Abstract
The aim of the study was to investigate the role of high-risk human papillomavirus (HR-HPV) infection in the etiopathogenesis of oral (OSCC) and oropharyngeal (OPSCC) squamous cell carcinoma in non-smoking and non-drinking patients (NSNDP). Twenty-four OSCCs and 22 OPSCCs were analyzed by immunohistochemistry for p16INK4a protein (p16) expression and by chromogene in situ hybridization (CISH) and polymerase chain reaction (PCR) for HR-HPV DNA presence. The series included 23 males and 23 females aged 35–93 years. p16 expression was seen in 7 out of 24 (29%) OSCCs and in 22 out of 22 (100%) OPSCCs. Using CISH, HR-HPV DNA was observed in 6 out of 24 (25%) OSCCs and in 21 out of 22 (95%) OPSCCs. HPV DNA was found in 3 out of 24 (13%) OSCCs and in 18 out of 22 (82%) OPSCCs using PCR. HPV 16 and 33 were detected in 16 and in two cases, respectively. Compared with OSCCs, OPSCCs more frequently showed basaloid morphology (p < 0.0001), lymph node involvement (p = 0.0063), diffuse p16 expression (p < 0.0001), HR-HPV DNA presence using both CISH and PCR (p < 0.0001; p < 0.0001), and better outcome. The sensitivity and specificity of p16 expression for HR-HPV DNA presence detected by CISH were 0.89 and 0.95, respectively, and 0.95 and 0.85 for PCR detected HPV DNA. The sensitivity and specificity of CISH for PCR detected presence of HPV DNA were 1.00 and 0.73, respectively. Our study is the first larger study analyzing OSCC and OPSCC in NSNDP. Our results indicate that unlike OSCC, a vast majority of OPSCCs may be associated with HR-HPV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous cell carcinomas (SCCs) are the most common malignancies in the head and neck area. Traditionally, they are associated with smoking and alcohol abuse, which still represent important risk factors. Since the 1980s, however, evidence has been emerging, suggesting that a subset of oropharyngeal SCCs (OPSCCs), in contrast to oral SCCs (OSCCs), is associated with oral high-risk human papillomavirus (HR-HPV) infection [1]. The prevalence of HPV-positive head and neck SCCs (HNSCCs) shows considerable regional variation. In the last decade, at least 20 studies on this topic were published, reporting a prevalence range of HPV-positive HNSCC as wide as 0% to 93% [2]. In a multicentre case–control study organized by the International Agency for Research on Cancer, a total of 70% of HNSCCs were shown to harbor HPV DNA, with HPV 16 being the most commonly observed type [3].
HPV-positive and HPV-negative HNSCCs seem to be separate clinicopathologic entities. The former occur in younger patients without sex predilection; show poorly differentiated and/or basaloid morphology; display minimal p53, cyclin D1, and epidermal growth factor receptor expression; and feature good prognosis despite frequent regional lymph node metastases. The reverse is true for HPV-negative HNSCCs in most cases [4].
The incidence of HPV-positive HNSCCs is increasing worldwide. A recent study from Sweden reports that 23% of tonsillar carcinomas were HPV positive in the 1970s whereas there was up to 93% positivity in 2006–2007 [5].
Because of the considerable regional prevalence differences and lack of large studies focused on patients without personal history of classic risk factors for HNSCC, we investigated HPV status and other clinicopathologic characteristics in a group of non-smoking and non-drinking patients with oral and oropharyngeal SCC in the Czech Republic using three different detection methods, with the aim to clarify the etiopathogenesis of HNSCCs in this patients’ cohort. Furthermore, we compared selected clinicopathological parameters of the patients and/or tumors with particular regard to the anatomic localization and HPV status of the tumors.
Material and methods
An institutional computer search revealed a total of 289 OSCCs and 214 OPSCCs diagnosed between 2000 and 2009 at The Fingerland Department of Pathology, Faculty Hospital, Hradec Kralove, Czech Republic in patients who had undergone surgical resection of these lesions. To eliminate the role of actinic damage, SCCs of lips were excluded from the search.
