounding mucosa were also noted.
As controls, we used 10 mucosal specimens from the nasal cavity and 10 from the maxillary sinus with features of chronic rhinitis and sinusitis, obtained from 10 males and 10 females aged 24–74 years (median 57 years; mean 59 ± 15 years).
Immunohistochemical staining for p16
A mouse monoclonal antibody against p16 (CINtec® Histology Kit, clone E6H4™, Roche, Mannheim, Germany) was used on 4-μm sections from formalin-fixed, paraffin-embedded tissue blocks on a Ventana BenchMark ULTRA immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA). For details, see Supplementary online material.
All cases were independently classified by two pathologists (J.L., A.R.), blinded to the results of other methods, as positive when ≥70 % of tumor cells showed strong nuclear and/or cytoplasmic staining, or negative, according to recent recommendations [31–35]. Discrepant cases were resolved by consensus review.
HPV ISH
ISH was performed on 4-μm whole tumor sections, cut from formalin-fixed paraffin-embedded tissue blocks. For detection of HPV DNA, this was done on a Ventana BenchMark ULTRA immunostainer (Ventana Medical Systems, Inc.) using the INFORM® HPV III Family 16 Probe (B) (Ventana Medical Systems, Inc.), a cocktail targeting HR-HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. For detection of HR-HPV E6/E7 mRNA, the RNAscope® Probe HPV-HR18 (Advanced Cell Diagnostics, Hayward, CA, USA), a cocktail targeting HR-HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82, and detection system RNAscope® 2.0 HD Reagent Kit (Brown) (Advanced Cell Diagnostics) were used manually, as previously described [36]. For details, see Supplementary material (available online).
All cases were independently assessed by two pathologists (J.L., A.R.), who were blinded to the results of other methods, and classified binarily as positive or negative. Any definitive nuclear diffuse and/or dot-like navy-blue precipitate (for HPV DNA) or nuclear and/or cytoplasmic brownish dots and/or clusters (for HPV E6/E7 mRNA) were considered positive. Discrepant cases were resolved by consensus review.
HPV DNA PCR and typing
HPV DNA was extracted from formalin-fixed, paraffin-embedded tumor tissue after deparaffinization in xylene and rehydration in ethanol using the commercial DNA Sample Preparation Kit (Roche, Basel, Switzerland) according to the manufacturer’s protocol. PCR amplification of β-globin sequences was performed to confirm sample fitness for PCR assay [37]. PCR amplification was performed with EIA Kit HPV GP HR (Diassay, Rijswijk, Netherlands) using as forward and reverse primers 5′-TTTGTTACTGTGGTAGATACTAC-3′ (GP5+) and 5′-GAAAAATAAACTGTAAATCATATT-3′ (GP6+) which detect HPV types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 45, 51, 52, 53, 56, 58, 59, 61, 66, 67, 68 (and 68a), 69, 71, 72, 73, 81, and 82 (MM4 and IS39).
Samples showing HPV DNA presence by PCR or ISH or immunohistochemical expression of p16 were analyzed using the Linear Array HPV SPF10 Genotyping Test (LBP, Rijswijk, Netherlands), which detects HPV types 3, 4, 5, 6, 7, 8, 11, 13, 16, 18, 26, 27, 30, 31, 32, 33, 34, 35, 37, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 65, 66, 67, 68, 69, 70, 71, and 74. For details, see Supplementary material (available online).
HPV E6/E7 mRNA RT-PCR
HPV RNA was extracted from paraffin-embedded tissue after deparaffinization in xylene and rehydration in ethanol using the commercial RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Reverse transcription of RNA was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). PCR amplification of β-globin sequences was performed to confirm sample fitness for PCR assay [37, 38]. PCR amplification was performed using Power SYBR Green qPCR Master Mix (2×) (Applied Biosystems, Carlsbad, CA, USA) with specific primers for each tested HPV type (for each HPV 16, 18, and 35: HPV E6-Forward, HPV E6-Reverse, HPV E7-Forward, HPV E7-Reverse) and HPV cDNA. For details, see Supplementary material (available online).
Statistical analysis
For the analysis of categorical data, we used median, mean, and 95 % confidence interval (CI), for continuous data, absolute and relative frequencies, and for survival analysis, Kaplan-Maier and Log-rank tests and Cox regression. For the purpose of statistical analysis, a case was considered HPV-positive if it was positive for HPV DNA ISH/PCR and/or HPV E6/E7 mRNA ISH/PCR. Relationships between HPV/p16 positivity and other independent factors were analyzed using the chi-square test, Fisher’s exact test, or logistic regression analysis. We considered p < 0.05 to be statistically significant. All statistical analyses were performed using the NCSS 8 statistical software program (NCSS, Kaysville, Utah, USA).
Results
Clinicopathological data
Clinicopathological data are listed in Table 1. Due to missing clinical data, sums in the entire study sample or in the SCC group or partial sums do not always add up to the total number of patients.
The study sample (n = 73) comprised 47 males and 26 females, aged 23–83 years (median 62 years). From those with a known history, 30 patients were nonsmokers (18 with SCC), 12 ex-smokers (7 with SCC), and 20 were current smokers (16 with SCC). In only 7/62 (11 %) patients, occupational exposure to wood dust or other air pollutants/irritants was recorded (2× joiner, 1× wood industry worker, 1× miller, 1× locksmith (SCC), 2× rubber industry worker (SCC)).
As regards the whole study sample, most of the tumors arose in the nasal cavity, but SCCs were slightly more common in the maxillary sinus. The majority of the patients were diagnosed with advanced tumors and four patients (two with SCC) had lung metastases (cM1) at the time of diagnosis.
The treatment modalities included radical surgery with radiotherapy (24×), radical surgery with chemoradiotherapy (19×), radical surgery only (11×), radiotherapy only (6×), and chemoradiotherapy only (5×). Positive surgical margins were found in 44/57 (77 %) patients treated by radical surgery.
Microscopic typing of the tumors resulted in 49 SCCs, 17 adenocarcinomas, 3 neuroendocrine tumors, 3 SNUCs, and 1 LEC. Vascular invasion was found in 11/73 (15 %) tumors and perineural spread in 5/73 (7 %).
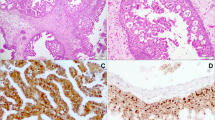
Among the SCC group, 27/49 (55 %) tumors were NK/NKM-SCCs (Fig. 1a), 16/49 (33 %) K-SCCs (3× grade 1, 11× grade 2, 2× grade 3) (Fig. 1b), 3/49 (6 %) basaloid, verrucous, papillary, and adenosquamous (one each) carcinomas. NK/NKM-SCC occurred more frequently within the nasal cavity, whereas K-SCC more commonly arose within the maxillary sinus. The findings in surrounding mucosa included dysplastic squamous epithelium (18×), inverted papilloma without dysplasia (1×), and inverted papilloma with dysplastic changes (4×).
a Nonkeratinizing squamous cell carcinoma (NK-SCC) is characterized by pushing borders and consists of smaller darkly appearing cells with little cytoplasm (hematoxylin-eosin (HE), original magnification ×100). b Keratinizing squamous cell carcinoma (K-SCC) is composed of larger cells with eosinophilic cytoplasm and typically shows keratin pearls (HE, original magnification ×100). c Diffuse strong p16 expression in NK-SCC (original magnification ×100). d Diffuse strong p16 expression in K-SCC (original magnification ×100). a, c The same NK-SCC harboring HPV16, and b, d the same K-SCC harboring HPV35
The follow-up period ranged from 1 to 241 months (median 19 months). Local recurrence was found in 24/63 (38 %) tumors, 5/62 (8 %) recurred regionally, and 7/61 (11 %) patients developed distant metastases in the lungs. During the follow-up period, 32/61 (52 %) patients died, of whom 16/59 (27 %) due to the tumor.
Regarding the SCC group, the follow-up period ranged from 1 to 104 months (median 16 months). Local recurrence was found in 14/42 (33 %) tumors, 3/41 (7 %) recurred regionally, and 3/40 (8 %) patients developed distant metastases in the lungs. During the follow-up period, 25/41 (61 %) patients died, of whom 10/40 (25 %) due to the tumor. Comparing NK/NKM-SCC with K-SCC, a total of 8/22 (36 %) versus 4/14 (29 %) tumors recurred locally, 2/21 (10 %) versus 0/14 recurred regionally, 1/21 (5 %) versus 0/13 patients developed distant metastases, a total of 14/22 (64 %) versus 9/13 (69 %) patients died and 8/21 (38 %) versus 2/13 (15 %) died due to the tumor.