Twenty-four out of 289 (8%) of the patients with OSCC and 22 out of 214 (10%) of the patients with OPSCC had no personal history of either smoking or chronic alcohol abuse. These cases were the subject of this study. Ethical approval was obtained from the Ethics Committee, Faculty Hospital Hradec Kralove.
For every patient, gender and age were recorded. Tumors occurring in the oral vestibulum, on the cheek, hard palate, floor of mouth, gingival/alveolar mucosa, and on the anterior tongue (the first 2/3 of the tongue) were classified as OSCCs, whereas OPSCCs included tumors of the root of tongue, palatine tonsils and arches, soft palate, and the posterior wall of the oropharynx [6]. In every case, tumor size (“pT” category), presence of microscopically confirmed regional lymph node metastases (“pN” category), and presence of distant metastases (“cM” category) were recorded. Histologic subtypes of SCC (“conventional”, verrucous, basaloid, papillary, spindle cell, acantholytic, adenosquamous, and carcinoma cuniculatum) and differentiation grade in cases of “conventional” SCC were recorded according to the current WHO classification of head and neck tumors [6]. Pathologic staging was done from the material from radical tumor resection with neck lymph node dissection at the time of presentation according to current TNM classification [7]. As controls, we examined 10 fibromas from various parts of oral cavity covered by normal squamous epithelium and 10 tonsils with chronic tonsillitis. The control lesions were obtained from gender- and age-matched patients.
During the follow-up period, data on local, regional, and locoregional recurrence; on occurrence of distant metastases; on development of other metachronous malignant tumors; and on tumor-associated death were recorded. After resection, the specimens were immediately fixed in 10% formalin, routinely processed, embedded in paraffin, and stained with hematoxylin–eosin. Paraffin blocks for further analysis were available in all cases.
For immunohistochemical detection of p16INK4a protein (p16), the CINtec® Histology Kit (mtm laboratories AG, Heidelberg, Germany) was used according to the manufacturer’s manual. Samples of cervical intraepithelial lesion grade III were used as positive controls. Control slides provided by the manufacturer were used as negative controls. Brown staining of tumor cell nuclei and/or cytoplasm was interpreted as positive. The p16 immunostaining was scored according to criteria set out in our previous study [8] as follows: 0 absent, + 1–5% tumor cells stained, ++ 6–20% tumor cells stained, +++ 21–50% tumor cells stained, and ++++ 51–100% tumor cells stained.
In addition, the samples were examined using molecular genetic methods. First, HR-HPV DNA detection was performed by chromogene in situ hybridization (CISH) using the GenPoint™ HPV biotinylated DNA probe (Dako, Glostrup, Denmark), which detects 13 HR-HPV types, namely 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, according to the manufacturer’s manual. Briefly, after sample preparation, the DNA probe was added and denaturated by placing the slides on a heating block at 92°C for 5 min. Following denaturation, the slides were transferred to a humid chamber for hybridization at 37°C for 18 h. Then, detection of the hybridized probe was performed using the Genpoint™ Detection System (Dako) according to the kit instructions using primary streptavidin-horseradish peroxidase (HRP; dilution 1:100), biotinyl tyramide, and secondary streptavidin-HRP. Finally, DAB was used as chromogene. Samples of cervical intraepithelial lesion grade III were used as positive controls. The correct performance of the staining procedure was checked using Positive Control (Human DNA) Biotinylated DNA Probe and Negative Control (Plasmid DNA) Biotinylated DNA Probe (Dako) instead of GenPoint™ HPV Biotinylated DNA Probe. Only the brown signals from tumor cell nuclei were interpreted as positive. The CISH results were scored according to our previous study [8] as follows: 0 absent, + 1–5% epithelial cells stained, and ++ >5% epithelial cells stained.
HPV DNA detection was then performed by polymerase chain reaction (PCR) as follows: HPV DNA was extracted from paraffin-embedded tissue after deparaffinization in xylene and rehydration in ethanol using the commercial QIAamp DNA FFPE Tissue Kit (Qiagen GmBH, Hilden, Germany) according to the manufacturer’s protocol. PCR amplification of β-globin sequences was performed to confirm sample fitness for PCR assay. All samples were screened for presence of HPV DNA by PCR amplification with primers GP5+/GP6+ located within the HPV L1 gene. The sequences of the forward and reverse primers used were 5′-TTTGTTACTGTGGTAGATACTAC-3′ (GP5+) and 5′-GAAAAATAAACTGTAAATCATATT-3′ (GP6+). PCR reaction was performed in a volume of 25 μL, containing 25 mM MgCl2, 2.5 mM of each dNTP, 2.5 U of Takara Taq polymerase (Takara Bio Inc., Shiga, Japan), 100 pmol of each primer (GP5+/GP6+), and 2 μL of HPV DNA at various dilutions. The PCR protocol was then carried out with an initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 60 s, and extension at 72°C for 45 s. Amplified products were run on 2% agarose gel and stained with ethidium bromide for size verification.
Samples showing HPV DNA presence by the above-mentioned procedure were subsequently analyzed by Linear Array HPV Genotyping Test (Roche, Basel, Switzerland). The manufacturer’s protocol was modified to adapt the test for use on paraffin-embedded tissue according to Siriaunkgul et al. [9]. The test involves three steps: PCR amplification of target DNA, nucleic acid hybridization, and detection of 37 HPV types, specifically 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73 (MM9), 81, 82 (MM4), 83 (MM7), 84 (MM8), IS39, and CP6108. PCR was performed in a total volume of 100 μL containing 50 μL of the manufacturer’s master mix and 50 μL of GP5+/GP6+ PCR product. The amplification program consisted of 2 min at 50°C and 9 min at 95°C, followed by 40 cycles of 30 s at 50°C, of 1 min at 55°C, and of 1 min at 72°C, with a final extension at 72°C for 5 min. The PCR product was denaturated with denaturation solution and hybridized on to a strip containing specific probes for the 37 above-mentioned HPV types and β-globin reference lines. Detection was carried out using streptavidin-HRP and 0.1% tetramethylbenzidine as a chromogen. Positive reaction was visible as a blue line on the strip.
Statistical analysis using NCSS 2007 program, chi-squared test, Fisher’s exact test, and two-sample t test was performed. Differences were considered statistically significant at p < 0.05.
Results
The study sample consisted of 23 male and 23 female patients aged 35–93 years (mean 63 ± 15 years; median 61 years). The clinicopathological data of the patients are shown in Table 1. None of the patients had a personal history of any precancerous disorder for OSCC or OPSCC, namely oral lichen planus, oral submucous fibrosis, and proliferative verrucous leukoplakia. The follow-up information was available in 44 out of 46 (96%) patients and ranged from 7 to 168 months (median 41 months). Twenty-eight patients underwent adjuvant radiotherapy only, 14 patients underwent adjuvant chemoradiotherapy, and two patients underwent adjuvant chemotherapy only. Among the OSCC patients, 9 out of 22 (41%) developed local, 10 out of 22 (46%) regional, and 12 out of 22 (55%) locoregional recurrences; 1 out of 22 (5%) patient developed distant metastases in skeleton; 5 out of 22 (23%) patients developed other metachronous malignancies (SCC of tongue, SCC of larynx, colonic adenocarcinoma, chronic B-lymphocytic leukemia/small cell lymphoma, and Langerhans cell histiocytosis each); and 16 out of 22 (73%) patients died due to OSCC. Among the OPSCC patients, 3 out of 22 (14%) developed local, 1 out of 22 (5%) regional, and 4 out of 22 (18%) locoregional recurrences; 2 out of 22 (9%) patients developed distant metastases in skeleton and in liver each; 3 out of 22 (14%) patients developed other metachronous malignancies (SCC of lung, neuroendocrine carcinoma of pancreas and chronic B-lymphocytic leukemia/small cell lymphoma each); and 7 out of 22 (32%) patients died due to OPSCC.