Immunohistochemical and molecular findings
Immunohistochemical and molecular findings are listed in Table 2
p16 IHC
All 73 study samples and 20 control cases were successfully stained and easily readable with unequivocal results.
Applying the 70 % threshold, 25/73 (34 %) tumors from the whole study cohort were scored as p16-positive: 16 SCCs, 6 adenoid cystic carcinomas, 2 SCNECs, and 1 SNUC. Comparing the subtypes of conventional SCC, 13/27 (48 %) of NK/NKM-SCCs were p16-positive (Fig. 1c), while only 1/16 (6 %) K-SCC was positive (Fig. 1d). Staining for p16 was generally diffuse and strong nuclear/cytoplasmic in nearly 100 % tumor cells, except for adenoid cystic carcinomas, where cells lining true ducts and/or duct-like structures were preferentially stained, while abluminal cells were rather negative. Dysplastic squamous epithelium adjacent to p16-positive invasive SCC was p16-positive as well.
Of the p16-negative group, p16 expression was completely absent in 28 cases, while the other 20 tumors featured variable expression in 10–60 % tumor cells. None of the control cases was scored as p16-positive. We observed only patchy p16 staining in mucinous cells of mucosal glands and focal staining of respiratory epithelium with goblet cell hyperplasia and of superficial layers of metaplastic squamous epithelium (one case).
HPV DNA ISH and PCR
All 73 study samples and 20 control cases were successfully stained for HPV DNA by ISH. Although most cases were interpreted without difficulties, in 13/73 (18 %) tumors, the staining result was considered equivocal and needed consensus review.
Twelve of all 73 (16 %) tumors—SCCs only—were HPV DNA ISH-positive, including 10/27 (37 %) NK/NKM-SCCs (Fig. 2a) and 2/16 (13 %) K-SCCs (Fig. 2b). In eight cases, the HPV DNA ISH nuclear signal was seen in max. 1–20 % tumor cells only at ×400 magnification, while in the remaining four cases, up to 80 % tumor cells showed nuclear signal clearly visible at ×40–200 magnification. In all cases, we observed dot-like nuclear signal (mostly up to four dots per nucleus) indicating integrated form of HPV DNA. An additional diffuse nuclear signal, indicating episomal form of HPV DNA, was seen in minority of tumor cells in only two cases. Dysplastic squamous epithelium adjacent to HPV DNA ISH-positive invasive SCC harbored HPV DNA as well.
a Positive HPV DNA ISH signal appears as mainly two navy-blue dots within nuclei of nonkeratinizing squamous cell carcinoma (NK-SCC) (original magnification ×400). b Positive HPV DNA ISH signal appears as mainly two navy-blue dots within nuclei of keratinizing squamous cell carcinoma (K-SCC) (original magnification ×400). c Positive HPV E6/E7 mRNA ISH signal appears as clusters in most of tumor cells of NK-SCC (original magnification ×400). d Positive HPV E6/E7 mRNA ISH signal appears as clusters or single dots in most of tumor cells of K-SCC (original magnification ×400). a, c The same NK-SCC harboring HPV16 and b, d the same K-SCC harboring HPV35
A total of 62/73 (85 %) study samples and all 20 control cases were suitable for detection of HPV DNA by PCR. Eight of all 62 (13 %) tumors—SCCs only—harbored HPV DNA PCR, including 6/22 (27 %) NK/NKM-SCCs and 2/16 (13 %) K-SCCs. DNA of types HPV16 (3×), HPV18 (4×), and HPV35 (1×) was detected. No low risk HPV DNA was found.
All non-SCC tumor types as well as all control cases were HPV DNA ISH/PCR-negative.
HPV E6/E7 mRNA ISH and RT-PCR
All 73 study samples and 20 control cases were successfully stained for HPV E6/E7 mRNA by ISH and easily readable without equivocal results.