The 24 OSCC cases included SCCs of the anterior tongue (4× left, 4× right), cheek (4× left, 2× right), gingival/alveolar mucosa (3× lower left, 1× upper left, 1× lower right), floor of mouth (1× left, 2× right), and the hard palate (2× left). The 22 OPSCC cases comprised SCCs of the tonsils (9× left, 8× right) and of the root of the tongue (3× left, 2× right).
The staging characteristics of the tumors are shown in Table 1. No tumor in the “pT4b” category was seen. Among the OSCCs, most tumors were advanced at presentation being classed as “pT4a” (8 out of 24), whereas the most common tumor size category of OPSCC was “pT2”, seen in 12 out of 22 cases. Regional lymph node metastases were found in 17 out of 22 of OPSCCs while the majority of OSCCs displayed no dissemination (16 out of 24 cases). All tumors characterized as “pN2” were actually “pN2b” except for one tonsillar tumor which featured “pN2c” status. No “pN2a” carcinoma was found. Distant metastases were not detected in any of the cases using imaging methods (“cM0”).
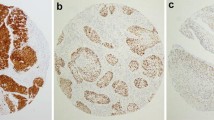
Microscopically, apart from one tumor of the floor of mouth which was classified as a spindle cell variant, all the cases of OSCCs were classified as “conventional” SCC. The “conventional” OSCCs were moderately differentiated in 17 out of 24 cases (Fig. 1a). Five of the tumors were graded as well differentiated and one as poorly differentiated. No tumor from the OSCC group featured basaloid morphology. Almost all OPSCCs showed basaloid morphology with pushing borders, frequent peripheral palisading of dark tumor cells, and common central comedo-like necroses (Fig. 2a). Only one tumor of right tonsil showed different morphological features and was classified as a moderately differentiated “conventional” SCC. The microscopic appearance of regional lymph node metastases followed that of the primary tumor in all cases.
a Moderately differentiated “conventional” squamous cell carcinoma of the anterior part of the tongue (HE, ×200). b Focal expression of p16 particularly in the superficial portion of the tumor (×200). This figure and Fig. 3 show identical case with HPV 16 subsequently detected by PCR
a Basaloid squamous cell carcinoma of the tonsil (HE, ×200). b Diffuse expression of p16 in the deeper portion of the tumor (×200). This figure and Fig. 4 show identical case with HPV 16 subsequently detected by PCR
The results of p16 expression and of HPV DNA presence, the latter detected using both CISH and PCR, are summarized in Table 2. p16 expression was detected in 7 out of 24 (29%) OSCCs and in 22 out of 22 (100%) OPSCCs. In OSCCs, the expression was usually weak and limited to less than 5% of tumor cells (four out of seven cases). Only one tumor of the anterior tongue was scored as +++, with 30% positive tumor cells (Fig. 1b). The p16-positive tumor cells appeared to be more frequently seen in the superficial portion of the tumors and at the periphery of the tumor nests. On the contrary, all the OPSCCs showed almost always strong diffuse p16 expression throughout the tumor tissue (Fig. 2b). Variable p16 expression was seen in dysplastic squamous epithelium adjacent to p16-positive invasive SCC in both groups. In the control group, isolated weakly p16-positive cells within non-dysplastic squamous epithelium were found in 3 out of 10 oral fibromas and in 2 out of 10 tonsils with chronic tonsillitis.
Using CISH, HR-HPV DNA was observed in 6 out of 24 (25%) OSCCs (Fig. 3) and in 21 out of 22 (95%) OPSCCs (Fig. 4). The distribution pattern of CISH positivity generally followed that of p16 expression in tumors in both groups. Positive CISH signals were seen as variably sized brown dots within the nuclei of neoplastic cells or as diffuse brown nuclear staining. All controls were uniformly CISH negative.