Thirteen of 73 (18 %) tumors—SCCs only—harbored HPV E6/E7 mRNA ISH transcripts, including 11/27 (41 %) NK/NKM-SCCs (Fig. 2c) and 1/16 (6 %) K-SCC (Fig. 2d). In seven cases, the nuclear/cytoplasmic signal (1 to 10 dots per cell) was seen in approx. 10 % tumor cells only at ×200–400 magnification, while in the remaining six cases, up to 90 % tumor cells showed nuclear/cytoplasmic signal (more than 10 dots per cell and/or dot clusters) clearly visible at ×40–100 magnification. The dysplastic squamous epithelium adjacent to the positive invasive SCC harbored HPV E6/E7 mRNA as well.
A total of 70/73 (96 %) study samples and all 20 control cases were suitable for detection of HPV E6/E7 mRNA by RT-PCR. Nine of 70 (13 %) tumors harbored HPV E6/E7 mRNA: eight SCCs and one SCNEC. E6/E7 mRNA transcripts of types HPV16 (5×), HPV18 (3×), and HPV35 (1×) were detected.
All other tumor samples as well as all control cases were HPV E6/E7 mRNA ISH/RT-PCR-negative.
Comparison of HPV detection methods and p16 IHC
The comparison between p16 IHC and HPV detection methods is listed in Table 3. Regarding the entire study sample, a total of 18/73 (25 %) tumors were HPV-positive. These included 17 SCCs (13× NK/NKM-SCCs, 2× K-SCCs, 2× basaloid SCCs) and 1 SCNEC. Altogether, we found HPV16 (5×), HPV18 (5×), and HPV35 (1×). In the other seven cases, the HR-HPV type remained unknown. In only 3/18 (17 %) HPV-positive cases, HPV E6/E7 mRNA ISH/PCR transcripts were not detected (2× NK/NKM-SCCs with unknown HPV type (1× p16-positive, 1× p16-negative), 1× K-SCC harboring HPV18 DNA) indicating that a total of 15/18 (83 %) HPV-positive cases showed evidence of transcriptionally active HR-HPV infection. Only 2/18 (11 %) HPV-positive cases were p16 IHC-negative (1× NK/NKM-SCC, 1× K-SCC). On the contrary, among the 25/73 (34 %) p16-positive cases from the whole study sample, 16/25 (64 %) were HPV-positive. The nine p16-positive/HPV-negative cases included six adenoid cystic carcinomas and NK/NKM-SCC, SCNEC, and SNUC, one each). Regarding the SCC group, among the 16/49 (33 %) p16-positive SCCs, 15/16 (94 %) were HPV-positive.
As expected, there was strong and statistically significant correlation between the HPV detection methods used and also between HPV methods and p16 IHC (p < 0.0001 in most cases; Fisher’s test) (Table 4). In general, the correlation between p16 IHC and HPV E6/E7 mRNA ISH/PCR was stronger than between p16 IHC and HPV DNA ISH/PCR. The correlation index between p16 IHC and HPV status was highest in the SCC group (R = 0.864).
Comparison between HPV-positive versus HPV-negative SCCs
The follow-up period for the SCC group ranged from 1 to 104 months (median 16 months). The median survival for all SCC patients was 24 months (95 % CI 14 to 36 months). Eight of 49 (16 %) patients were lost to follow-up. The follow-up period for the HPV-positive SCC patients ranged from 2 to 91 months (median 23 months) and for the HPV-negative SCC patients from 1 to 104 months (median 14 months). The median survival for the HPV-positive SCC patients was 30 months (95 % CI 19 to 71 months), while for the HPV-negative SCC patients, it was 14 months (95 % CI 10 to 24 months) (p = 0.23, Log-rank test) (Fig. 3). HPV-positive SCCs occurred more frequently in smokers/ex-smokers (p = 0.04) and were more frequently NK/NKM-SCCs (p = 0.02) and p16-positive (p < 0.0001). The follow-up for the p16-positive SCC patients ranged from 2 to 91 months (median 19 months) and for the p16-negative patients from 1 to 104 months (median 16 months). The median survival for the p16-positive SCC patients was 71 months (95 % CI 23 to 73 months), while for the p16-negative SCC patients, it was 16 months (95 % CI 10 to 30 months) (p = 0.13, Log-rank test) (Fig. 4). The p16-positive SCCs occurred more frequently in smokers/ex-smokers (p = 0.04), were localized in the nasal cavity (p = 0.05), and were NK/NKM-SCCs (p = 0.005). No other relationships between the HPV/p16 status and the parameters studied were found (p > 0.05).
Similar analysis was also performed for the whole study sample but did not reveal any additional significant findings (not shown).
Discussion
HR-HPV is an important risk factor for a subset of head and neck cancer, particularly in oropharyngeal SCC with highly variable detection rates, reaching up to 80 % [12, 13, 39, 40]. HPV-positive oropharyngeal SCC usually affects slightly younger patients, nonsmokers or light smokers, nondrinkers, patients with good oral hygiene, and probably higher (oral) sexual exposure [11]. HPV-positive carcinomas carry better prognosis than HPV-negative SCCs of the oropharynx [41]. HPV-positive oropharyngeal SCC is currently regarded as a distinct clinicopathologic tumor entity and merits to be included as a new entity in the forthcoming WHO classification of head and neck tumors [30]. In contrast, HPV DNA and/or transcriptionally active HPV is only rarely found in SCCs of the oral cavity, larynx, and hypopharynx [42–45].
Sinonasal carcinomas comprise only about 3 % of all head and neck carcinomas, and their incidence rate seems to be decreasing [24]. It is a heterogenous group of malignant tumors, the etiopathogenesis of which remains largely unknown. Most sinonasal SCCs arise de novo, but some may develop from inverted or oncocytic preexisting papillomas [4]. One paper reported that 11 % of inverted papillomas, mostly of the SCC type, were complicated by carcinoma [46]. HPV DNA was found in 20–40 % inverted papillomas, more frequently in recurring papillomas and those with dysplastic changes or frankly invasive carcinoma [47, 48].
Surprisingly, however, most SCCs arising from inverted papillomas do not contain transcriptionally active HPV [21]. In our series, 5/49 SCCs (10 %) arose with/from inverted papillomas (4× with dysplastic changes). All were HPV ISH/PCR-negative, but one was p16- and HPV E6/E7 mRNA ISH-positive. These data indicate that although HR-HPV may contribute to the formation of inverted papillomas, SCCs that develop in that background mostly do not retain transcriptionally active HPV. Some authors regard HPV presence in inverted papillomas rather as incidental colonization than as etiological factor [49]. Focal mild-to-moderate p16 expression is seen in most inverted papillomas regardless of HPV status, which is not useful for identification of high-risk lesions [50]. Oncocytic and exophytic papillomas rarely harbor HR-HPV, although the latter commonly contain low-risk HPV types [47]. Regarding the prevalence of HPV infection in sinonasal mucosa in healthy persons, Jenko et al. [49] detected HPV in 15 % of their control cases, whereas all our control cases were HPV ISH/PCR-negative.
Regardless of the associated lesion, 27 % of sinonasal carcinomas were HPV-positive in a recent meta-analysis [2]. We found HPV-positive SCCs more often in smokers/ex-smokers (p = 0.04). The HPV types most commonly detected include HPV16, HPV18, HPV35, and HPV45 [16, 18, 22], which corresponds with the findings observed in our study. Limited data have been published on detailed morphology of HPV-positive sinonasal carcinomas [16, 18, 21–23]. Conventional sinonasal SCC may also be subclassified into NK-SCC, NKM-SCC, and K-SCC subtypes, following recently proposed criteria [28, 29]. NK-SCC and NKM-SCC probably correspond to the cylindrical cell, transitional, and Schneiderian carcinomas [24]. While subtype, p16 expression, and HPV status are associated in oropharyngeal SCC [39, 40], in sinonasal SCC, this is less clear [16, 18, 21, 22]. We found a statistically highly significant correlation between p16 IHC and HPV status (p < 0.0001, R = 0.864) and therefore confirm p16 IHC as a reliable surrogate marker for HPV status also in sinonasal SCCs.
As regards our 49 SCC cases, 27 (55 %) were NK/NKM-SCC, and 16 (33 %) were K-SCCs. NK/NKM-SCCs occurred more frequently in the nasal cavity (p = 0.025), but the significance of this finding remains unclear. Of the 16 K-SCCs, only two (13 %) were HPV DNA ISH/PCR-positive (HPV18 and HPV35), but transcriptionally active HPV(35) infection was detected in only one case. In contrast, of the 27 NK/NKM-SCC cases, 11 (41 %) showed transcriptionally active HR-HPV infection (HPV16 and HPV18), 10 of which were p16-positive. The difference regarding HPV/p16 status was statistically significant (p = 0.02; p = 0.005).