Using PCR, HPV DNA was found in 3 out of 24 (13%) OSCCs and in 18 out of 22 (82%) OPSCCs. Subsequent HPV typization was successful in 18 out of 21 cases. HPV 16 was the predominant type, detected in 16 cases (11× tonsil, 3× root of tongue, 1× anterior tongue, and 1× gingiva). Two tonsillar SCCs showed the presence of HPV 33. No low-risk HPV types were found. No HPV co-infection was observed.
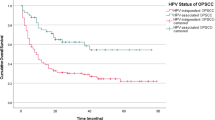
There was no statistically significant difference regarding the gender and age of patients and the “pT” tumor status between OSCC and OPSCC groups. OSCCs frequently showed “conventional” morphology whereas the basaloid variant (p < 0.0001) was the principal morphologic subtype of OPSCCs. Furthermore, OPSCCs showed more frequent regional lymph node involvement (p = 0.003), more frequent p16 expression (p < 0.0001), and more frequent HR-HPV DNA presence using both CISH and PCR (p < 0.0001; p < 0.0001), when compared to OSCCs. For the purpose of statistical analysis, “pN2b” and “pN2c” subcategories were regarded as one “pN2” category. Regarding the follow-up data, OPSCC patients experienced less frequent local (p = 0.042), regional (p = 0.002), and locoregional (p = 0.030) tumor recurrences and tumor-associated death (p = 0.007). There were no significant differences between OSCC and OPSCC patients regarding distant metastases development and occurrence of metachronous malignancies.
With regard to the HPV status assessed by PCR, the HPV-positive SCCs occurred more frequently in oropharynx (p < 0.0001), displayed more frequently basaloid morphology (p < 0.0001), showed more frequent lymph node dissemination (p = 0.006), and more frequent p16 expression (p < 0.0001), when compared to HPV-negative tumors. During the follow-up period, the patients with HPV-positive SCCs experienced less frequent regional recurrence (p = 0.005), when compared to the patients with HPV-negative tumors. There were no significant differences between HPV-positive and HPV-negative SCCs regarding the gender and age of the patients, the “pT” tumor status, the frequency of both local and locoregional recurrences, the development of distant metastases, the occurrence of metachronous malignancies, and the frequency of tumor-associated death.
The optimal sensitivity, specificity, positive predictive value, and negative predictive value of p16 expression for HR-HPV DNA presence were detected at positivity threshold level “more than 20% positive tumor cells” and were calculated 0.89, 0.95, 0.96, and 0.86 when compared to CISH (CISH used as gold standard) and 0.95, 0.85, 0.83, and 0.96 when compared to PCR (PCR used as gold standard). The optimal sensitivity, specificity, positive predictive value, and negative predictive value of CISH for HR-HPV DNA presence compared to PCR (when PCR used as gold standard) were detected at positivity threshold level of “any positive tumor cell” and were calculated 1.00, 0.73, 0.74, and 1.00. Calculated at the above-mentioned levels of threshold, the correlation index between p16 expression and HR-HPV DNA presence detected by CISH and PCR was 0.82 and 0.80, respectively. The correlation index between CISH and PCR was 0.74.
Discussion
Nowadays, it is widely accepted that persistent HR-HPV infection is the major cause of uterine cervix carcinoma, HPV 16 and 18 being responsible for about 70% of these tumors [10]. In addition, HR-HPVs have been shown to be associated with a subset of vulvar, penile, and anal carcinomas, as well [11]. The first study analyzing HPV status in HNSCCs was published in 1983 by Syrjänen et al. who suggested that HPV infection may be involved in the development of a subset of these lesions [12]. Subsequently in 1985, two series were published by de Villiers et al. [13] and by Löning et al. [14] who both found HPV infection in their cases of oropharyngeal cancer. Since that time, at least 30 studies on this topic were published in the English literature providing good evidence that oral HPV infection is an independent risk factor for HNSCCs [2,15]. The precise proportion of HPV-positive HNSCCs, however, varies considerably, ranging from 0% up to 93% [2]. This discordance may be partially explained by true regional differences in oral HPV infection prevalence probably influenced by different sexual habits in particular cultures, by precise anatomic localization of the tumors, and by different detection methods used in the studies [16]. In a multicentre case–control study on HNSCCs, 70% of tumors harbor HPV DNA, with HPV 16 being most commonly observed [3].