The true prevalence of HPV in sinonasal malignant tumors other than SCC is unknown. HPV is present in a minority of SCNEC and SNUC cases [24]. However, although these tumors usually lack HPV, they may be p16-positive. We observed diffuse p16 expression in 2/2 SCNECs; only one of which was HPV16-positive and in 1/3 SNUC. As reported earlier, we found that adenoid cystic carcinoma of the salivary glands often strongly express p16 expression without containing HPV [51]. It is the predominant p16 expression in cells lining the true ductal structures and HPV absence in adenoid cystic carcinoma which differentiates it from the recently described peculiar sinonasal tumor called “HPV-positive adenoid cystic-like carcinoma,” characterized by diffuse p16 expression [52]. In general, as the specificity (=0.65) of p16 IHC for HPV-positive status and the association between p16 IHC and HPV status (R = 0.269) were both low, p16 IHC cannot be recommended as a surrogate marker of HPV status in non-SCC sinonasal carcinoma.
HPV status seems to be associated with better outcome in sinonasal cancer. Alos et al. and Larque et al. found better progression free survival and overall survival for HPV-positive compared to HPV-negative [18, 22], while in the series reported by Bishop et al., this was not statistically significant [21]. Likewise, Takahashi et al. did not find any significant difference regarding HPV status, but the number of HPV/p16-positive SCC patients was too small to allow reliable conclusions [23]. We found a median survival for HPV-positive SCC patients of 30 months, while for HPV-negative patients, this was 14 months, but this difference was not statistically significant (p = 0.23), most probably due to incomplete follow-up data.
In summary, we confirm that HR-HPV is actively involved in the etiopathogenesis of a significant subset of sinonasal SCCs, mostly of nonkeratinizing morphology. Detection of HPV E6/E7 mRNA transcripts by ISH strongly correlated with results of other HPV detection methods and p16 expression. In oropharyngeal and sinonasal SCCs, p16 expression is a reliable surrogate marker for HPV status but not in non-SCC sinonasal carcinomas. As SCCs in smokers and in the nasal cavity are more often HPV-positive, we hypothesize that chemical substances from cigarette might activate preexisting HPV infection and contribute to the pathogenesis. Alternatively, nasal mucosa chronically damaged by cigarette smoke derivatives might be more susceptible for HPV infection. Although we observed a trend toward better overall survival in HPV-positive SCCs, the prognostic impact of HPV status in sinonasal carcinomas needs to be elucidated by further studies.
References
Zvolsky M (2014) Incidence of malignant neoplasms in the Czech Republic in 2011. http://www.uzis.cz/rychle-informace/zhoubne-nadory-roce-2011. Accessed 1 March 2015
Syrjänen K, Syrjänen S (2013) Detection of human papillomavirus in sinonasal carcinoma: systematic review and meta-analysis. Hum Pathol 44:983–991. doi:10.1016/j.humpath.2012.08.017
Turner JH, Reh DD (2012) Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 34:877–885. doi:10.1002/hed.21830
Barnes L, Eveson JW, Reichart P, Sidransky S, World Health Organization (2005) Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. IARC Press, Lyon, pp 9–80
’t Mannetje A, Kogevinas M, Luce D, Demers PA, Bégin D, Bolm-Audorff U et al (1999) Sinonasal cancer, occupation, and tobacco smoking in European women and men. Am J Ind Med 36:101-107
de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B et al (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11:1048–1056. doi:10.1016/S1470-2045(10)70230-8
de Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM et al (2013) Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer 49:3450–3461. doi:10.1016/j.ejca.2013.06.033
Alemany L, de Sanjosé S, Tous S, Quint W, Vallejos C, Shin HR et al (2014) Time trends of human papillomavirus types in invasive cervical cancer, from 1940 to 2007. Int J Cancer 135:88–95. doi:10.1002/ijc.28636
Alemany L, Saunier M, Tinoco L, Quirós B, Alvarado-Cabrero I, Alejo M et al (2014) Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer 50:2846–2854. doi:10.1016/j.ejca.2014.07.018
Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin HR et al (2015) Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 136:98–107. doi:10.1002/ijc.28963