HPV-positive oral and oropharyngeal SCCs seem constitute a distinct entity, different from HPV-negative tumors [17]. They tend to occur in younger patients without sex predilection. Up to 20% of these cancers develop in patients without traditional risk factors, i.e., smoking and alcohol abuse. Conversely, their risk factors include young age at first intercourse, promiscuity, and history of genital warts in men and number of sexual partners in women [18]. As positive personal history of oral–genital and oral–anal sexual contact, during which the HPV infection may be transmitted to the oral cavity, increases the risk for HPV-positive HNSCCs, they may be regarded as sexually transmitted disease [2]. It is assumed that long-lasting oral HPV infection, which prevalence increases after the onset of sexual activity, precedes the development of HPV-positive HNSCC for about 10 years [3]. In addition, these tumors seem to be related to immunosuppression [17]. The influence of smoking on HPV-positive HNSCCs is yet unclear, suggesting either synergistic or additional effect [2].
Despite intensive research, larger studies analyzing selective cohorts of non-smoking and non-drinking patients with HNSCCs in detail are lacking. The results of previous studies with these patients included are summarized in Table 3 [19–32]. The number of examined cases is, however, low, not exceeding 20 patients in most studies. We found data on patients that were non-smokers and concurrently non-drinkers in only three studies [19–21]. All the other studies report either non-smokers or non-drinkers, making interpretation of the results and correlation even more difficult. The precise anatomic localization of the patients’ tumors is also difficult to identify. The HPV DNA detection rate ranged as wide as from 20% to 100% in non-smokers and from 0% to 82% in non-drinkers. In our study, HPV DNA was found in 3 out of 24 (13%) of OSCCs and in 18 out of 22 (82%) of OPSCCs using PCR, which was the most common detection method used in previous studies. The result differences may be caused by several factors, namely (1) different prevalence of oral HPV infection in studied populations which may range from 0% to about 11% [16], (2) low numbers of non-smokers and non-drinkers, (3) precise anatomical localization of the tumors (oral cavity versus oropharynx, see below), and (4) variations in the detection methods. Our findings indicate that the vast majority of OPSCCs developing in this cohort of patients are caused by HR-HPV infection. On the contrary, as only 13% of OSCCs in our patients showed HR-HPV DNA presence, other yet unidentified risk factors must exist for SCCs arising in this anatomic area which need to be elucidated by further studies.
HPV-positive HNSCCs show tendency to occur in the oropharynx, particularly in palatine tonsils and at the root of the tongue, both areas rich on lymphoid tissue [1]. This phenomenon was observed in our selective cohort as HPV infection was detected in 82% of OPSCCs, in contrast with only 13% HPV-positive cases of OSCC. The affinity of HPV to these anatomic areas may be explained by production of currently unspecified cytokines by lymphoid tissue and/or by deep invagination of the tonsillar surface and incompact squamous epithelium at the base of the tonsillar crypts, the latter factors blocking simple mechanical clearing of the epithelium and making the epithelium more susceptible to HPV infection [14].
Microscopically, HPV-positive HNSCCs tend to display basaloid and/or poorly differentiated morphology. This finding was confirmed in our series as this growth pattern was observed in 17 out of 21 (81%) of HPV-positive SCCs whereas it was seen in only 4 out of 25 (16%) of HPV-negative tumors. Interestingly, all four of these tumors occurred in the tonsil and at the root of the tongue, but PCR assay failed to prove HPV DNA presence. Conversely, only 4 out of 21 (19%) of “conventional” SCCs, arising on the gingiva, cheek, anterior part of the tongue, and in the tonsil, were found to contain HPV DNA by PCR.