Nováková V, Laco J (2008) Role of human papillomavirus in carcinogenesis of head and neck cancer. Klin Onkol 21:141–148
Laco J, Vosmikova H, Novakova V, Celakovsky P, Dolezalova H, Tucek L et al (2011) The role of high risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch 458:179–187. doi:10.1007/s00428-010-1037-y
Laco J, Nekvindova J, Novakova V, Celakovsky P, Dolezalova H, Tucek L et al (2012) Biologic importance and prognostic significance of selected clinicopathological parameters in patients with oral and oropharyngeal squamous cell carcinoma, with emphasis on smoking, protein p16INK4a expression, and HPV status. Neoplasma 59:398–408. doi:10.4149/neo_2012_052
Buchwald C, Lindeberg H, Pedersen BL, Franzmann MB (2001) Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish Epidemiological study 1980-1998. Laryngoscope 111:1104–1110
Syrjänen KJ (2003) HPV infections in benign and malignant sinonasal lesions. J Clin Pathol 56:174–181
El-Mofty SK, Lu DW (2005) Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol 29:1367–1372
McKay SP, Grégoire L, Lonardo F, Reidy P, Mathog RH, Lancaster WD (2005) Human papillomavirus (HPV) transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope 115:1428–1431
Alos L, Moyano S, Nadal A, Alobid I, Blanch JL, Ayala E et al (2009) Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favourable outcome. Cancer 115:2701–2709. doi:10.1002/cncr.24309
Jo VY, Mills SE, Stoler MH, Stelow EB (2009) Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol 33:1720–1724. doi:10.1097/PAS.0b013e3181b6d8e6
Cheung FM, Lau TW, Cheung LK, Li AS, Chow SK, Lo AW (2010) Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J 89:E5–E12
Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI et al (2013) Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol 37:185–192. doi:10.1097/PAS.0b013e3182698673
Larque AB, Hakim S, Ordi J, Nadal A, Diaz A, del Pino M et al (2014) High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol 27:343–351. doi:10.1038/modpathol.2013.155
Takahashi Y, Bell D, Agarwal G, Roberts D, Xie TX, El-Naggar A et al (2014) Comprehensive assessment of prognostic markers for sinonasal squamous cell carcinoma. Head Neck 36:1094–1102. doi:10.1002/hed.23423
Lewis JS Jr, Westra WH, Thompson LD, Barnes L, Cardesa A, Hunt JL et al (2014) The sinonasal tract: another potential “hot spot“ for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol 8:241–249. doi:10.1007/s12105-013-0514-4
Sobin LH, Gospodarowicz MK, Wittekind C (eds). TNM Classification of Malignant Tumours, 7th ed. Wiley-Blackwell, 2009, pp. 47-50 (Czech ed. 2011)
Cordes B, Williams MD, Tirado Y, Bell D, Rosenthal DI, Al-Dhahri SF et al (2009) Molecular and phenotypic analysis of poorly differentiated sinonasal neoplasms: an integrated approach for early diagnosis and classification. Hum Pathol 40:283–292. doi:10.1016/j.humpath.2008.07.019
Franchi A, Palomba A, Cardesa A (2011) Current diagnostic strategies for undifferentiated tumours of the nasal cavities and paranasal sinuses. Histopathology 59:1034–1045. doi:10.1111/j.1365-2559.2011.03813.x
Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS Jr (2009) HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol 3:186–194. doi:10.1007/s12105-009-0126-1
Lewis JS Jr, Khan RA, Masand RP, Chernock RD, Zhang Q, Al-Naief NS et al (2012) Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma – a prospective cohort and interobserver variability study. Histopathology 60:427–436. doi:10.1111/j.1365-2559.2011.04092.x
Lewis JS Jr, Chernock RD (2014) Human papillomavirus and Epstein-Barr virus in head and neck carcinomas: suggestions for the new WHO classification. Head Neck Pathol 8:50–58. doi:10.1007/s12105-014-0528-6
Singhi AD, Westra WH (2010) Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 116:2166–2173. doi:10.1002/cncr.25033
Lewis JS Jr (2012) p16 immunohistochemistry as a standalone test for risk stratification in oropharyngeal squamous cell carcinoma. Head Neck Pathol 6:S75–S82. doi:10.1007/s12105-012-0369-0
Lewis JS Jr, Chernock RD, Ma XJ, Flanagan JJ, Luo Y, Gao G et al (2012) Partial p16 staining in oropharyngeal squamous cell carcinoma: extent and pattern correlate with human papillomavirus RNA status. Mod Pathol 25:1212–1220. doi:10.1038/modpathol.2012.79