The immunohistochemical characteristics of HPV-positive HNSCCs seem to be different from those of HPV-negative tumors. The former display minimal p53, cyclin D1, and epidermal growth factor receptor expression whereas the reverse is mostly true for the HPV-negative tumors [4].
The HPV types detected in HNSCCs include most frequently HPV 16, 18, and 33 [2,17]. Less commonly, HPV 35, 38, 52, and 59 as well as low-risk types 6 and 11 were observed. In our study, the HPV types observed were HPV 16 and 33 only, detected in 16 and in two cases, respectively.
PCR assay and the FDA-approved Hybrid Capture 2 assay may serve as gold standard detection methods for HPV testing [33]. In routine biopsy practice, the immunohistochemical detection of p16INK4a protein (p16), which is regarded as a sensitive marker of active HPV replication, is widely used as a surrogate marker, particularly in cervical pathology [34]. However, little is known about the application of this marker in HNSCCs. The sensitivity and specificity of p16 expression for HR-HPV DNA presence when compared to PCR were in our study 0.95 and 0.85, confirming the use of this marker as an acceptable tool in testing the HPV status of head and neck tumors. As the specificity of the particular chromogene in situ hybridization method used in our study for HR-HPV DNA presence when compared to PCR was only 0.73, this appears to be of little additional diagnostic value.
Importantly, HPV-associated HNSCCs show better outcome and reduced risk of recurrence, compared to HPV-negative tumors [1,17], as they were shown to display enhanced radiochemosensitivity. This finding was in part confirmed in our study, as despite frequent initial lymph node dissemination, HPV-positive SCCs showed less frequent regional recurrence rate whereas the opposite was true for HPV-negative tumors. Furthermore, the nodal status of HPV-positive HNSCCs was reported to be of little prognostic value [35]. It seems that HPV-positive HNSCC represents a distinct clinicopathological entity, for which more effective treatment options may develop in the near future different from HPV-negative tumors. Thus, HPV testing in every case of HNSCC is of particular importance.
In summary, we report the first large study analyzing in detail a selective cohort of patients with oral and oropharyngeal squamous cell carcinoma without positive personal history of smoking and alcohol abuse, classic risk factors for this type of malignancy. Our results indicate that the majority of oropharyngeal tumors developing in these patients are related to oral HPV infection whereas the viral etiology is responsible for a substantially smaller subset of tumors occurring in oral cavity, stressing the need for identification of further, still unknown, risk factors playing role in the pathogenesis of squamous cell carcinoma of the oral cavity.
References
Mannarini L, Kratochvil V, Calabrese L et al (2009) Human papilloma virus (HPV) in head and neck region: review of literature. Acta Otorhinolaryngol Ital 29:119–126
Lajer CB, von Buchwald C (2010) The role of human papillomavirus in head and neck cancer. APMIS 118:510–519
D’Souza G (2007) Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356:1125–1131
Nguyen NP, Chi A, Nquyen LM et al (2010) Human papillomavirus-associated oropharyngeal cancer: a new clinical entity. Q J Med 103:229–236
Nasman A, Attner P, Hammarstedt L et al (2009) Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer 125:362–366
Slootweg PJ, Eveson JW (2005) Tumours of the oral cavity and oropharynx. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) World Health Organization classification of tumours. Pathology and genetics of head and neck tumours, 1st edn. IARC, Lyon, pp 166–175
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, Chichester, pp 25–38
Laco J, Slaninka I, Jirasek M et al (2008) High risk human papillomavirus infection and p16INK4a protein expression in laryngeal lesions. Pathol Res Pract 204:545–552
Siriaunkgul S, Suwiwat S, Settakorn J et al (2008) HPV genotyping in cervical cancer in northern Thailand: adapting the linear array HPV assay for use on paraffin-embedded tissue. Gynecol Oncol 108:555–560
zur Hausen H (2002) Papillomavirus and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350