Dreyer JH, Hauck F, Oliveira-Silva M, Barros MH, Niedobitek G (2013) Detection of HPV infection in head and neck squamous cell carcinoma: a practical proposal. Virchows Arch 462:381–389. doi:10.1007/s00428-013-1393-5
Seethala RR, Weinreb I, Carlson DL, McHugh JB, Harrison LB, Richardson MS et al (2013) Protocol for the examination of specimens from patients with carcinomas of the pharynx. http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/pharynx-13protocol-3300.pdf .Accessed 1 March 2015
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A et al (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin embedded tissues. J Mol Diagn 14:22–29. doi:10.1016/j.jmoldx.2011.08.002
Sato Y, Sugie R, Tsuchiya B, Kameya T, Natori M, Mukai K (2001) Comparison of the DNA extraction methods for polymerase chain reaction amplification from formalin-fixed and paraffin-embedded tissues. Diagn Mol Pathol 10:265–271
Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H et al (2013) A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer 132:882–890
Lewis JS Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q et al (2010) p16 positive oropharyngeal squamous cell carcinoma: an entity with a favourable prognosis regardless of tumor HPV status. Am J Surg Pathol 34:1088–1096. doi:10.1097/PAS.0b013e3181e84652
Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS Jr (2011) High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol 35:1343–1350. doi:10.1097/PAS.0b013e318220e59d
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. doi:10.1056/NEJMoa0912217
Laco J, Slaninka I, Jirasek M, Celakovsky P, Vosmikova H, Ryska A (2008) High-risk human papillomavirus infection and p16INK4a protein expression in laryngeal lesions. Pathol Res Pract 204:545–552. doi:10.1016/j.prp.2008.03.001
Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M (2012) Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol 6(Suppl 1):S104–S120. doi:10.1007/s12105-012-0368-1
Lewis JS Jr, Ukpo OC, Ma XJ, Flanagan JJ, Luo Y, Thorstad WL et al (2012) Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas – a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology 60:982–991. doi:10.1111/j.1365-2559.2011.04169.x
Combes JD, Franceschi S (2014) Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol 50:370–379. doi:10.1016/j.oraloncology.2013.11.004
Barnes L (2002) Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol 15:279–297
Syrjänen K, Syrjänen S (2013) Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope 123:181–192. doi:10.1002/lary.23688
Lawson W, Schlecht NF, Brandwein-Gensler M (2008) The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol 2:49–59. doi:10.1007/s12105-008-0048-3
Jenko K, Kocjan B, Zidar N, Poljak M, Strojan P, Žargi M et al (2011) In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Arch 459:529–538. doi:10.1007/s00428-011-1139-1
Shah AA, Evans MF, Adamson CS, Peng Z, Rajendran V, Cooper K (2010) HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16INK4a immunoreactivity. Head Neck Pathol 4:106–112. doi:10.1007/s12105-010-0176-4
Skalova A, Kaspirkova J, Andrle P, Hosticka L, Vanecek T (2013) Human papillomaviruses are not involved in the etiopathogenesis of salivary gland tumors. Cesk Patol 49:72–75
Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI et al (2013) Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol 37:836–844. doi:10.1097/PAS.0b013e31827b1cd6
Acknowledgments
The authors are very grateful to Professor Ivo Šteiner, MD, PhD, and to Folakemi A. Sobande, MD, PhD, for English language correction.
The experiments carried out in this study comply with the current laws of the Czech Republic.
This study is based on the experiments conducted by one of the co-authors, Kateřina Sieglová, during her undergraduate studies for Master degree at the Charles University in Prague, Faculty of Pharmacy in Hradec Kralove. The study was supported by the programs PRVOUK P37/11 and PRVOUK P27/LF1/1 and by the project BBMRI LM2010004. The funding sources had no involvement on the study design, collection, analysis, and interpretation of data, on the writing of the report, and on the decision to submit the article for publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Laco, J., Sieglová, K., Vošmiková, H. et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch 467, 405–415 (2015). https://doi.org/10.1007/s00428-015-1812-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1812-x