zur Hausen H (2009) Papillomaviruses in the causation of human cancers—a brief historical account. Virology 384:260–265
Syrjänen K, Syrjänen S, Lamberg M et al (1983) Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 12:418–424
de Villiers E, Weiddauer H, Otto H et al (1985) Papillomavirus DNA in human tongue carcinomas. Int J Cancer 36:575–578
Löning T, Ikenberg H, Becker J et al (1985) Analysis of oral papillomas, leukoplakias, and invasive carcinomas for human papillomavirus type related DNA. J Invest Dermatol 84:417–420
Glombitza F, Guntinas-Lichius O, Petersen I (2010) HPV status in head and neck tumors. Pathol Res Pract 206:229–234
Novakova V, Laco J (2008) Role of human papillomavirus in carcinogenesis of head and neck cancer. Klin Onkol 21:141–148 [in Czech]
Gillison ML (2004) Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol 31:744–754
Swartz SM, Daling JR, Doody DR et al (1998) Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst 90:1626–1636
Cruz IB, Snijders PJ, Steenbergen RD et al (1996) Age-dependence of human papillomavirus DNA presence in oral squamous cell carcinomas. Eur J Cancer B Oral Oncol 32B:55–62
Smith EM, Ritchie JM, Summersgill KF et al (2004) Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer 108:766–772
Koppikar P, de Villiers EM, Mulherkar R (2005) Identification of human papillomaviruses in tumors of the oral cavity in an Indian community. Int J Cancer 113:946–950
Tachezy R, Klozar J, Salakova M et al (2005) HPV and other risk factors of oral cavity/oropharyngeal cancer in the Czech Republic. Oral Dis 11:181–185
Slebos RJ, Yi Y, Ely K et al (2006) Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinomas. Clin Cancer Res 12(3Pt1):701–709
Weinberger PM, Yu Z, Haffty BG et al (2006) Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favourable prognosis. J Clin Oncol 24:736–747
Luo CW, Roan CH, Liu CJ (2007) Human papilloma viruses in oral squamous cell carcinoma and precancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg 36:153–158
Chuang AY, Chuang TC, Chang S et al (2008) Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol 44:915–919
Simonato LE, Garcia JF, Sundefeld ML et al (2008) Detection of HPV in mouth floor squamous cell carcinoma and its correlation with clinicopathologic variables, risk factors and survival. J Oral Pathol Med 37:593–598
Gudleviciene Z, Smailyte G, Mickonas A et al (2009) Prevalence of human papillomavirus and other risk factors in Lithuanian patients with head and neck cancer. Oncology 76:205–208
Lohavanichbutr P, Houck J, Fan W et al (2009) Genomewide gene expression profiles of HPV-positive and HPV-negative oropharyngeal cancer: potential implications for treatment choices. Arch Otolaryngol Head Neck Surg 135:180–188
Weinberger PM, Yu Z, Kountourakis P et al (2009) Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: validation of three class hypothesis. Otolaryngol Head Neck Surg 141:382–389
Luginbuhl A, Sanders M, Spiro JD (2009) Prevalence, morphology, and prognosis of human papillomavirus in tonsillar cancer. Ann Otol Rhinol Laryngol 118:742–749
Straetmans JM, Olthof N, Mooren JJ et al (2009) Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope 119:1951–1957
Zaravinos A, Mammas IN, Sourvinos G et al (2009) Molecular detection methods of human papillomavirus (HPV). Int J Biol Markers 24:215–222
von Knebel Doeberitz M (2002) New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infection. Eur J Cancer 38:2229–2242
Klozar J, Kratochvil V, Salakova et al (2008) HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol 265(Suppl 1):S75–S82
Acknowledgments
The authors thank Mrs. M. Zakova for her excellent technical support and Dr. F.A. Sobande for English language correction. The experiments carried out in this study comply with the current laws of the Czech Republic. This study was supported by the Research Project of the Ministry of Health of the Czech Republic No. 00179906.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laco, J., Vosmikova, H., Novakova, V. et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch 458, 179–187 (2011). https://doi.org/10.1007/s00428-010-1037-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-1037-y